| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 2, June 2015, pages 226-231
Is There a Role for Estradiol With Progesterone in Luteal Phase Support With Intracytoplasmic Sperm Injection Cycles? A Retrospective Controlled Study With Reviewing the Literature
Mohammed El-Mahdy Abdel-Moneima, c, Ahmed Samy El-Agwanya, Ashraf Hassan Abo Alib
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Alexandria University, Alexandria, Egypt
bMadina Fertility Center, Alexandria, Egypt
cCorresponding Author: Mohammed El-Mahdy Abdel-Moneim, Department of Obstetrics and Gynecology, El-Shatby Maternity University Hospital, Faculty of Medicine, Alexandria University, Alexandria, Egypt
Manuscript accepted for publication June 10, 2015
Short title: Estradiol and Progesterone in Luteal Phase
doi: http://dx.doi.org/10.14740/jcgo333w
| Abstract | ▴Top |
Background: Luteal phase support (LPS) after intracytoplasmic sperm injection (ICSI) is a mandatory step. Progesterone is the standard LPS. There is a debate as regards the use of estradiol. The present study was conducted to evaluate the effect of adding estradiol to LPS on pregnancy rate in patients treated by ICSI.
Methods: A retrospective controlled study was conducted on 68 infertile females scheduled for ICSI through the luteal long agonist protocol. They were divided into two groups as regards the LPS: group 1 (control): 42 patients using progesterone injections intramuscular (100 mg daily) together with two vaginal pessaries 400 mg daily twice daily; group 2 (estradiol): 26 patients where midluteal oral 4 mg estradiol valerate tablet in addition to progesterone as group 1 was used. Clinical pregnancy rate was the primary outcome.
Results: There was no significant difference between the two studied groups as regards number of MII oocytes, the cleavage rate, number of embryos and number of class A embryos. As regards the pregnancy rate, it was higher in the estradiol group than in the control group but did not reach the statistical significance, 16 of 26 (61.5%) and 20 of 42 (47.6%) in the treated group versus non-treated group (P = 0.264).
Conclusion: Since estradiol level declines in the midluteal phase, patients could benefit from adding estradiol to progesterone in LPS, and at that time the effect of human chorionic gonadotropin (hCG) used for ovulation triggering on the corpus luteum decreases. The benefit includes an increase in the probability of pregnancy. There is a need for further RCTs that will assess the effect of estrogen addition to progesterone during the luteal phase on the probability of pregnancy.
Keywords: Estrogen; ICSI; Luteal phase support; Progesterone
| Introduction | ▴Top |
Ovarian stimulation for in vitro fertilization (IVF) is associated with very low luteinizing hormone (LH) concentrations during the luteal phase [1]. Luteal phase support (LPS), after controlled ovarian stimulation (COS) for IVF, has been a routine practice in IVF - embryo transfer (ET) because stimulated IVF cycles are associated with a defective luteal phase in almost all patients [2, 3]. There is a worldwide controversy concerning the type of hormones used for LPS, its dose and duration, and the time of starting and stopping it [4].
LPS traditionally was in the form of progesterone support after ovum pickup. Four formulations of progesterone are currently used for assisted reproduction, including vaginal, intramuscular (IM), oral and rectal preparations. Vaginal progesterone was used for LPS as a single agent in 64% of cycles and in another 16% of cycles in combination with either IM (15%) or oral progesterone (1%). As a single agent, IM progesterone was used in 13% of cycles, oral progesterone in another 2% and human chorionic gonadotropin (hCG) in 5% [5]. Vaginal progesterone can result in a similar pregnancy rates as IM progesterone and is more comfortable and tolerable to patients [6, 7], but it is more expensive. Conversely, IM progesterone is often associated with a number of side effects, including painful injections, severe inflammatory reactions, and sterile abscesses [8].
Although luteal hCG supplementation has proven to be an effective way to overcome luteal phase defects, this treatment is frequently associated with an increased risk of ovarian hyperstimulation syndrome (OHSS) [9].
As serum progesterone level declines after ovum pickup due to luteal phase defect, serum estradiol level also declines. It was found that the lower the ratios of estradiol levels measured on post-transfer days 4, 7 and 9 to the maximum follicular phase level, the lower the probability of pregnancy (P < 0.01, P < 0.01 and P < 0.01, respectively) [9]. Several studies tried the addition of estradiol to progesterone during luteal phase but the results suggested that adding oral estradiol to vaginal progesterone supplementation does not improve the chemical and clinical pregnancy rates of IVF/ICSI cycles [10]. The addition of transdermal estradiol to the luteal phase progesterone support of IVF cycles did not improve cycle outcomes in terms of implantation and pregnancy rates [11].
But another randomized trial showed that adding 4 mg of oral estradiol to progesterone during the luteal phase significantly increased the pregnancy and implantation rates and decreased the miscarriage rate compared with the use of progesterone only [12].
The purpose of the current study is to assess whether the probability of pregnancy is increased by adding estrogen to progesterone for LPS in IVF cycles performed with the use of gonadotrophins and gonadotrophin releasing hormone.
| Material and Methods | ▴Top |
This retrospective controlled study was conducted in a private ICSI center in Alexandria on 68 infertile women scheduled for ICSI through long agonist protocol and was approved by the Ethics Committee. The inclusion criteria were: age 20 - 40 years, basal FSH less than 12 and BMI less than 35. The exclusion criteria were: patients with male factor infertility, endometriosis, polycystic ovary, previous failed ICSI or previous history of OHSS, medical history of previous DVT, cardiac or respiratory problems.
This retrospective study was conducted on 68 patients scheduled for ICSI. Where luteal long agonist protocol was started in the midluteal phase of the preceding cycle by using daily triptorelene (decapeptyl 0.1 mg) subcutaneous injection till pituitary desensitization is confirmed by FSH ≤ 5 IU/mL, LH ≤ 5 IU/mL, progesterone ≤ 1 ng/mL, estradiol ≤ 50 pg/mL), then stimulation phase was started by using half the dose of gonadotropin releasing hormone agonist (GnRH agonist) and 300 IU (hMG and FSH) ampoules intramuscular and subcutaneous.
Follow-up till the criteria of hCG is confirmed for administration (most of the follicles more than 19 mm and serum estradiol level 150 - 200 pg/mL per large follicle). hCG (10,000 IU) was used to induce oocyte maturation.Oocyte retrieval was done transvaginally after 36 h of hCG administration. Ultrasound-guided embryo transfer was done on days 2 or 3 after oocyte retrieval. The average number of embryos transferred was 4. LPS started after oocyte retrieval. Beta-hCG was checked 2 weeks after embryo transfer. Clinical pregnancy was confirmed by the appearance of intrauterine gestational sac and pulsating fetal pole by sixth week.
According to LPS patients were classified into two groups: group 1 (control): 42 patients where progesterone intramuscular injections (100 mg daily) together with two vaginal pessaries 400 mg twice a day started on the day after oocyte retrieval and continued until the 10th week if the chemical pregnancy test was positive; group 2 (estradiol): 26 patients where midluteal oral 4 mg estradiol valerate tablets in addition to progesterone as group 1 were given.
Outcome measure
Clinical pregnancy was confirmed by the appearance of intrauterine gestational sac and pulsating fetal pole by sixth week.
Statistical analysis
Data were fed to the computer using the Predictive Analytics Software (PASW Statistics 18). Quantitative data were described using mean and standard deviation.
| Results | ▴Top |
This retrospective study was conducted on 68 patients (26 in estradiol group and 42 in control group). Both groups were homogenous as regards the mean age: 33.04 ± 5.79 years and 30.50 ± 5.48 years in estradiol and control groups respectively (P = 0.074). There was no significant difference between both groups as regards final serum estradiol level where the levels were 2,031 ± 940 pg/mL and 2,150 ± 915 pg/mL in estradiol and control groups respectively (P = 0.461).
Table 1 shows that there was no significant difference between the two studied groups as regards number of MII oocytes, the cleavage rate, number of embryos and number of class A embryos (Fig. 1, 2). As regards the pregnancy rate, it was higher in the estradiol group than in the control group but did not reach the statistical significance, 16 of 26 (61.5%) and 20 of 42 (47.6%) in the treated group versus non-treated group (P = 0.264) (Fig. 3).
 Click to view | Table 1. Comparison Between the Two Studied Groups According to Different Parameters |
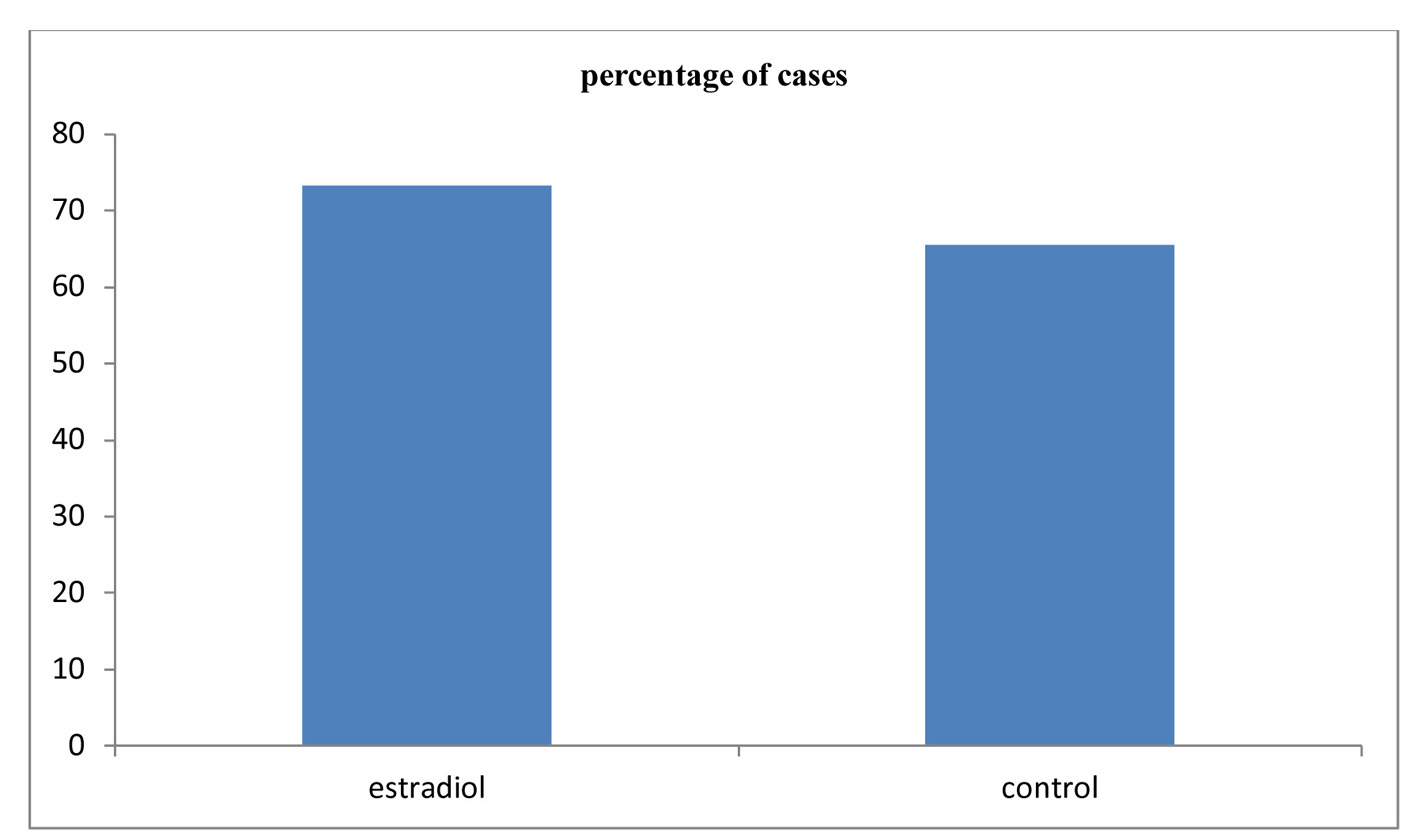 Click for large image | Figure 1. Comparison between the two studied groups according to cleavage rate. |
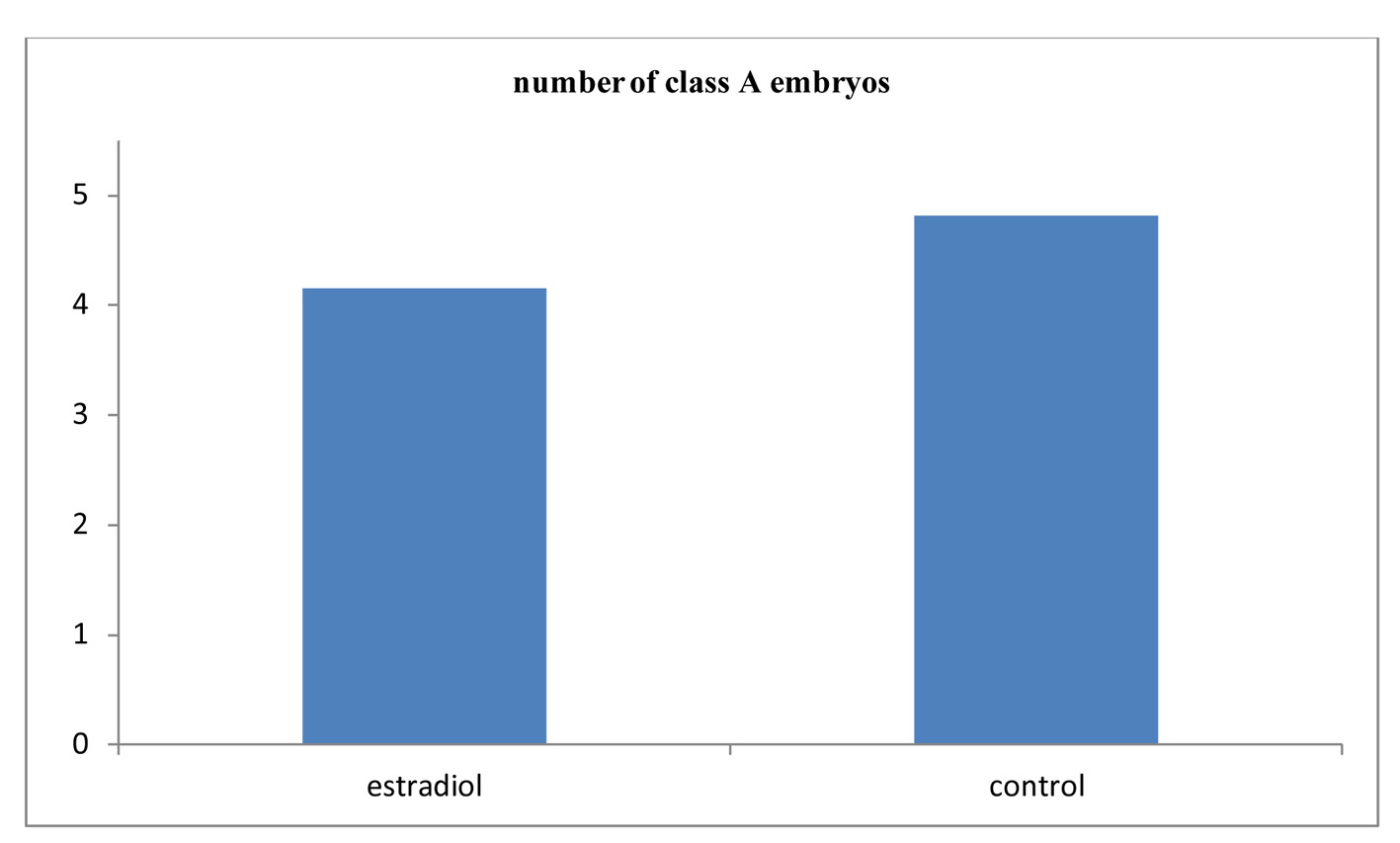 Click for large image | Figure 2. Comparison between the two studied groups according to class A embryo. |
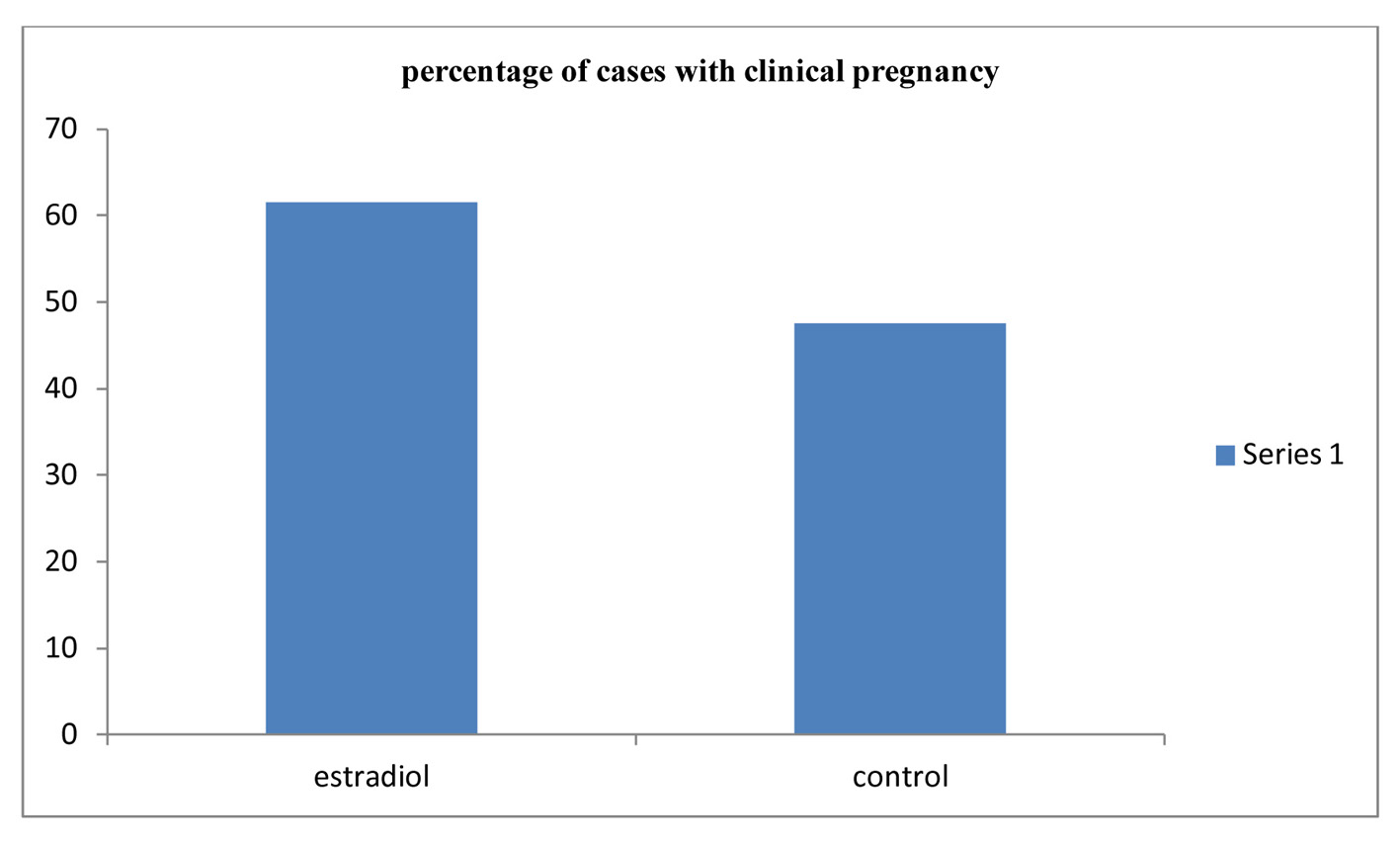 Click for large image | Figure 3. Comparison between the two studied groups according to clinical pregnancy. |
As regards the number of class A embryos, it was significantly higher in the pregnant than the non-pregnant cases in the estradiol group and in the whole study population (2.60 ± 2.67 and 5.13 ± 2.60; P = 0.016) and in the treated and the whole population (3.78 ± 2.84 and 5.25 ± 3.07; P = 0.032) (Table 2).
 Click to view | Table 2. Comparison Between the Two Studied Groups According to Different Embryos Parameters |
| Discussion | ▴Top |
LPS is a mandatory step after COH and ICSI due to the associated luteal phase defect. In COH and ICSI there is a supraphysiologic level of sex steroid that suppresses LH secretion. There is no doubt that progesterone supplementation till now is the standard LPS used. Till now, there is no consensus for the dose, route of administration or the timing of discontinuation [1-4].
Estradiol is essential for endometrial priming, with proliferation of the glands, stroma, and vessels and is observed when estrogen is administered during the follicular phase [13]. What is not clear is the role of estrogens during the luteal phase. Estrogen during the luteal phase has a modulatory effect on the secretory endometrial progesterone receptor concentration and may serve to replenish and maintain a requisite level of the receptors to mediate and complete the progesterone response [14, 15].
It has been shown that the high estrogen level during the early luteal phase of an IVF cycle induces a strong negative feedback on the pituitary, decreasing LH secretion to very low levels [16, 17]. It was found that serum estradiol level showed two declines in the luteal phase, the first after ovulation and the second at the midluteal phase. This was also shown by Turgut et al [12] who found a decline in serum estradiol level in the midluteal phase [18]. Midluteal estradiol (E2) level has a place in successful implantation and a low midluteal E2 level decreases endometrial receptivity [19, 20].
Estradiol in addition to progesterone was tried in many studies, but most studies did not prove its role in improving implantation rate and pregnancy rate. Serna et al [11] found that there were no statistically significant differences in terms of implantation rate (34.9% (51 of 146) vs. 28.9% (41 of 142)), ongoing pregnancy rate (42% (34 of 81) vs. 41.8% (33 of 79)), early pregnancy loss (15% (6 of 40) vs. 13.2% (5 of 38)), or multiple pregnancy rate (28.6% (12 of 42) vs. 24.4% (10/41)) in patients receiving progesterone versus estrogen and progesterone. Gelbaya et al [21] found the same results. These studies used estradiol supplementation from the early luteal phase which may be deleterious for embryo implantation [18, 21-23].
Since estradiol level declines in the midluteal phase, Friedler et al suggested that a subgroup of patients with a large decline in midluteal estradiol levels could benefit from this approach instead of administering estradiol universally [23].
In the present study, 4 mg oral estradiol valerate tablets were given from day 7 post-transfer in addition to progesterone which was started after oocyte retrieval. It was given empirically whatever the level of midluteal estradiol. There was a better implantation and clinical pregnancy rate in the treated group than the non-treated but not statistically significant. These results parallel to what was found by the randomized trial of Turgut et al [12] supplementing progesterone with 4 mg oral estradiol in the luteal phase significantly increased the pregnancy and implantation rates decreasing miscarriage rate compared with the use of progesterone alone. Although supplementing progesterone with hCG as a luteal support also yielded similar results to estradiol, the latter should be preferred because of OHSS and multiple pregnancy risks. Farhi et al [24] showed that when estrogen was added to progesterone in the luteal phase, the implantation rate was significantly higher, with a relative risk of 1.49 (95% CI: 1.02 - 2.19). Lukaszuk et al [25] showed significantly better support of the lutealphase when oral estradiol was added to progesterone. This result may be explained by midluteal E2 supplementation coincided with the decline in the serum E2 level which may affect implantation, and at that time the effect of hCG used for ovulation triggering on the corpus luteum decreases. Vlahos et al [26] found that the combination of progesterone and E2 seemed to increase endometrial L-selectin ligand expression in the luminal endothelium compared with progesterone alone, suggesting a scientific rationale for this approach.
Turgut et al found that the pregnancy and implantation rates in the E2 + progesterone versus hCG + progesterone groups were similar, but these rates in the E2 + progesterone and hCG + progesterone groups were significantly higher than in the progesterone only group. Farhi et al [24] and Lukaszuk et al [25] demonstrated significantly higher pregnancy and implantation rates in the E2 + progesterone group compared with the progesterone only group. However, Elgindy et al [27] found that when E2 valerate was taken orally, the pregnancy was higher than in the only group, but the difference was not statistically significant.
Conclusion
Since estradiol level declines in the midluteal phase, patients could benefit from adding estradiol to progesterone in LPS, and at that time the effect of hCG used for ovulation triggering on the corpus luteum decreases. The benefit includes an increase in the probability of pregnancy. There is a need for further RCTs that will assess the effect of estrogen addition to progesterone during the luteal phase on the probability of pregnancy.
| References | ▴Top |
- Tavaniotou A, Albano C, Smitz J, Devroey P. Comparison of LH concentrations in the early and mid-luteal phase in IVF cycles after treatment with HMG alone or in association with the GnRH antagonist Cetrorelix. Hum Reprod. 2001;16(4):663-667.
doi pubmed - Kolibianakis EM, Devroey P. The luteal phase after ovarian stimulation. Reprod Biomed Online. 2002;5(Suppl 1):26-35.
doi - Pabuccu R, Akar ME. Luteal phase support in assisted reproductive technology. Curr Opin Obstet Gynecol. 2005;17(3):277-281.
doi pubmed - Aboulghar M. Luteal support in reproduction: when, what and how? Curr Opin Obstet Gynecol. 2009;21(3):279-284.
doi pubmed - Vaisbuch E, Leong M, Shoham Z. Progesterone support in IVF: is evidence-based medicine translated to clinical practice? A worldwide web-based survey. Reprod Biomed Online. 2012;25(2):139-145.
doi pubmed - Polyzos NP, Messini CI, Papanikolaou EG, Mauri D, Tzioras S, Badawy A, Messinis IE. Vaginal progesterone gel for luteal phase support in IVF/ICSI cycles: a meta-analysis. Fertil Steril. 2010;94(6):2083-2087.
doi pubmed - Doody K, Bush M, Collins M. Progesterone supplementation for luteal support: Efficacy and patient experiences with vaginal inserts (Endometrin(registered trademark)) versus intramuscular injection. FertilSteril. 2012;97:S18.
doi - Propst AM, Hill JA, Ginsburg ES, Hurwitz S, Politch J, Yanushpolsky EH. A randomized study comparing Crinone 8% and intramuscular progesterone supplementation in in vitro fertilization-embryo transfer cycles. Fertil Steril. 2001;76(6):1144-1149.
doi - Soliman S, Daya S, Collins J, Hughes EG. The role of luteal phase support in infertility treatment: a meta-analysis of randomized trials. Fertil Steril. 1994;61(6):1068-1076.
pubmed - Moini A, Zadeh Modarress S, Amirchaghmaghi E, Mirghavam N, Khafri S, Reza Akhoond M, Salman Yazdi R. The effect of adding oral oestradiol to progesterone as luteal phase support in ART cycles - a randomized controlled study. Arch Med Sci. 2011;7(1):112-116.
doi pubmed - Serna J, Cholquevilque JL, Cela V, Martinez-Salazar J, Requena A, Garcia-Velasco JA. Estradiol supplementation during the luteal phase of IVF-ICSI patients: a randomized, controlled trial. Fertil Steril. 2008;90(6):2190-2195.
doi pubmed - Var T, Tonguc EA, Doganay M, Gulerman C, Gungor T, Mollamahmutoglu L. A comparison of the effects of three different luteal phase support protocols on in vitro fertilization outcomes: a randomized clinical trial. Fertil Steril. 2011;95(3):985-989.
doi pubmed - Adams SM, Terry V, Hosie MJ, Gayer N, Murphy CR. Endometrial response to IVF hormonal manipulation: comparative analysis of menopausal, down regulated and natural cycles. Reprod Biol Endocrinol. 2004;2:21.
doi pubmed - Fritz MA, Westfahl PK, Graham RL. The effect of luteal phase estrogen antagonism on endometrial development and luteal function in women. J Clin Endocrinol Metab. 1987;65(5):1006-1013.
doi pubmed - Goldstein D, Zuckerman H, Harpaz S, Barkai J, Geva A, Gordon S, Shalev E, et al. Correlation between estradiol and progesterone in cycles with luteal phase deficiency. Fertil Steril. 1982;37(3):348-354.
pubmed - de Ziegler D, Bergeron C, Cornel C, Medalie DA, Massai MR, Milgrom E, Frydman R, et al. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74(2):322-331.
pubmed - Beckers NG, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, et al. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab. 2003;88(9):4186-4192.
doi pubmed - Valbuena D, Martin J, de Pablo JL, Remohi J, Pellicer A, Simon C. Increasing levels of estradiol are deleterious to embryonic implantation because they directly affect the embryo. Fertil Steril. 2001;76(5):962-968.
doi - de Ziegler D, Bouchard P. Understanding endometrial physiology and menstrual disorders in the 1990s. Curr Opin Obstet Gynecol. 1993;5(3):378-388.
doi pubmed - De Hertogh R, Vanderheyden I, Glorieux B, Ekka E. Oestrogen and progestogen receptors in endometrium and myometrium at the time of blastocyst implantation in pregnant diabetic rats. Diabetologia. 1989;32(8):568-572.
doi pubmed - Gelbaya TA, Kyrgiou M, Tsoumpou I, Nardo LG. The use of estradiol for luteal phase support in in vitro fertilization/intracytoplasmic sperm injection cycles: a systematic review and meta-analysis. Fertil Steril. 2008;90(6):2116-2125.
doi pubmed - Forman RG, Eychenne B, Nessmann C, Frydman R, Robel P. Assessing the early luteal phase in in vitro fertilization cycles: relationships between plasma steroids, endometrial receptors, and endometrial histology. Fertil Steril. 1989;51(2):310-316.
pubmed - Friedler S, Zimerman A, Schachter M, Raziel A, Strassburger D, Ron El R. The midluteal decline in serum estradiol levels is drastic but not deleterious for implantation after in vitro fertilization and embryo transfer in patients with normal or high responses. Fertil Steril. 2005;83(1):54-60.
doi pubmed - Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2000;73(4):761-766.
doi - Lukaszuk K, Liss J, Lukaszuk M, Maj B. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2005;83(5):1372-1376.
doi pubmed - Vlahos NF, Lipari CW, Bankowski B, Lai TH, King JA, Shih Ie M, Fragakis K, et al. Effect of luteal-phase support on endometrial L-selectin ligand expression after recombinant follicle-stimulating hormone and ganirelix acetate for in vitro fertilization. J Clin Endocrinol Metab. 2006;91(10):4043-4049.
doi pubmed - Elgindy EA, El-Haieg DO, Mostafa MI, Shafiek M. Does luteal estradiol supplementation have a role in long agonist cycles? Fertil Steril. 2010;93(7):2182-2188.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
