| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 2, Number 2, September 2013, pages 84-89
Cerebellopontine Angle Chondroid Meningioma Apoplexy Complicating a Third Trimester Pregnancy: Case Report and Review of the Literature
Mark Rivkina, Alexandra Tomsb, Oleg Bronovb, Michel Lacroixc, Hope Hueizhi Wud, Steven A Tomsa, e
aDepartment of Neurosurgery, Geisinger Health System, 100 North Academy Avenue, Danville, PA 17822, USA
bDepartment of Radiology, Geisinger Health System, 100 North Academy Avenue, Danville, PA 17822, USA
cDepartment of Neurosurgery, Geisinger Wyoming Valley, 1000 E. Mountain Drive, Wilkes Barre, PA 18711, USA
dDepartment of Laboratory Medicine, Geisinger Health System, 100 North Academy Avenue, Danville, PA 17822, USA
eCorresponding author: Steven A. Toms, 100 North Academy Avenue, Danville, PA 17822, USA
Manuscript accepted for publication May 3, 2013
Short title: Meningioma Apoplexy
doi: https://doi.org/10.4021/jcgo138w
| Abstract | ▴Top |
We review a case of tumor apoplexy complicating third trimester pregnancy in order to highlight management of a rare complication of meningiomas during pregnancy. This case illustration along with a review of literature is presented for recognition of this unusual case. Although intratumoral hemorrhage in meningioma is not uncommon, life threatening apoplexy can occur during periods of rapid tumor growth, such as during pregnancy. Prompt recognition and obstetrical and neurosurgical coordination are required to achieve excellent outcomes for both patient and child.
Keywords: Apoplexy; Brain tumor; Intracranial hemorrhage; Intratumor hemorrhage
| Introduction | ▴Top |
Meningiomas account for 15 - 20% of all primary intracranial neoplasms [1]. They are commonly regarded as benign, slow growing neoplasms consisting of meningoepithelial cells originating from intracranial meninges [2, 3]. Meningioma occurrence is associated with previous trauma, radiation exposure, viruses, and genetic variations [4]. The most common location for these tumors is the cerebral convexity, which accounts for over 30% of cases, followed by parasagittal, and sphenoid ridge lesions [4, 5]. Meningiomas are more common in women by 2:1 ratio [4, 6].
Meningiomas are classified as Grade I (benign), II (atypical), or III (anaplastic) [7, 8]. Grade II meningiomas are defined by increased mitotic activity, brain infiltration, small cell changes, necrosis, or clear cell or chordoid histologic subtype [8, 9]. Grade III meningiomas may make up to 30% of all meningiomas [7], but the chordoid meningiomas are rare, accounting for 0.5 - 1.0% of intracranial meningiomas [10]. Chordoid meningiomas are predominantly tumors of young adults with supratentorial locations. There has been only one other report suggesting a propensity towards intratumoral hemorrhage in chordoid meningioma [11].
Clinical presentations in a majority of these lesions are secondary to local mass effect [4, 12]. Although the onset of symptoms is usually gradual, acute clinical decompensation can develop secondary to seizures or tumor associated hemorrhage that sometimes mimics pituitary apoplexy [3, 12]. Hormonal fluctuations during pregnancy, menopause, and menstrual cycle have been implicated in facilitating accelerated tumor growth leading to clinical symptoms [2, 6, 13-15]. Fewer than 3% of meningiomas have been reported to bleed in previous reports [1, 16-18].
Here, we present a case of a 26 years old female with an incidentally discovered cerebellopontine angle meningioma during third trimester pregnancy. Two weeks after initial neurosurgical evaluation, she presented acutely to an outside institution with a decreased level of consciousness and a dilated pupil. The patient underwent a CT scan showing an intratumoral hemorrhage and obstructive hydrocephalus. Transfer to a tertiary care center with subsequent neurosurgical and obstetrical interventions facilitated good clinical outcome for both mother and child.
| Case Report | ▴Top |
Initial presentation
A 26 years old 30-week pregnant female initially presented for neurosurgical evaluation of an incidentally discovered right cerebellopontine (CP) angle mass. She underwent a head CT as part of routine trauma evaluation after a motor vehicle accident (Fig. 1). During her emergency room evaluation, she admitted to progressive decreased hearing in right ear with intermittent tinnitus the past several months. She also described occasional pain behind the right eye and pain along right side of the face. A non-contrast MRI confirmed a large right CP angle mass consistent with meningioma (Fig. 2a, b, c).
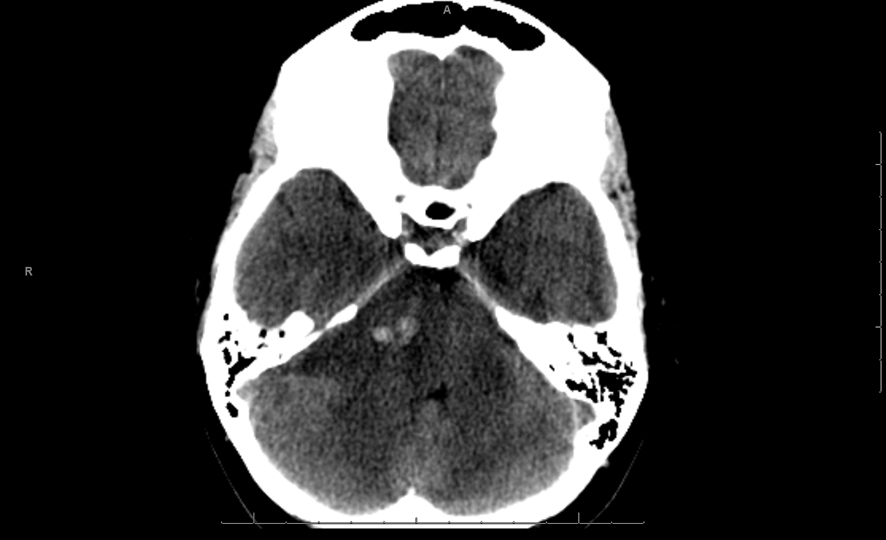 Click for large image | Figure 1. Single axial CT image of the posterior fossa demonstrates extra-axial mass centered on the right cerebellopontine cistern. The mass is hypodense to brain parenchyma with small central hyperdense foci of acute hemorrhage. There is moderate mass effect on the adjacent pons and ipsilateral cerebellar hemisphere. |
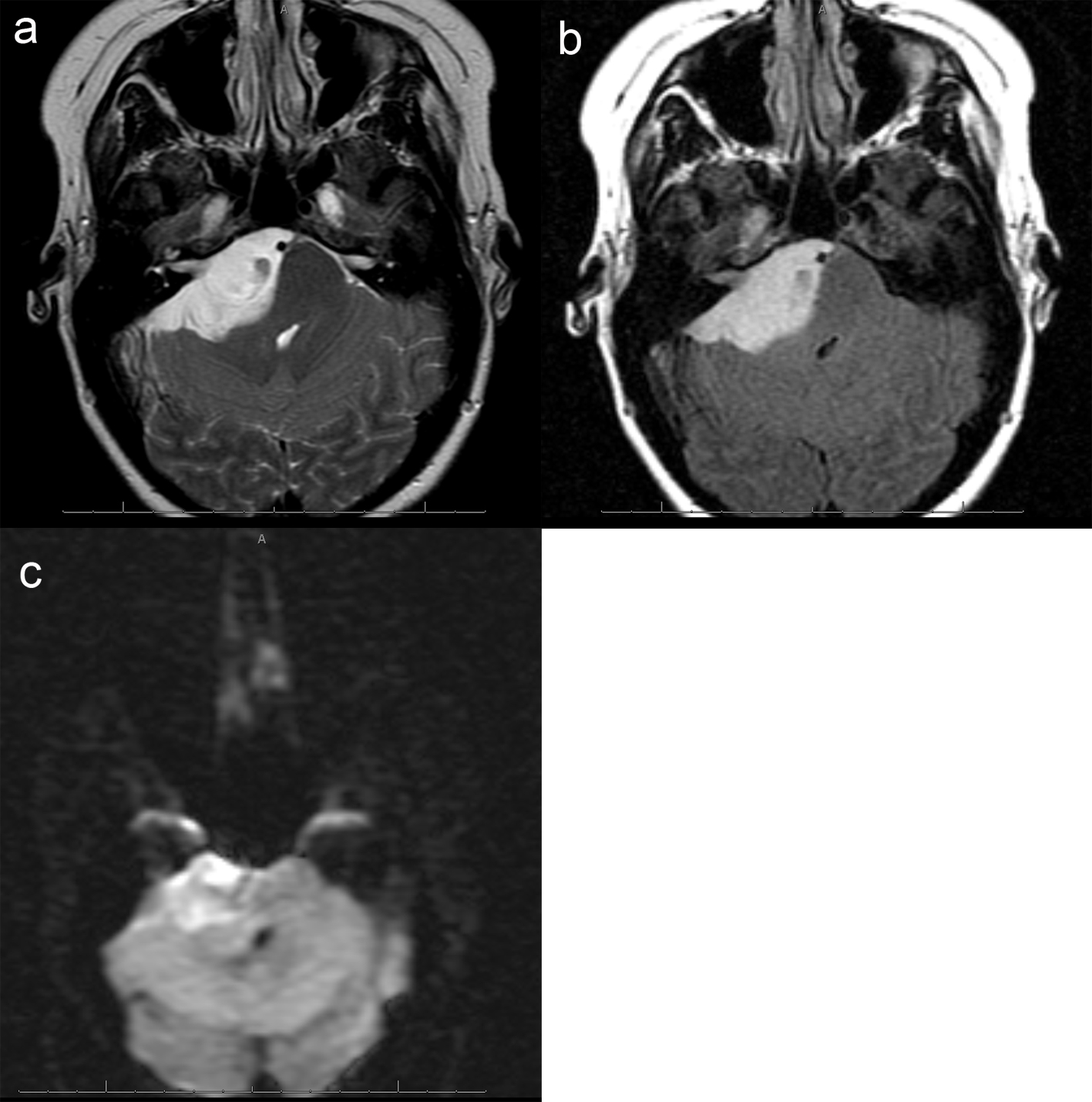 Click for large image | Figure 2. (a), Axial T2 image of the posterior fossa confirms the presence of a large extra-axial T2 hyperintense mass. There is mass effect on the adjacent pons and right cerebellar hemisphere. Foci of hemorrhage are present within the medial aspect of the mass. (b), Axial FLAIR image shows no surrounding edema. Focal abnormal T2/FLAIR signal is seen within the internal auditory canal of unclear significance. Abnormal signal with the same characteristics as the dominant mass extends within the right Meckel's cave. (c), Axial DWI image demonstrates mild, diffuse restricted diffusion is noted. The ADC map is not shown, however confirms the DWI findings. |
Upon referral to neurosurgery, the neurological examination was unremarkable other than decreased auditory acuity in the right compared to left ear. The patient was advised to undergo cesarean section at term. Following the delivery, she was to return to our care with a contrasted MRI to discuss surgical intervention of the CP angle meningioma.
Clinical deterioration and surgical intervention
Two weeks after her initial evaluation, the patient presented to an outside emergency department with an acute deterioration in mental status. She required endotracheal intubation and was noted to have a fixed, nonreactive right pupil measuring 7 mm along with dense right hemiparesis. A head CT revealed acutely hemorrhagic right CP angle lesion with significant compression of brainstem and upward transtentorial herniation, and obstructive hydrocephalus (Fig. 3a, b).
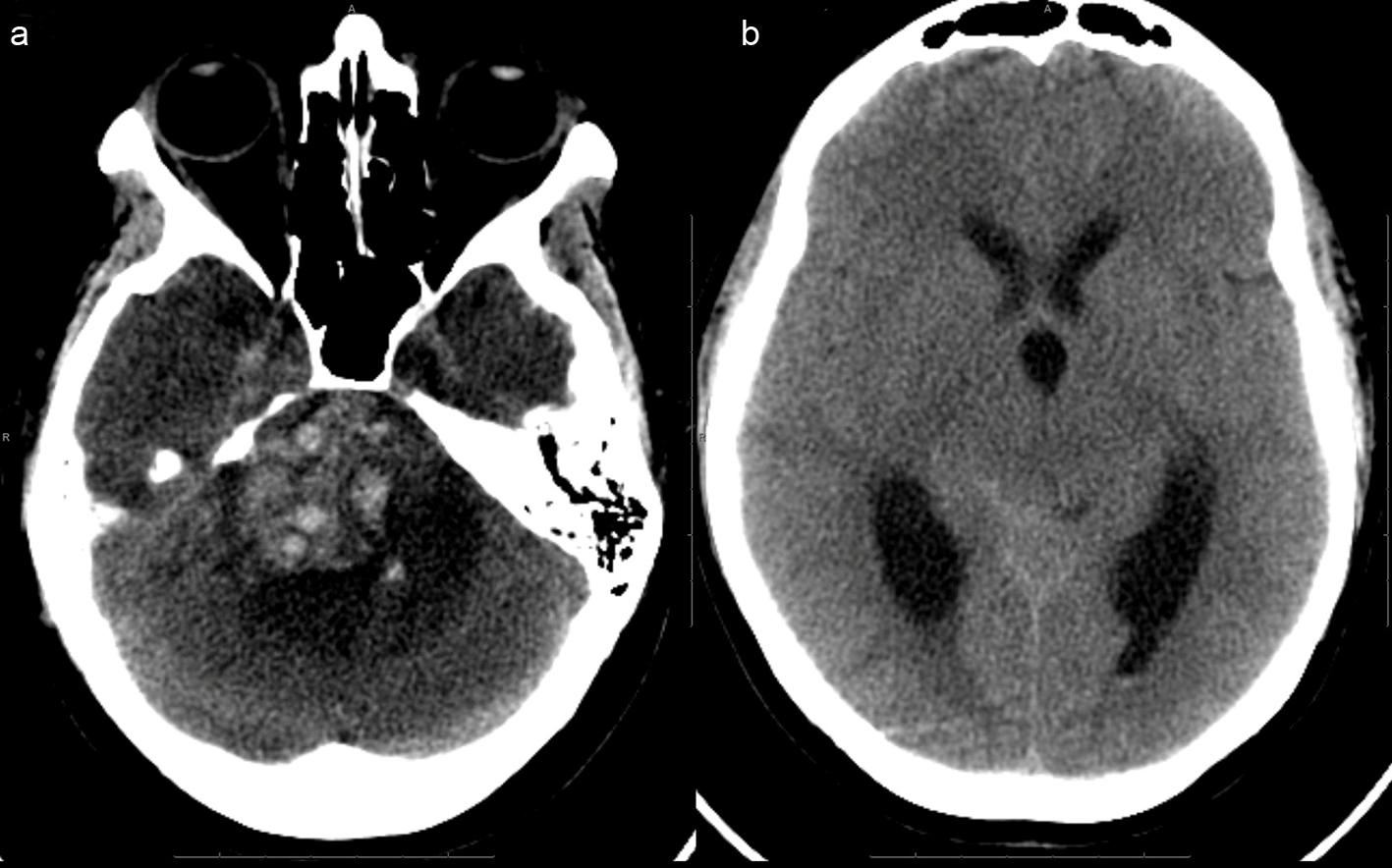 Click for large image | Figure 3. (a), (picture without the ventricles) Extensive acute hemorrhage is seen at the site of the right cerebellopontine cistern mass, causing mass effect within the posterior fossa, including compression of the pons and upward transtentorial herniation. There is surrounding hypodense rim, indicative of edema.(b), (picture of dilated ventricles) - Effacement of the basilar cisterns and compression of the aqueduct and fourth ventricle are noted, indicative of obstructive hydrocephalus. |
She was given mannitol and airlifted to the nearest hospital with neurosurgery and a Level I nursery. Upon arrival, simultaneous caesarean section and right frontal ventriculostomy were performed. After successful delivery of the child, the patient was placed in left lateral decubitus position and right suboccipital craniectomy with a C1 laminectomy performed for resection of the hemorrhagic lesion (Fig. 4a, b).
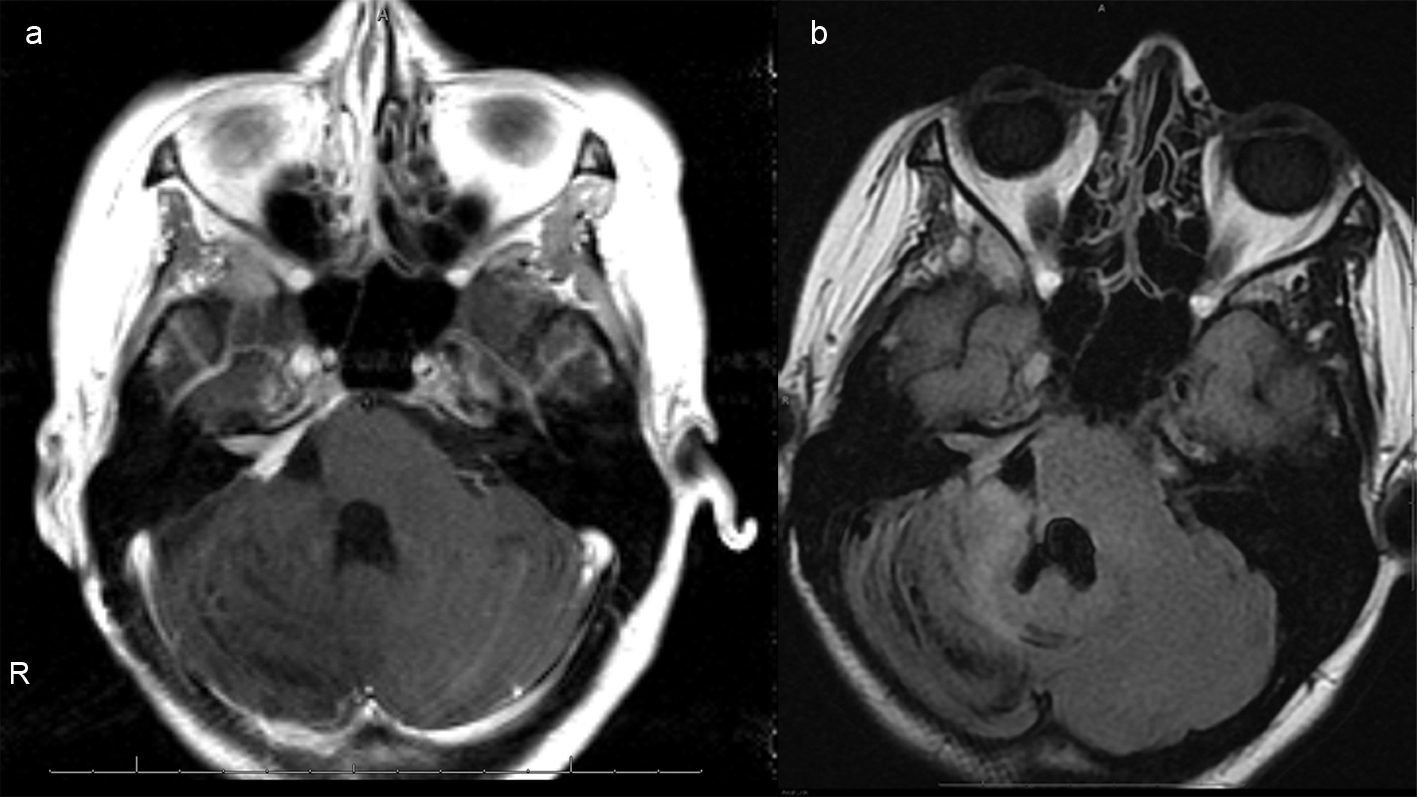 Click for large image | Figure 4. (a), Axial T1 postcontrast images of the brain status post gross total resection of the mass with mild residual enhancement along the right petrus ridge, internal auditory canal and Meckel's cave, indicative of residual tumor. (b), Axial FLAIR image demonstrates reexpansion of previously compressed brainstem and right cerebellar hemisphere. Susceptibility artifacts within the surgical bed indicate hemosiderin deposition. There is no hydrocephalus. |
Pathology
Microscopic examination revealed a tumor admixed with unorganized blood clot/hemorrhage (Fig. 5). The tumor is composed of whorl formation and chordoid growth pattern with eosinophilic tumor cells in a myxoid matrix (Fig. 6). The tumor cells are positive for vimentin, epithelial membrane antigen (EMA), and Progesterone receptor (PR) (Fig. 7) immunostains. The tumor cells are negative for Oestrogen receptor (ER), glial fibrillary acidic protein (GFAP), S-100, and Anti-Cytokeratin(CAM 5.2) immunostains. The histological features and the immunostain profiles are consistent with chordoid meningioma [8, 19].
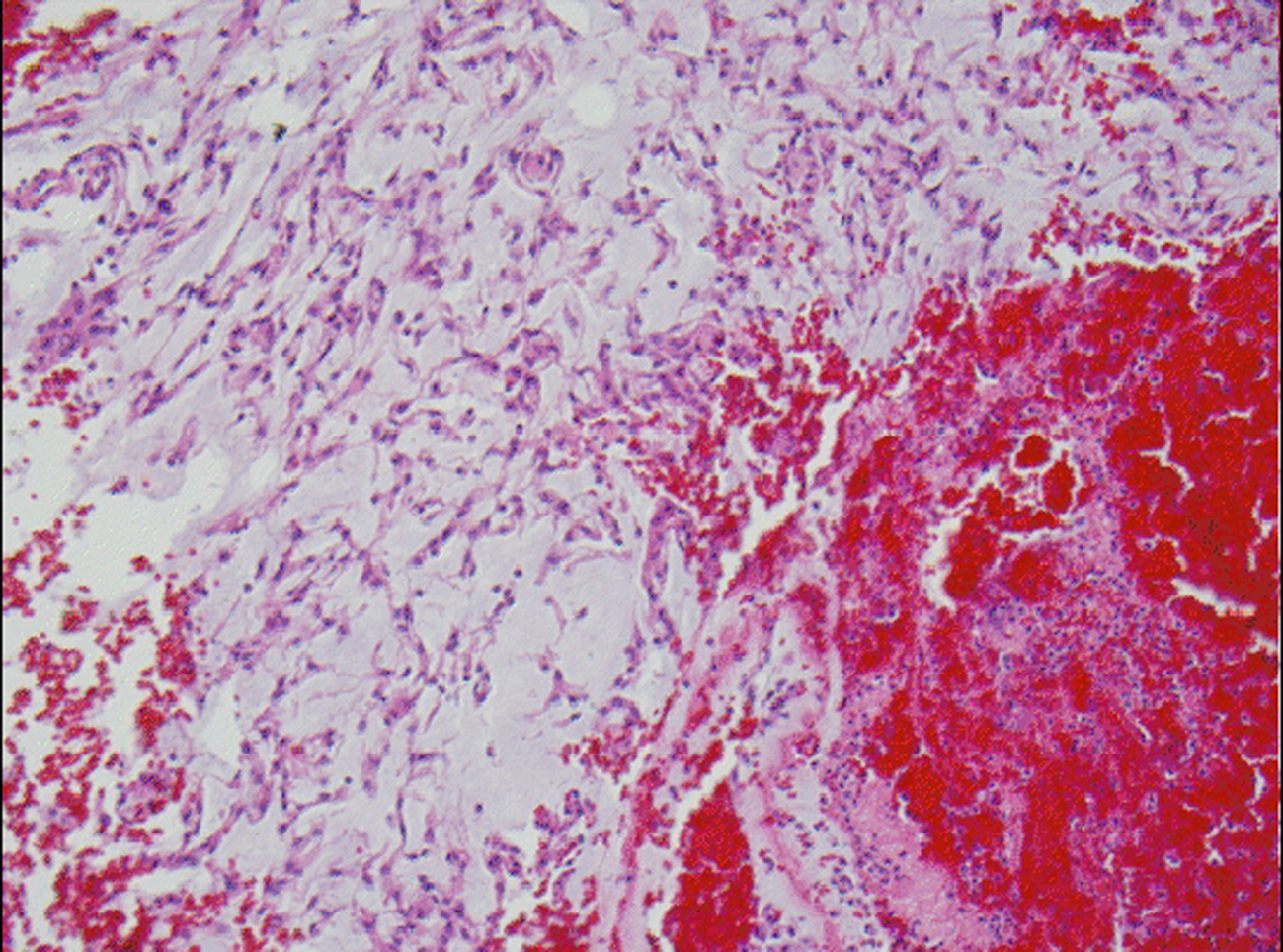 Click for large image | Figure 5. H&E stained slide demonstrates chordoid meningioma admixed with hemorrhage. |
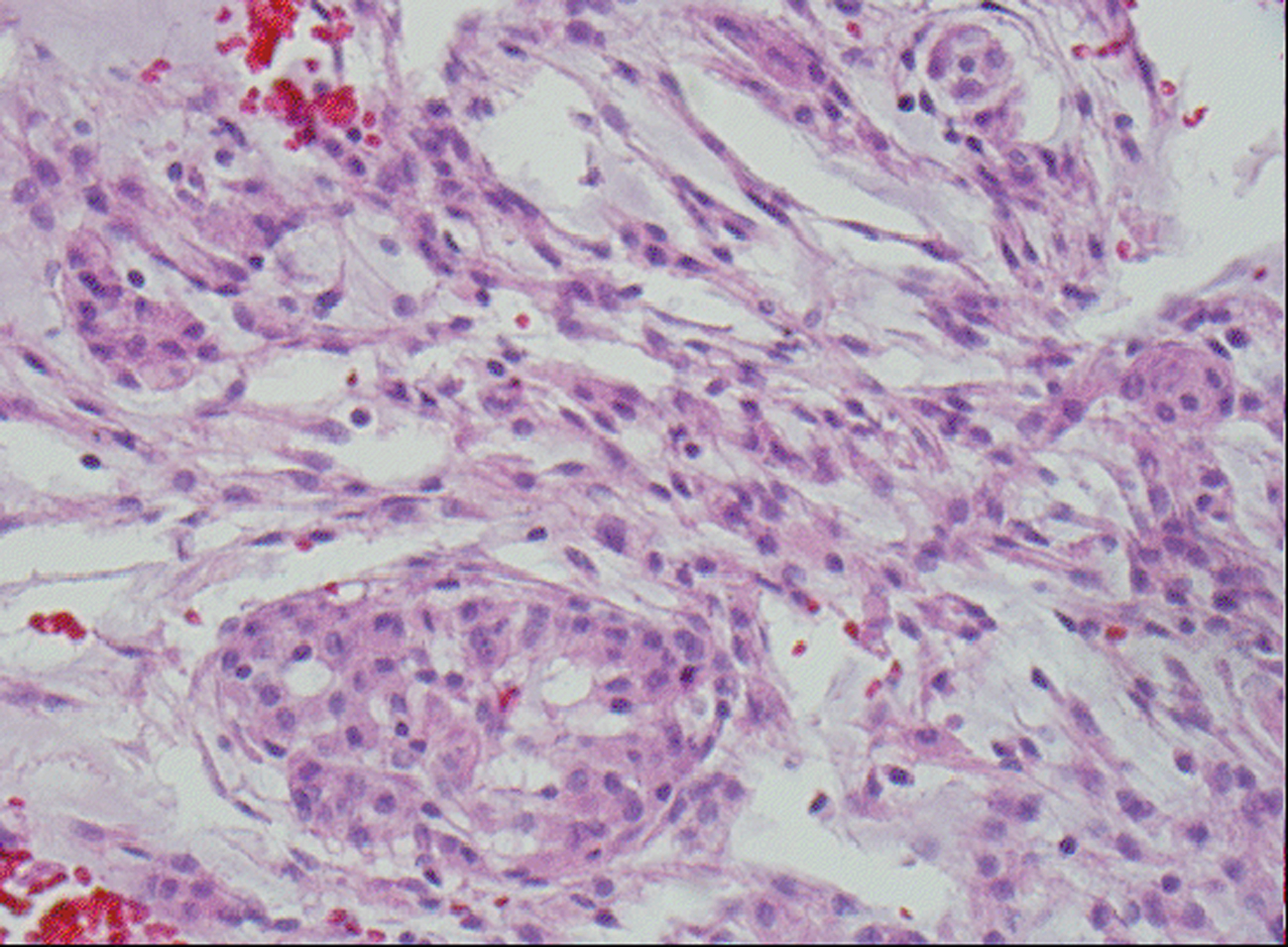 Click for large image | Figure 6. H&E stained slide demonstrates chordoid meningioma composed whorl formation and chordoid growth pattern with eosinophilic tumor cells in a myxoid matrix. |
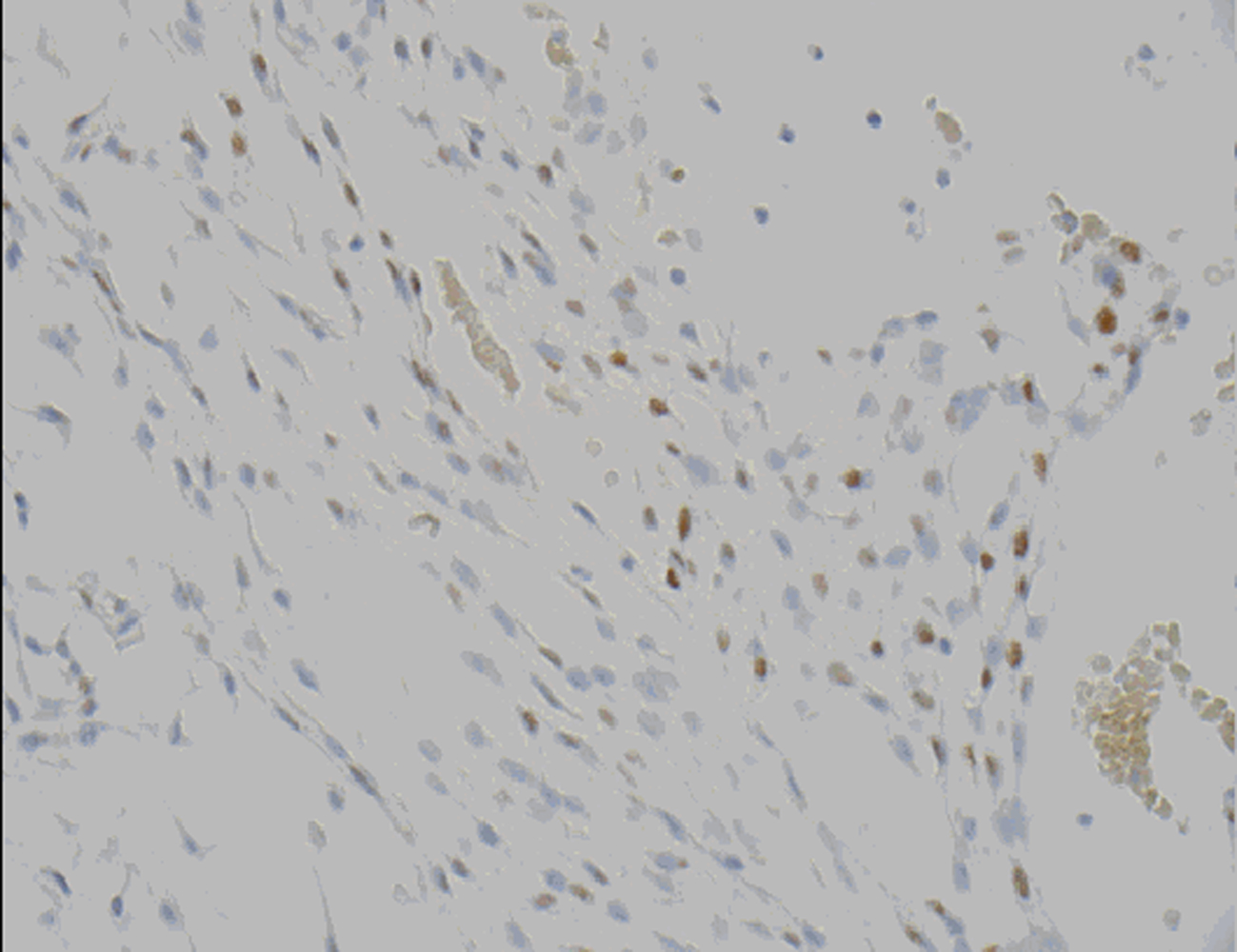 Click for large image | Figure 7. Progesterone receptor (PR) demonstrates nuclear positivity in chordoid meningioma tumor cells. |
Clinical course
The patient remained intubated and postoperatively transferred to Intensive Care Unit. Within the first week, the patient was extubated and her ventriculostomy removed. Her hemiparesis nearly resolved within the first week, other than a mild facial weakness (House-Brackmann 2/6). At one year follow-up, all deficits had resolved other than her right hearing deficit. MRI showed no evidence of tumor recurrence.
| Discussion | ▴Top |
Meningioma is the second most common primary brain tumor. Intracranial tumor associated hemorrhage has been reported in up to 4% of the cases, most notably in high grade gliomas and metastatic lesions [17]. Due to their slow growth and benign nature, meningiomas are typically not regarded as a hemorrhagic entity although spontaneous bleeds from cranial and spinal meningiomas are reported in the literature [20]. Intracranial bleeds are infrequent, but carry a significant mortality of almost 14% in the CT era and morbidity of 36% [1]. Additionally, fatal hemorrhagic transformation of meningiomas has been reported during both neurosurgical as well as non-neurosurgical procedures [21]. Even though older age correlates with increased incidence of meningiomas, a recent study suggested bimodal incidence of hemorrhage with twofold risk in patients younger than 30 years old and those older than 70 [1, 2, 5]. Hence, the patient in the present report fits into the increased risk age group. Histopathologic diagnosis of fibrous, malignant, and angioblastic meningiomas have been demonstrated to carry the highest propensity for hemorrhage [1, 17, 22]. However, even most rare subtypes such as myxoid lesions can develop intratumoral hemorrhage [23].
In a recent report by Bosnjak et al., patient outcomes were associated with location of hemorrhagic meningioma, the type of hemorrhage observed, as well as level of consciousness. Intraventricular and convexity lesions were noted to have the highest bleeding incidence while tumors presenting with associated intracranial hemorrhage (ICH) had a mortality of 28%. Skull base location, as described in our patient, accounted for only 13% of all lesions reviewed [1, 17]. Among patients who did not regain consciousness, the mortality rate topped 74% compared to just 3.8% in preoperatively awake patients. Moreover, just over 53% of comatose patients survived urgent surgical interventions [1].
Clinical management in this case was further complicated by a third trimester pregnancy. The first reported association between symptomatic meningiomas and pregnancy was by Cushing and Eisenhardt in 1938 [24]. Yet brain tumors remain a rarely reported entity during pregnancy. In a review of over 126,000 pregnancies, Isla et al. noted only seven total brain tumors and two meningiomas [25]. However, some studies have suggested that the incidence is similar to age-matched nonpregnant females [13].
Kanaan et al. had successfully managed 18 pregnant patients from a series of 600 meningiomas. Seven patients underwent surgical intervention during pregnancy. The authors maintained that surgery should be limited to patients with malignancy, acute hydrocephalus, and lesions associated with signs of imminent herniation or deteriorating neurological picture. Attention should also be directed to pharmacotherapy in these patients. While dexamethasone is generally well tolerated, mannitol crosses the placenta and many antiepileptic drugs are associated with neural tube defects [13]. Of note, infertility therapy is also implicated with adverse outcomes. Montegi et al. described an interesting case of a woman who experienced spontaneous intratumoral hemorrhage shortly after initiating clomiphene citrate necessitating acute neurosurgical intervention [22].
Hormones are implicated in the etiology of meningiomas with increased growth patterns that have been observed in pregnancy, menopause, and menstrual cycle [2, 6, 13-15].
Specifically, progesterone has been shown to contribute to worsening symptoms [6]. Benzel et al. demonstrated tumor growth via retention of fluid facilitated by effects of progesterone [26]. Similarly, Roelvink et al. reported a decrease in tumor size associated with postpartum progesterone decline [27]. Up to 90% of benign meningiomas stain positive for progesterone receptors [2]. Interestingly, the presence of progesterone receptors has been implicated as a positive prognostic factor for tumor recurrence [28].
The pathophysiology responsible for hemorrhage of a benign tumor has been poorly characterized in the literature. Various reports implicate stretching of peritumoral vessels during lesional growth that renders vessel walls more friable and susceptible for rupture [1, 17, 29, 30]. Additionally, focal mass effect may lead to venous outflow obstruction, distal vessel necrosis, and resultant bleeding [16, 17]. Shaffrey et al. demonstrated direct tumor growth into the vessel wall causing rupture [31]. Interestingly, hypertension, diabetes, blood dyscrasias, and oral anticoagulation have also been implicated [1, 16, 32, 33].
Conclusion
Intratumoral hemorrhage is an exceedingly rare complication of meningioma. Rapid tumor growth during pregnancy may increase the risk of hemorrhagic complications. In the event of hemorrhagic complications in meningioma during pregnancy, prompt recognition and coordinated obstetrical and neurosurgical interventions can prove life saving for the patient and fetus.
Conflict of Interests
The authors have no conflict of interest and observed ethical adherence.
Grant Support and Financial Disclosure
None.
| References | ▴Top |
- Bosnjak R, Derham C, Popovic M, Ravnik J. Spontaneous intracranial meningioma bleeding: clinicopathological features and outcome. J Neurosurg. 2005;103(3):473-484.
doi pubmed - Barnholtz-Sloan JS, Kruchko C. Meningiomas: causes and risk factors. Neurosurg Focus. 2007;23(4):E2.
doi pubmed - Marano SR, Sonntag VK, Spetzler RF. Planum sphenoidale meningioma mimicking pituitary apoplexy: a case report. Neurosurgery. 1984;15(6):859-862.
doi pubmed - Rockhill J, Mrugala M, Chamberlain MC. Intracranial meningiomas: an overview of diagnosis and treatment. Neurosurg Focus. 2007;23(4):E1.
doi pubmed - Bondy M, Ligon BL. Epidemiology and etiology of intracranial meningiomas: a review. J Neurooncol. 1996;29(3):197-205.
doi - Alluwimi I, Al-Anazi AR. Meningioma in Pregnancy. In: Bahrain Med Bull [Internet]. 2004 [cited 2012 Feb 17];26(2):17. Available from: http://www.bahrainmedicalbulletin.com/june_2004/Meningioma.pdf.
- Backer-Grondahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5(3):231-242.
pubmed - Perry A, Louis DN, Scheithauer BW, Budka H, von Deiming A. Meningioma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, eds. WHO classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer; 2007:164-172.
- Ko KW, Nam DH, Kong DS, Lee JI, Park K, Kim JH. Relationship between malignant subtypes of meningioma and clinical outcome. J Clin Neurosci. 2007;14(8):747-753.
doi pubmed - Lin JW, Lu CH, Lin WC, Wu YT, Huang YJ, Shih FY, Ho JT, et al. A clinicopathological study of the significance of the proportion of choroid morphology in chordoid meningioma. J Clin Neurosci. 2012;19(6):836-843.
doi pubmed - Lee KH, Lall RR, Chandler JP, Bigio EH, Mao Q. Pineal chordoid meningioma complicated by repetitive hemorrhage during pregnancy: Case report and literature review. Neuropathology. 2013;33(2):192-198.
doi pubmed - Orakdogeny M, Karadereler S, Berkman Z, Ersahin M, Ozdogan C, Aker F. Intra-suprasellar meningioma mimicking pituitary apoplexy. Acta Neurochir (Wien). 2004;146(5):511-515.
doi pubmed - Kanaan I, Jallu A, Kanaan H. Management Strategy for Meningioma in Pregnancy: A Clinical Study. Skull Base. 2003;13(4):197-203.
doi pubmed - Pliskow S, Herbst SJ, Saiontz HA, Cove H, Ackerman RT. Intracranial meningioma with positive progesterone receptors. A case report. J Reprod Med. 1995;40(2):154-156.
pubmed - Schrell UM, Adams EF, Fahlbusch R, Greb R, Jirikowski G, Prior R, Ramalho-Ortigao FJ. Hormonal dependency of cerebral meningiomas. Part 1: Female sex steroid receptors and their significance as specific markers for adjuvant medical therapy. J Neurosurg. 1990;73(5):743-749.
doi pubmed - Kim DG, Park CK, Paek SH, Choe GY, Gwak HS, Yoo H, Jung HW. Meningioma manifesting intracerebral haemorrhage: a possible mechanism of haemorrhage. Acta Neurochir (Wien). 2000;142(2):165-168.
doi - Mangubat EZ, Byrne RW. Major intratumoral hemorrhage of a petroclival atypical meningioma: case report and review of literature. Skull Base. 2010;20(6):469-474.
doi pubmed - Wakai S, Nakamura K, Niizaki K, Nagai M, Nishizawa T, Yokoyama S, Katayama S. Meningioma of the anterior third ventricle presenting with parkinsonism. Surg Neurol. 1984;21(1):88-92.
doi - Wu H, Schuerch C, Miller DC. Central and peripheral nerve system tumors. In: Lin F and Prichard J, eds: Handbook of practical immunohistochemistry. New York: Springer; 2011:119-135.
doi - Vij M, Jaiswal S, Jaiswal AK, Kumar S, Behari S. Meningioma with hemorrhagic onset: two case reports. J Cancer Res Ther. 2012;8(1):145-147.
doi pubmed - Sun H, Ross DA. Fatal hemorrhagic infarction of posterior fossa meningioma during cardiopulmonary bypass. Ann Thorac Surg. 2012;93(2):653-656.
doi pubmed - Motegi H, Kobayashi H, Terasaka S, Ishii N, Ito M, Shimbo D, Kubota K, et al. Hemorrhagic onset of rhabdoid meningioma after initiating treatment for infertility. Brain Tumor Pathol. 2012;29(4):240-244.
doi pubmed - Krisht KM, Altay T, Couldwell WT. Myxoid meningioma: a rare metaplastic meningioma variant in a patient presenting with intratumoral hemorrhage. J Neurosurg. 2012;116(4):861-865.
doi pubmed - Cushing H, Eisenhardt L. Meningiomas. Am J Med Sci. 1938;196(5):741-742.
doi - Isla A, Alvarez F, Gonzalez A, Garcia-Grande A, Perez-Alvarez M, Garcia-Blazquez M. Brain tumor and pregnancy. Obstet Gynecol. 1997;89(1):19-23.
doi - Benzel EC, Gelder FB. Correlation between sex hormone binding and peritumoral edema in intracranial meningiomas. Neurosurgery. 1988;23(2):169-174.
doi - Roelvink NC, Kamphorst W, van Alphen HA, Rao BR. Pregnancy-related primary brain and spinal tumors. Arch Neurol. 1987;44(2):209-215.
doi pubmed - Hsu DW, Efird JT, Hedley-Whyte ET. Progesterone and estrogen receptors in meningiomas: prognostic considerations. J Neurosurg. 1997;86(1):113-120.
doi pubmed - Helle TL, Conley FK. Haemorrhage associated with meningioma: a case report and review of the literature. J Neurol Neurosurg Psychiatry. 1980;43(8):725-729.
doi - Pluchino F, Lodrini S, Savoiardo M. Subarachnoid haemorrhage and meningiomas. Report of two cases. Acta Neurochir (Wien). 1983;68(1-2):45-53.
doi - Shaffrey ME, Dolenc VV, Lanzino G, Wolcott WP, Shaffrey CI. Invasion of the internal carotid artery by cavernous sinus meningiomas. Surg Neurol. 1999;52(2):167-171.
doi - Everett BA, Kusske JA, Pribram HW. Anticoagulants and intracerebral hemorrhage from an unsuspected meningioma. Surg Neurol. 1979;11(3):233-235.
pubmed - Martinez-Lage JF, Poza M, Martinez M, Esteban JA, Antunez MC, Sola J. Meningiomas with haemorrhagic onset. Acta Neurochir (Wien). 1991;110(3-4):129-132.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
