| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 3, Number 2, May 2014, pages 73-75
Cellular Placental Chorioangioma and Adverse Fetal Outcome
Giampaolo Passalacquaa, Laura Donatia, d, Manila Ferrettia, Giulia Giovanninib, Marta Sbaragliac, Graziano Clericib
aDepartment of Obstetrics/Gynecology, Santa Maria Hospital, 05100 Terni, Italy
bCentre of Reproductive and Perinatal Medicine, Department of Obstetrics/Gynecology, Polo Unico Ospedaliero Santa Maria della Misericordia, University Hospital, 06132 San Sisto, Perugia, Italy
cDepartment of Pathological Anatomy, Santa Maria Hospital, 05100 Terni, Italy
dCorresponding author: Laura Donati, Department of Obstetrics/Gynecology, Santa Maria Hospital, 05100 Terni, Italy
Manuscript accepted for publication April 1, 2014
Short title: Cellular Placental Chorioangioma
doi: https://doi.org/10.14740/jcgo242w
| Abstract | ▴Top |
Placental chorioangioma is the most common benign tumor of the placenta, and it is generally asymptomatic and an incidental finding. Large chorio angioma conversely is much less common, and it needs a close follow-up cause of a high risk for maternal and fetal complications. We report a case of cellular chorioangioma larger than 5 cm and its unexpected evolution, associated with a dramatically rapid increasing of amniotic fluid volume and a poor fetal outcome.
Keywords: Placenta; Tumor; Cellular chorioangioma; Adverse fetal outcome; Polyhydramnios
| Introduction | ▴Top |
Placental chorioangioma is a benign vascular tumor with a prevalence of 1%. Most of placental tumor is vascular type, and its evolution generally does not complicate the pregnancy. The following case draws attention to cellular chorioangioma unusually associated with maternal and fetal complications.
| Case Report | ▴Top |
A 28-year-old, gravida 2 para 0, at 21 + 4 weeks of gestation was admitted to our Obstetric and Gynaecologic Department for general abdominal pain, uterine contractions and vaginal bleeding. The woman had no stressful job, was leading a quiet life without particular risk factors. She was neither diabetic nor anemic, and her blood pressure was regular. Obstetric anamneses revealed a previous abortion at 22 weeks due to the presence of genetic disease osteogenesis imperfecta related. The woman underwent a previous morphological scan at 20 weeks and no abnormality of the placenta and of the fetus was detected. Amniocentesis was performed at 16 weeks for alpha-fetoprotein (NTDs), karyotyping and research for main genetic mutation of cystic fibrosis, muscular dystrophy, deaf-mutism, X-fragile syndrome and gene 1 a 1 of type 1 of osteogenesis imperfecta. The result was: 46 XY, negative for genetic disorders. The admission obstetric visit showed no actual signs of a real miscarriage, uterine contractions were irregular and not intense, cervix uteri was tightly closed, and there were both fetal movements and normal fetal heart rate. Ultrasound showed a single live fetus with regular biometry, with no evidence of structural abnormalities. Amniotic fluid appeared slightly increased AFI about 220 mm. Placenta was on the posterior wall and a well-defined echogenic mass size 45 × 44 mm, with no vascularization by Doppler, was seen on the fetal side. After three days of tocolytic treatment, abdominal discomfort and vaginal bleeding stopped. The patient was discharged without signs of bleeding and uterine contractions, and she was recommended to perform a new US scan within one week, in order to check AFI and the placental mass. Just six days later, she had a new admission to our Department, at 22 + 2 weeks for abdominal pain due to overdistended abdomen, moderate dyspnea, uterine contractions and vaginal bloody mucous discharge. Obstetric examination showed a closed, 20% shorted cervix, not appreciable fetal presenting part, and uterus increased in size, fetal parts were not palpable, and there were signs of cervical bleeding. Ultrasound examination detected a slight but significative increase in both of placental mass 43 × 56 mm and of AFI 280 mm. Cervical tunneling was documented by TV scan. The patient once again was treated with tocolytic drugs, bed rest, but there was not an effective control of sintomatology. There was a dramatically rapid and unexpected evolution of the clinical condition within 72 h of admission: abdomen became overdistended up to xiphisternum, preterm labour was starting with regular, painful, valid uterine contractions. Dyspnea became so important that the patient showed discomfort for supine position and could sleep just in a seated position. For this reason, a therapeutic amniocentesis was performed with the reduction of more than 500 mL of amniotic fluid. Just few hours later, the patient delivered a male fetus (600 g, Apgar 0/0). Histopathological examination of products of conception observed the placental disc 170 × 150 × 40 mm. The fetal side showed a brownish mass, size 60 × 50 mm, and it is close to cord insertion (Fig. 1). Cut section presented a red-brown surface and cotyledons partially fragmented on the maternal side. No fetal anomaly was detected. This hypoechoic placental tumor was identified as a cellular type of chorioangioma (Fig. 2).
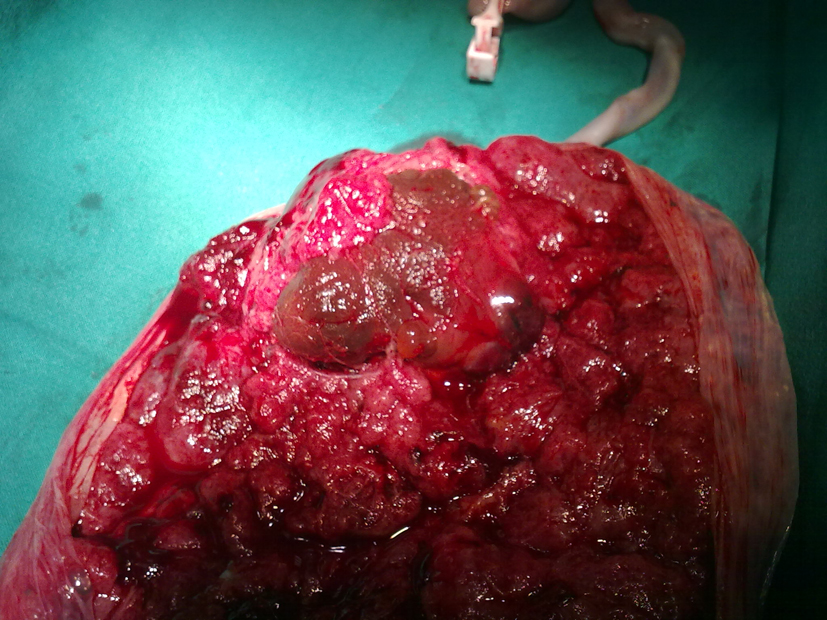 Click for large image | Figure 1. Histopathologic examination of the tumor at delivery. |
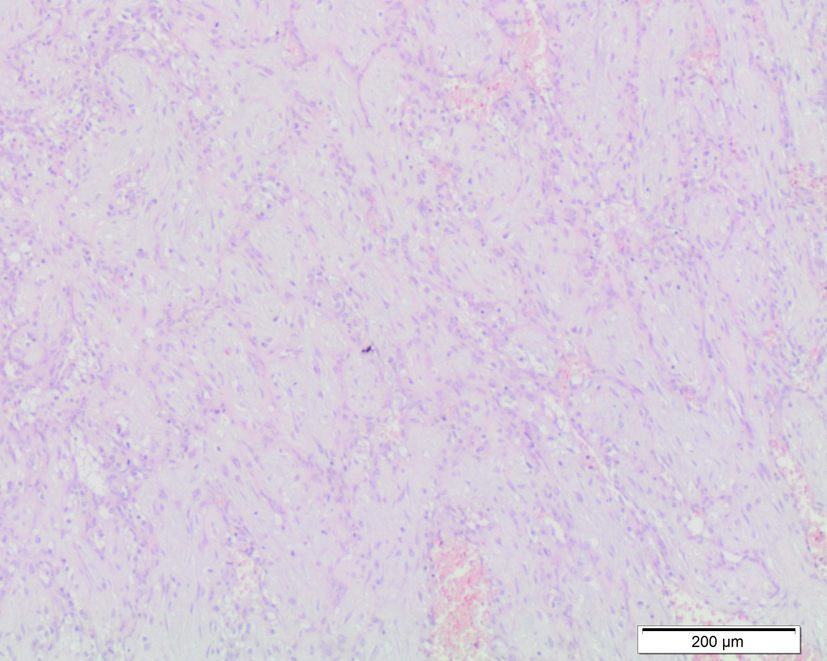 Click for large image | Figure 2. Microscopic examination of cellular chorioangioma. |
| Discussion | ▴Top |
Placental chorioangioma is a benign vascular tumor with a prevalence of 1%. Most of placental tumor is an incidental finding. They are more seen in multiple pregnancies and in female babies. The differential diagnosis of placental masses includes teratoma, placental hemorrhage, metastasis from a primary maternal tumor (very rare), degenerate myoma and partial hydatidiform mole [1, 2].
Chorioangioma is believed to arise by 16th day of fertilization, although there has been no documentation of this tumor during the first trimester. Large, clinically significant chorioangiomas (> 5 cm) occur much less frequently with a reported incidence ranging from 1:3,500 to 1:9,000 [3], and there is a high risk of pregnancy complications.
In the literature, it has described three histological patterns of chorioangioma (believed to represent various steps of tumor development): angiomatous, cellular and degenerative [4]. The cellular type is small, circular, encapsulated and situated in the placental parenchyma. It consists of loose mesenchymal tissue and only few vessels, especially located in the periphery of the lesion. It is less frequent than the angiomatous one, less differentiated or more immature [5, 6].
Ultrasound examination of the angiomatous type shows a well-circumscribed hypoechoic mass protruding from the fetal side of the placenta close to the insertion of the umbilical cord. This type presents an echostructure complex with a diameter greater than 4 cm and numerous vessels inside the mass assessed by color Doppler [6]. Degenerative type presents changes, such as necrosis, calcification, hyalization or mixed inside the tumor mass. They are more common in large tumors [5]. Most of the literature shows a prevalence of angiomatous types of chorioangioma, due to the strong association with maternal and fetal complications, that can be traced to the presence of arteriovenous shunts and a rapid growth of the tumor. Maternal complications are preeclampsia, preterm labor, placental abruption and polyhydramnios [7]. There are different theories that attempt to explain the origin of the polyhydramnios. According to some, polyhydramnios is due to the major transudate that escapes through the walls of the vessels of chorioangioma and the accumulation of fluid caused by retrograde compression of the umbilical vein by the tumor itself [8]. The latter hypothesis regards the role played by an increased production of fetal urine or by congestive heart failure [6].
Fetal risks include anemia, growth restriction, hydrops, congenital anomalies, thrombocytopenia, cardiomegaly and fetal congestive heart failure, which can be developed by the presence of arteriovenous shunt, and cause low vascular resistance and a relative increase blood flow [7].
In the literature, it has described many clinical cases of chorioangioma associated with maternal and fetal complications; however, the most common histological pattern of this tumor reported is angiomatous type. In fact, this type of chorioangioma usually has a rapid growth and a large size (also 10 cm), but it is the presence of many vessels, and especially of the arteriovenous shunt that facilitates the development of complications.
For this reason, the evolution of our case was totally unexpected. The woman had the first admission at 21 + 4 weeks, and the ultrasound examination showed a placental mass around 4 cm without vascularization and after six days, during the second admission, the tumor showed an increasing in size of almost 50 mm. Nevertheless, we have to consider that the morphological ultrasound at 20 weeks did not detect anomaly of the placenta.
The progression of this chorioangioma, especially its growth and the rapid increase of fluid amniotic volume, causing dyspnea, was completely unpredictable. This evolution is more appropriate for angiomatous chorioangioma than a cellular type, which was in this case histologically confirmed. The treatment chosen for this cellular chorioangioma was a close follow-up, notwithstanding an amnioreduction was carried out to reduce polyhydramnios.
In the literature, the therapy of chorioangioma needs yet to be defined. Many placental tumors are small in size, and they are usually asymptomatic. In this condition, it is recommended only observation while the presence of mass larger than 4 cm presents a high risk to have clinical complications. In this case, it is advised an immediate treatment to promote the development of the fetus and the progression of pregnancy. Many types of treatments have been reported to improve the outcome of pregnancy, such as tocolytic drugs to avoid miscarriage, cordocentesis in case of fetal anemia and amnioreduction in case of polyhydramnios, and thrombosis of nutritive vessel to reduce the vascularization of the tumor and stop its growth [9]. There are many possibilities to determinate a thrombosis of tumoral vessel: alcoholization, laser coagulation, endoscopic suture or using microcoils [9]. Despite these therapeutic opportunities, it remains impossible to predict the future of the pregnancy.
Conclusion
Placental chorioangioma is certainly the most common benign tumor of the placenta. Ultrasound examination with color Doppler helps in timely diagnosis, and it is recommended during the follow-up. However, the progression of this tumor is often unpredictable. We suggest a close observation of placental mass, especially if it is larger than 4 cm, even when the Doppler scan does not show a vascularization of itself. A regular monitoring could be useful to treat clinical complications immediately, and to achieve better outcomes in complicated pregnancies.
| References | ▴Top |
- Jauniaux E, Campbell S. Ultrasonographic assessment of placental abnormalities. Am J Obstet Gynecol. 1990;163(5 Pt 1):1650-1658.
doi - Harris RD, Barth RA. Sonography of the gravid uterus and placenta: current concepts. AJR Am J Roentgenol. 1993;160(3):455-465.
doi pubmed - Quintero RA, Reich H, Romero R, Johnson MP, Goncalves L, Evans MI. In utero endoscopic devascularization of a large chorioangioma. Ultrasound Obstet Gynecol. 1996;8(1):48-52.
doi pubmed - Marchetti AA. A consideration of certain types of benign tumors of the placenta. Surgery, Gynecology and Obstetrics. 1939; 68:733-743.
- Fox H. Pathology of Placenta. 2nd edn. Philadelphia: WB. Saunders, 1997.
- Jauniaux E, Ogle R. Color Doppler imaging in the diagnosis and management of chorioangiomas. Ultrasound Obstet Gynecol. 2000;15(6):463-467.
doi pubmed - Kodandapani S, Shreshta A, Ramkumar V, Rao L. Chorioangioma of placenta: a rare placental cause for adverse fetal outcome. Case Rep Obstet Gynecol. 2012;2012:913878.
- Wehrens XH, Offermans JP, Snijders M, Peeters LL. Fetal cardiovascular response to large placental chorioangiomas. J Perinat Med. 2004;32(2):107-112.
doi pubmed - Munoz Lopez M, Comas Gabriel C, Torrents Muns M, Munoz Prades A, Garcia Gallardo M, Mallafre Dols J. Diagnostico prenatal de corioangioma placentario y gestacion a termino. Progresos de Obstetricia y Ginecología. 2013;56(2):94-100.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
