| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 3, Number 2, May 2014, pages 55-61
Intracervical Foley Catheter Versus Vaginal Isosorbid Mononitrate for Induction of Labor in Women With Previous One Cesarean Section
Mohamed Rezka, b, Zakaria Sanada, Ragab Dawooda, Alaa Masooda, Mohamed Emarha, Alaa Al Halabya
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Menoufia University, Egypt
bCorresponding author: Mohamed Rezk, Department of Obstetrics and Gynecology, Faculty of Medicine, Menoufia University, Egypt
Manuscript accepted for publication April 1, 2014
Short title: Foley Catheter Versus Isosorbid Mononitrate
doi: https://doi.org/10.14740/jcgo243w
| Abstract | ▴Top |
Background: To compare the efficacy, safety and acceptability of intracervical Foley catheter versus vaginal isosorbid mononitrate (IMN) for induction of labor in women with previous one caesarean section at term.
Methods: A prospective clinical trial including 80 term pregnant women who were assigned randomly to receive either intracervical Foley catheter or moistened one tablet of IMN 40 mg vaginally was carried out. Induction to delivery interval and outcomes of labor, adverse effects and acceptability were assessed.
Results: There was shorter induction to delivery interval in the catheter group (22.2 ± 4.99 h) when compared to the IMN group (26.1 ± 3.98 h) (P < 0.05). There was a significant headache in the IMN group (10, 25%) in comparison to the catheter group (3, 7.5%). There was a significant maternal pyrexia in catheter group (12, 30%) when compared to IMN group (5, 12.5%). There was no difference between both groups regarding mode of delivery, neonatal outcome and method acceptability.
Conclusion: Intracervical Foley catheter is effective, safe and acceptable for labor induction in women with previous one lower segment caesarean section at term when compared to vaginal IMN but, with more maternal pyrexia.
Keywords: Foley catheter; Isosorbid mononitrate; Labor induction; Caesarean section
| Introduction | ▴Top |
It is well documented that the risks of caesarean section for women increase with increasing numbers of caesarean deliveries. These include potentially life-threatening complications including hemorrhage, surgical complications and placenta accreta [1, 2].
The induction of labor (IOL) is common in the obstetric practice and it is aimed to deliver a healthy baby and to maintain the health of the mother. In the absence of a ripe or a favorable cervix, a successful vaginal birth is less likely. The cervix is considered to be unfavorable if the Bishop’s score is less than 6 and if the cervical ripening is indicated prior to the artificial rupture of the membranes (AROM) and the production of oxytocin, to reduce the incidence of a failed induction and a caesarean delivery [3].
An agent that ripens the cervix without stimulating uterine activity would be ideal for induction. Nitric oxide (NO) is a free radical with a short half-life for cervical ripening, and its main effect is rearrangement of collagen, thereby allowing NO to soften the cervix without causing uterine contractions [4, 5].
In animal studies, compounds like isosorbide mononitrate (IMN) and glyceryl trinitrate facilitate the production of NO to induce cervical ripening [6, 7].
A study in which women with term pregnancy self-administered 40 mg IMN or placebo vaginally at 48 h, 32 h and 16 h before scheduled hospital admission provides evidence on the efficacy of outpatient IMN for pre-induction cervical ripening [8].
Mechanical methods are used to dilate the cervix, but may also increase prostaglandin and/or oxytocin release by causing localized inflammation [9].
IOL with a Foley catheter is as effective as induction with intravaginal prostaglandin E2 gel, with fewer maternal and neonatal side-effects [10].
The aim of this study was to compare the effectiveness, the safety and the acceptability of intracervical Foley catheter versus vaginal IMN tablets on cervical ripening and labor induction in women with a previous one cesarean section with an unfavorable cervix at term.
| Materials and Methods | ▴Top |
This was a single center balanced randomized parallel group study carried out at the Department of Obstetrics and Gynecology, Menoufia University Hospital, Egypt between January 2013 and January 2014. The institutional review board approved the study protocol and an informed consent was obtained from all participants prior to commencing the study.
Power was set at 0.8, alpha level at 0.05 and the confidence interval (CI) at 95%. A total sample size 80 subjects was needed for this trial (40 subjects in each group).
Participants
The study was conducted on 80 healthy pregnant women with previous one lower segment cesarean section at 37 weeks and beyond, with a Bishop’s score of ≤ 6, intact membranes, reactive non-stress test, normal umbilical arterial Doppler indices, absence of labor and willingness of women to participate in the study. The indications for the IOL were pregnancy-induced hypertension, oligohydramnios, intrauterine growth restrictions and controlled diabetes mellitus.
A detailed history including age, parity and period of gestation was noted and details of clinical examination (maternal vital signs, BMI and vaginal examination to obtain the Bishop score) were also recorded. Ultrasonography (Acuson 128 XP 10, computed sonography system, Mountain View, California, USA) was done to confirm gestational age, presentation, estimated fetal weight, placental localization and umbilical arterial Doppler indices. Patients with intrauterine foetal death, twins pregnancy, polyhydramnios, placenta previa, severe anemia, severe hypertension, uncontrolled diabetes, coagulopathy and any contraindication for the labor induction were excluded from the study.
Randomization
Enrolled women were randomly assigned to two groups according to the method of treatment. Randomization in 1:1 ratio was carried out using computer-generated simple random tables. It was not possible to blind the study participants from knowledge of which intervention a participant received because methods were clearly different (Fig. 1).
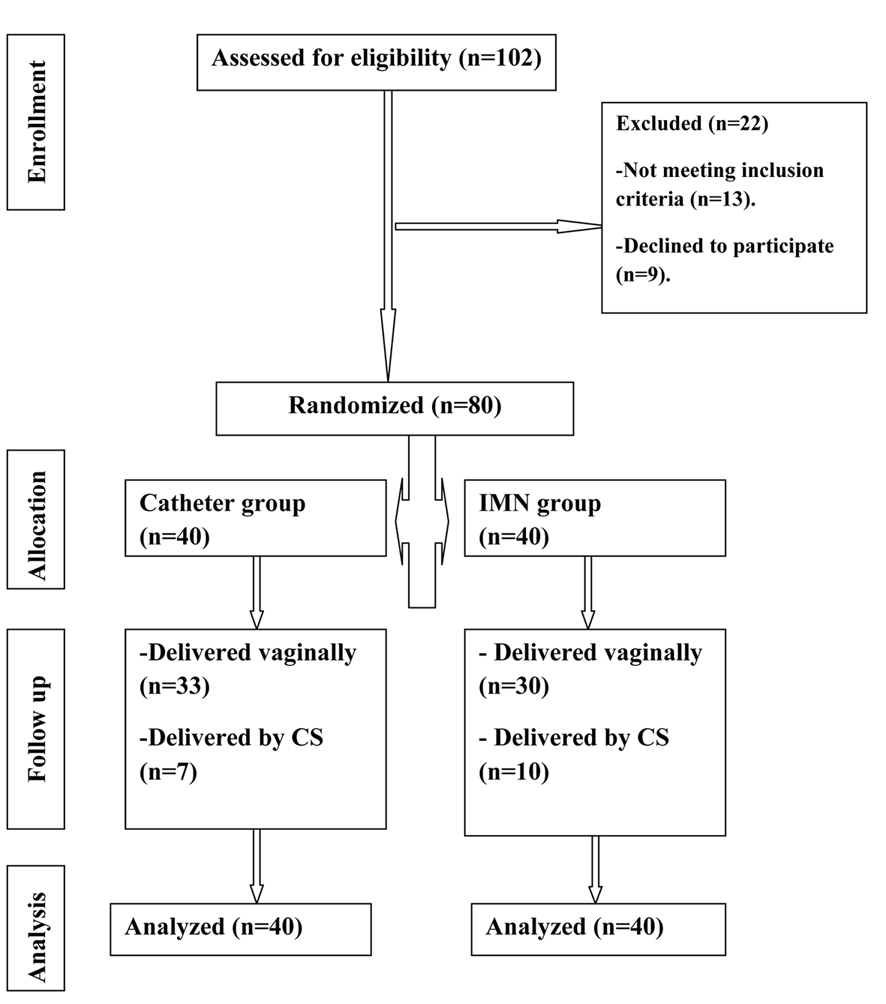 Click for large image | Figure 1. Flow diagram. |
Intervention
Group 1 (catheter group)
There were 40 pregnant women in whom intracervical Foley catheter was inserted, inflated and placed on traction. Under aseptic conditions, with the patients lying in the lithotomy position, the cervix was assessed and Foley catheter No. 14-16 Fr Ch was inserted into the endocervical canal, beyond the internal os and the balloon was inflated with 50-60 mL of normal saline. The catheter was strapped to the thigh with gentle traction. The catheter was checked for its position and the traction at 3 - 6 h intervals. The catheter was either removed at 12 h or expelled spontaneously and it was checked whether the Bishop’s score had improved or whether a spontaneous rupture of the membranes had occurred.
Group 2 (IMN group)
The other 40 pregnant women received moistened one tablet of IMN 40 mg (Monomak, October Pharma, Egypt) inserted into the posterior fornix of the vagina once.
Subjects were examined regularly at 3, 6, 9, 12 and 24 h after starting the method of induction to evaluate the change in Bishop score. Vital signs were monitored every 30 min. AROM was performed for all women when their cervical dilatation reached 3 - 4 cm and intravenous oxytocin infusion was started if there is no efficient uterine contractions. An oxytocin infusion was started at 2 mU/min and increased in increments of 1 - 2 mU/min at 15 - 30 min intervals as needed to achieve adequate uterine contraction pattern (≥ 200 MVU). Opiate and epidural analgesia was given on the patient’s request and at the discretion of the obstetrician. Continuous CTG was done during delivery and the modified WHO partograph was followed up for the labor management.
Outcome measures
Primary outcome measures included changes in Bishop score, time from initiation till the onset of labor, the induction to delivery interval, the mode of delivery and the length of the second and third stages of labor.
Maternal adverse effects, acceptability and neonatal outcome (Apgar score at 5 min, neonatal weight and admission to neonatal intensive care unit) were recorded as secondary outcomes.
Statistical analysis
Data entry and analysis was carried out using SPSS version 16 (2006, SPSS Inc., Chicago, IL, USA).
Descriptive statistics
Quantitative data are expressed to measure the central tendency of data and diversion around the mean, mean (x) and standard deviation (SD).
Qualitative data are expressed in number and percentage.
Analytic statistics
T test was used for comparison of two groups of normally distributed variables. Mann-Whitney test was used for comparison of two groups of non-normally distributed variables.
All these tests were used as tests of significance at: P value > 0.05 was considered statistically non significant. P value ≤ 0.05 was considered statistically significant. P value ≤ 0.001 was considered statistically highly significant.
| Results | ▴Top |
Table 1 displays the maternal characteristics. Outcomes of induction and labor dynamics are shown in Table 2 in which there was a significant improvement in the Bishop score after 12 h in both groups. Shorter induction to delivery interval was noted in the catheter group (22.2 ± 4.99 h) when compared to the IMN group (26.1 ± 3.98 h), with significant number of women delivered within 24 h in the catheter group. There was a significant number of patients who required oxytocin augmentation after AROM in the IMN group (33, 82.5%) in comparison to the catheter group (20, 50%). The analgesia, mode of delivery and the rate of failed induction were not significantly different between the two groups.
 Click to view | Table 1. Maternal Characteristics |
 Click to view | Table 2. Outcomes of Induction and Labor Dynamics |
Table 3 displays the maternal complications which were not significantly different between the two groups regarding major complications. There was a significant headache in the IMN group (10, 25%) in comparison to the catheter group (3, 7.5%). There was a significant maternal pyrexia in catheter group (12, 30%) when compared to IMN group (5, 12.5%). The neonatal outcome was not significantly different between the two groups.
 Click to view | Table 3. Maternal Adverse Effects and Neonatal Outcome |
Table 4 shows the maternal acceptability of both methods which was not statistically significant between the two groups.
 Click to view | Table 4. Maternal Acceptability |
| Discussion | ▴Top |
IOL is one of the commonest obstetric interventions, occurring in approximately 25% of term pregnancies in developed countries [11]. For women with an unfavorable cervix requiring IOL, cervical preparation is usually recommended by either prostaglandins or mechanical methods [9].
The use of intracervical Foley catheter reduces the risk of uterine hypertonicity and rupture in women with previous one caesarean section as the intracervical placement of the Foley catheter induces the cervical ripening without inducing any uterine contractions [3].
Also, NO donors as IMN inhibit uterine contractions, and promote uterine blood flow [12]. Thus, both methods appear to be ideal cervical ripening agents in women with previous one caesarean section.
In our study, both the use of intracervical Foley catheter and vaginal IMN tablets achieved cervical ripening, significant improvement of Bishop score after 12 h and successful vaginal delivery with shorter induction to delivery interval in the catheter group (22.2 ± 4.99 h) when compared to IMN group (26.1 ± 3.98 h).
In a study of 70 pregnant women with a previous one caesarean section with an unfavorable cervix at term, comparing transcervical Foley catheter and prostaglandin E2 (PGE2) gel for the IOL, the Foley catheter and the PGE2 gel had a comparable effect on the Bishop’s score after 12 h and the induction to the delivery interval was slightly shorter with the Foley catheter (18.15 h) as compared to 21.06 h with the PGE2 gel. There was no case of uterine rupture or scar dehiscence [3].
The Cochrane review of mechanical methods of IOL [9] including 71 randomized controlled trials suggests that mechanical methods have equivalent clinical effectiveness to prostaglandins with no overall significant difference in caesarean section rates, vaginal delivery within 24 h of induction, or need for oxytocin, and lower rates of hyperstimulation with fetal heart rate abnormalities.
A randomized, double-blind, placebo-controlled trial was conducted on 90 primiparous women with Bishop score ≤ 5, term pregnancy and no sign of labor. The women were allocated into two groups to receive either a 40 mg (2 × 20 mg) IMN tablet vaginally (n = 45) or placebo (n = 45) at 0 and 12 h, revealing a significant difference between the IMN group and the controls with respect to the Bishop score, induction to active phase interval and the length of induction [13].
In a study conducted by Eddama et al, the proportion of women with an unripe cervix after 24 h of outpatient treatment was significantly lower in the IMN group as compared with the placebo group [14].
Two studies were performed to test the effectiveness of vaginal IMN in conjunction with dinoprostone [15] and misoprostol [16] for IOL at term with reported faster cervical ripening and shortening of induction-labor interval.
IOL at term with Foley catheter is associated with a significant increase in intracervical pathogenic organisms despite undertaking routine aseptic measures [17]. In our study, 12 patients in the catheter group suffered pyrexia which may be due to prolonged placement of the catheter (about 12 h) or lack of administration of prophylactic antibiotics prior to insertion of the catheter.
The use of vaginal IMN was reported with a significant increase in headache, severe enough to interrupt the study [18]. This may be due to repeated dosage in this trial (two to three doses); in our study, we use only one IMN tablet once, and four out of 10 patients required analgesics in our study which is comparable to previous studies [13, 19].
In our study, the fetal heart rate abnormalities, caesarean delivery rate, Apgar scores and neonatal outcomes were similar in the two groups.
A recent Cochrane review about the methods of term labor induction for women with a previous caesarean section [20] did not make any recommendations due to shortage of randomized trials.
Our study is the first to test the use of vaginal IMN for IOL in women with previous one caesarean section at term in comparison to intracervical Foley catheter with promising success rates and fewer adverse effects. Also, both methods can be the first choice before prostaglandins when the storage, cost and the complications of prostaglandins are problematic. Studies with more power need to be conducted to evaluate both methods.
Acknowledgments
The authors would like to acknowledge the contribution of the residents and nursing staff of the labor and delivery ward of Menoufia University Hospital.
Disclosure
We certify that no actual or potential conflicts of interest in relation to this article exist.
| References | ▴Top |
- Knight M, Kurinczuk JJ, Spark P, Brocklehurst P, United Kingdom Obstetric Surveillance System Steering C. Cesarean delivery and peripartum hysterectomy. Obstet Gynecol. 2008;111(1):97-105.
doi pubmed - Silver RM, Landon MB, Rouse DJ, Leveno KJ, Spong CY, Thom EA, Moawad AH, et al. Maternal morbidity associated with multiple repeat cesarean deliveries. Obstet Gynecol. 2006;107(6):1226-1232.
doi pubmed - Ziyauddin F, Hakim S, Beriwal S. The transcervical foley catheter versus the vaginal prostaglandin e2 gel in the induction of labour in a previous one caesarean section - a clinical study. J Clin Diagn Res. 2013;7(1):140-143.
pubmed - Chwalisz K, Garfield RE. Nitric oxide as the final metabolic mediator of cervical ripening. Hum Reprod. 1998;13(2):245-248.
doi - Ledingham MA, Denison FC, Kelly RW, Young A, Norman JE. Nitric oxide donors stimulate prostaglandin F(2alpha) and inhibit thromboxane B(2) production in the human cervix during the first trimester of pregnancy. Mol Hum Reprod. 1999;5(10):973-982.
doi pubmed - Shi L, Shi SQ, Saade GR, Chwalisz K, Garfield RE. Studies of cervical ripening in pregnant rats: effects of various treatments. Mol Hum Reprod. 2000;6(4):382-389.
doi pubmed - Chwalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor. Role of progesterone and nitric oxide. Ann N Y Acad Sci. 1997;828:238-253.
doi pubmed - Bollapragada S, Mackenzie F. Randomized placebo-controlled trial of outpatient (at home) cervical ripening with Isosorbide Mononitrate (IMN) prior to induction of labor-clinical trial with analyses of efficacy and acceptability: The IMOP study. Obstet Gynecol Surv. 2009;64:699-700.
doi - Jozwiak M, Bloemenkamp KW, Kelly AJ, Mol BW, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database Syst Rev. 2012;3:CD001233.
pubmed - Jozwiak M, Oude Rengerink K, Benthem M, van Beek E, Dijksterhuis MG, de Graaf IM, van Huizen ME, et al. Foley catheter versus vaginal prostaglandin E2 gel for induction of labour at term (PROBAAT trial): an open-label, randomised controlled trial. Lancet. 2011;378(9809):2095-2103.
doi - World Health Organization: WHO recommendations for induction of labour, Department of reproductive health and research. Geneva, Switzerland:World Health Organisation. 2011:32.
- Bollapragada S, Mackenzie F, Norrie J, Petrou S, Reid M, Greer I, Osman I, et al. IMOP: randomised placebo controlled trial of outpatient cervical ripening with isosorbide mononitrate (IMN) prior to induction of labour - clinical trial with analyses of efficacy, cost effectiveness and acceptability. BMC Pregnancy Childbirth. 2006;6:25.
doi pubmed - Yazdizadeh H, Abedi P, Najar S, Angali KA. The impact of isosorbide mononitrate on cervical ripening and labor induction in primiparous women with term pregnancy: A double-blind, randomized, controlled trial. Iran J Nurs Midwifery Res. 2013;18(3):246-250.
pubmed - Eddama O, Petrou S, Schroeder L, Bollapragada SS, Mackenzie F, Norrie J, Reid M, et al. The cost-effectiveness of outpatient (at home) cervical ripening with isosorbide mononitrate prior to induction of labour. BJOG. 2009;116(9):1196-1203.
doi pubmed - Wolfler MM, Facchinetti F, Venturini P, Huber A, Helmer H, Husslein P, Tschugguel W. Induction of labor at term using isosorbide mononitrate simultaneously with dinoprostone compared to dinoprostone treatment alone: a randomized, controlled trial. Am J Obstet Gynecol. 2006;195(6):1617-1622.
doi pubmed - Abdellah MS, Hussien M, Aboalhassan A. Intravaginal administration of isosorbide mononitrate and misoprostol for cervical ripening and induction of labour: a randomized controlled trial. Arch Gynecol Obstet. 2011;284(1):25-30.
doi pubmed - Siddiqui S, Zuberi NF, Zafar A, Qureshi RN. Increased risk of cervical canal infections with intracervical Foley catheter. J Coll Physicians Surg Pak. 2003;13(3):146-149.
pubmed - Hatanaka AR, Moron AF, Auxiliadora de Aquino MM, de Souza E, de Silva Bussamra LC, Araujo Junior E, Mattar R. Interruption of a study of cervical ripening with isosorbide mononitrate due to adverse effects. Clin Exp Obstet Gynecol. 2012;39(2):175-180.
pubmed - Bullarbo M, Orrskog ME, Andersch B, Granstrom L, Norstrom A, Ekerhovd E. Outpatient vaginal administration of the nitric oxide donor isosorbide mononitrate for cervical ripening and labor induction postterm: a randomized controlled study. Am J Obstet Gynecol. 2007;196(1):50 e51-55.
- Jozwiak M, Dodd JM. Methods of term labour induction for women with a previous caesarean section. Cochrane Database Syst Rev. 2013;3:CD009792.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
