| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 4, December 2015, pages 283-289
Dysmenorrhea as a Risk Factor for Hyperemesis Gravidarum
Christopher Atsaboghena Enakpenea, Mudar Dalloulb, Carla Petterkin-Caldwellc, Jenny Anopab, Ozgul Muneyyirci-Delaleb, d, e
aDivision of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Illinois at Chicago, 820 S Wood Street, M/C 808, Chicago, IL 60612, USA
bDepartment of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, State University of New York, Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203, USA
cDivision of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Kings County Hospital Center, 451 Clarkson Avenue, Brooklyn, NY 11203, USA
dDepartment of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, Kings County Medical Center, 451 Clarkson Avenue, Brooklyn, NY 11203, USA
eCorresponding Author: Ozgul Muneyyirci-Delale, Department of Obstetrics and Gynecology, Division of Reproductive Endocrinology and Infertility, State University of New York, Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, NY 11203, USA
Manuscript accepted for publication October 02, 2015
Short title: Dysmenorrhea and Hyperemesis Gravidarum
doi: http://dx.doi.org/10.14740/jcgo356w
| Abstract | ▴Top |
Background: The aims of the study were to assess the association of dysmenorrhea and hyperemesis gravidarum (HG) and to determine other factors that may influence its onset and severity.
Methods: This is a prospective case-control IRB approved study of 344 consecutive singleton pregnant women with and without hyperemesis gravidarum in pregnancy from February 2011 to April 2012. The association between HG and dysmenorrhea in adolescent and adult was examined using Pearson’s Chi-square with Yates correlation, Student’s t-test and Mann-Whitney U test. Bivariate analysis and odd ratios (ORs) were calculated to evaluate the strength of their association, and multivariate logistic regression analysis while correcting for confounders. P-value of 0.05 was considered statistically significant.
Results: A total of 344 consecutive singleton pregnant women were recruited. Significant association was found between HG and adolescent dysmenorrhea: 77.8% versus 43.4% of controls (P < 0.0001, OR: 4.6, 95% CI: 2.3 - 8.9). Also, there was a significant association between HG and adult dysmenorrhea: 76.4% versus 38.1% controls (P < 0.0001, OR: 5.3, 95% CI: 2.7 - 10.2). The association of severe adolescent and adult dysmenorrhea with HG was stronger (P < 0.0001, OR: 8.8, 95% CI: 3.9 - 19.9 and P < 0.0001, OR: 12.2, 95% CI: 5.0 - 29.7 respectively). There was a modest association with moderate dysmenorrhea (P = 0.004, OR: 3.1, 95% CI: 1.4 - 6.9) which was not sustained but no associations were found between HG and all mild dysmenorrhea of both adolescent and adult.
Conclusion: This study found an association between adolescent and adult dysmenorrhea and HG. These associations were stronger with severe dysmenorrhea.
Keywords: Dysmenorrhea; Hyperemesis gravidarum; Adolescent; Adult; Onset; Severity
| Introduction | ▴Top |
Nausea and vomiting are common in pregnancy and affect 70-85% of pregnant women [1]. Hyperemesis gravidarum (HG) is an extreme of the spectrum of nausea and vomiting in pregnancy that affects approximately 0.5-2% of pregnancies [2]. This is characterized by persistent vomiting not related to other causes resulting in dehydration, acute starvation evidence by ketonemia and large ketonuria, weight loss of at least 5% of pre-pregnancy weight and electrolytes disorders. There may also be thyroid and liver abnormalities [3, 4]. Nausea and vomiting symptoms usually manifest before 9 weeks of gestation and it is the commonest indication for hospital admission in the first trimester of pregnancy. HG causes significant maternal and fetal morbidity and possibly mortality.
Though the etiology of HG is unknown, various theories have been adduced to HG including psychological predisposition [5], evolutional adaptation [6], and hormonal stimulus [5, 6]. Hormonal stimuli such as human chorionic gonadotrophin (hCG) and estrogen have been established to have varying influences on nausea and vomiting [7, 8]. Other hormones such as progesterone, adrenal and pituitary hormones may also trigger HG. However, the links between these hormones and hyperemesis are not straightforward because patients with hyperemesis may either have elevated or lower level of progesterone [9-11]. Other researchers found no association between hyperemesis and progesterone concentration because progesterone treatment does not improve complaints [12, 13]. Risk factors include an increase in placenta mass such as advance molar pregnancy and multiple gestation, genetic and family history, first pregnancy, overweight, history of HG in previous pregnancies, carrying of a female fetus, daughters and sisters of women with HG, motion sickness and migraines [14, 15].
The association between increased maternal serum concentration of hCG and nausea and vomiting of pregnancy has been studied for more than half a century. Shoeneck et al and other researchers have demonstrated a positive correlation between maternal serum level of hCG and nausea and vomiting of pregnancy. Their findings were based on the fact that the peak maternal serum and urinary hCG levels are from 6 to 14 weeks of pregnancy which corresponds to when nausea and vomiting of pregnancy is usually encountered [16-19]. However, Soules et al and Bagshawe did not find this correlation based on the fact that there is no nausea and vomiting in chorio-carcinoma where there is extremely high maternal serum and cerebrospinal fluid concentration of hCG. This leads to the suspicion that other factors which increase the synthesis and release of hCG may be responsible for nausea and vomiting of pregnancy [20, 21].
Primary dysmenorrhea is defined as pain during menses in the absence of an identifiable pathologic lesion which usually begins during adolescence. The prevalence of dysmenorrhea is highest in adolescents ranging 20-90% with the severe form reported to be as high as 15% in the age group of 13 - 19 years [22-24]. It is characterized by cramping lower abdominal pain and may be associated with nausea, vomiting, headaches, diarrhea, and myalgia. The actual cause of primary dysmenorrhea is unknown, but several studies have proven that primary dysmenorrhea is as a result of inflammatory responses mediated by prostaglandin and leukotrienes which cause myometrial contraction and vasoconstriction. This will invariably generate cramps and systemic symptoms such as nausea, vomiting, bloating, diarrhea and headaches [25, 26]. Dawood et al in their study demonstrated that the intensity of menstrual cramps and the associated symptoms are directly proportional to the amount of PGF2α and PGE2 levels measured in menstrual fluid [27]. Symptoms of dysmenorrhea such as nausea, vomiting, and diarrhea occur in approximately 60% of patients. These symptoms are similar to those experienced when exogenous prostaglandins are used as abortifacient drugs [28].
Cunningham and Muneyyirci-Delale hypothesized that there is an association between dysmenorrhea and HG [29]. This is based on the hypothesis that the prostaglandin stimulated symptoms of nausea and vomiting experienced in dysmenorrhea are affiliated with excessive nausea and vomiting during pregnancy and/or HG. Hence, the objectives of this study were to assess the association of dysmenorrhea and HG and to assess other factors that may influence onset of HG and its severity.
| Methodology | ▴Top |
Study design
This is a prospective case-control IRB approved study of 72 consecutive singleton pregnant women admitted for HG in their index pregnancy from February 2011 to April 2012 at the State University of New York (SUNY) Downstate Medical Center and King’s County Hospital Center. Also recruited were participants with various spectrum of nausea and vomiting in pregnancy and a control group without any symptoms of nausea and vomiting during the study period.
Study population
The study population consists of a total of 344 participants: 72 with HG, 104 moderate and 55 with mild nausea and vomiting and 113 as controls without nausea and vomiting. They were recruited from the prenatal clinics or on admission to State University of New York (SUNY) Downstate Medical Center and King’s County Hospital Center. The emesis gravidarum and dysmenorrhea in adolescent and adulthood were classified into three categories: 1) mild emesis gravidarum: nausea only without vomiting; 2) moderate emesis gravidarum: nausea and vomiting but not requiring hospital admission; and 3) severe emesis gravidarum (HG): nausea and vomiting requiring hospital admission due to dehydration and electrolytes imbalance, and others.
The three categories of dysmenorrhea were based on pain intensity using visual analogue scale (VAS): 1) mild dysmenorrhea: pain intensity of 1 - 3; 2) moderate dysmenorrhea: pain intensity of 4 - 7; and 3) severe dysmenorrhea: pain intensity of 8 - 10.
Sample size
The sample size was determined based on the prevalence of emesis gravidarum of 70-85%; a total of 300 participants were required in addition to 10% calculated attrition rate and a margin of error of 5% at a confidence level of 95% and a power of 80%.
Data collection
Written informed consent was obtained from individual participant and they were interviewed with structured IRB approved self-administered questionnaire about dysmenorrhea in adolescent and adulthood and emesis gravidarum symptoms. The questionnaires were distributed by two research assistants who were not privy to the objectives of the study. The research assistants were trained to guide participants to respond to questions that they needed more clarifications. Some of pertinent data collected were socio-demographic data, parity, history of nausea and vomiting in the index pregnancy, severity of the symptoms, gestational age of onset of symptoms, history of hospital admission for nausea and vomiting, gestational age at admission, number of fetuses, medical, psychiatric and surgical history, gynecologic and obstetric history, medications, pre-pregnancy weight and weight at admission, laboratory results at presentation such as complete blood count, comprehensive chemistry panel, TSH, serum β-hCG, duration of admission and history of nausea and vomiting in previous pregnancies. Information in regards to adolescent and adulthood dysmenorrhea, severity of dysmenorrhea using VAS for pain assessment, the age of onset of dysmenorrhea, history of chronic pelvic pain, dyspareunia, history of dysmenorrhea and HG in first degree relatives were collected.
Data management
Data obtained were entered into a computer running Statistical Package for Social Sciences version 20 (SPSS 20) software. Descriptive statistics of the baseline characteristics of participants were displayed in frequency tables. Participants were classified into four categories: HG, moderate nausea and vomiting in pregnancy not requiring hospitalization, nausea only and absent of nausea and vomiting in pregnancy used as control group. Comparisons of the baseline characteristics of each of the individual group with the control group were done using Chi-square for categorical variables and Student’s t-test for normally distributed continuous variables. Continuous variables that were not normally distributed were examined using Mann-Whitney U test. The proportions of primary dysmenorrhea were calculated for each group and compared, and tested for statistical significance using McNemar’s Chi-square test of 2 × 2 contingency table with Yates correlation for continuity. The odds ratio (OR) and 95% confidence interval (CI) were calculated to determine the strength of association between primary dysmenorrhea and nausea and vomiting, and HG. Multiple logistic regression analysis was used to determine the association between primary dysmenorrhea and HG while correcting for cofounding variables.
| Results | ▴Top |
A total of 344 consecutive sonogram proven singleton pregnant women were recruited with a mean age of 27.9 years, parity ranging from 0 to 7 with a mean parity of 1.07, and mean weight of 176.5 lbs. In the participants, 76.3% were African American, 14.2% were Hispanic and 9.5% were Caucasian. Seventy-two participants were hospitalized for HG, 104 had nausea and vomiting but not hospitalized, 55 had only nausea and 113 who neither experienced nausea nor vomiting served as controls. A significant association was found between HG and adolescent dysmenorrhea; 77.8% of participants with HG gave history of adolescent dysmenorrhea versus 43.4% of controls (P < 0.0001, OR: 4.6, 95% CI: 2.3 - 8.9), as shown in Table 1. Similarly, there was a significant association between HG and adult dysmenorrhea; 76.4% of participants with HG had adulthood dysmenorrhea versus 38.1% controls (P < 0.0001, OR: 5.3, 95% CI: 2.7 - 10.2) as shown in Table 2.
 Click to view | Table 1. Relationship Between Adolescent Dysmenorrhea and Hyperemesis Gravidarum |
 Click to view | Table 2. Relationship Between Adult Dysmenorrhea and Hyperemesis Gravidarum |
The impacts of the severity of dysmenorrhea on HG were examined using a VAS classified into mild, moderate and severe (scores 1 - 3, 4 - 7 and 8 - 10 respectively). Examining only those with severe dysmenorrhea, the association with HG was stronger for both adolescent and adult dysmenorrhea (P < 0.0001, OR: 8.8, 95% CI: 3.9 - 19.9 and P < 0.0001, OR: 12.2, 95% CI: 5.0 - 29.7) respectively (Tables 3 and 4). A modest association between moderate adult dysmenorrhea with HG was also found (P = 0.004, OR: 3.1, 95% CI: 1.4 - 6.9) as shown in Table 4. There was no significant association between HG with mild and moderate adolescent dysmenorrhea and mild adult dysmenorrhea (P = 0.08, 0.076 and 0.12 respectively). The adjusted ORs for adolescent and adult dysmenorrhea after controlling for confounders were 6.93 and 8.34 respectively (P < 0.0001).
 Click to view | Table 3. Nausea and Vomiting and Intensity and Hyperemesis Gravidarum |
 Click to view | Table 4. Nausea and Vomiting and Intensity of Adult Dysmenorrhea |
Further bivariate analysis showed statistically significant associations between nausea and vomiting in pregnancy and family history of nausea and vomiting in first degree relatives, personal history of nausea and vomiting in previous pregnancies (P < 0.0001), and past surgical history such as cesarean section and laparotomies for any indication (P = 0.011). There were no statistically significant associations between nausea and vomiting and medical history such as diabetics, hypertension, GERD, etc. (P = 0.357), marital status (P = 0.457), new father of baby as different from previous pregnancies (P = 0.161), contraceptive usage of any form (P = 0.356) and dyspareunia (P = 0.451). Multivariate logistic regression analyses using personal and family histories of nausea and vomiting of pregnancy and surgical history as cofounders showed persistent statistically significant association between nausea and vomiting of pregnancy and adult and adolescent dysmenorrhea. The adjusted ORs for adolescent and adult dysmenorrhea after controlling for cofounders were 6.93 and 8.35 respectively (P < 0.0001).
| Discussion | ▴Top |
Our study shows that there is a positive association between nausea and vomiting in pregnancy and history of dysmenorrhea as adult and adolescent. This association remains consistent even when controlled for confounders. There is also a linear relationship between the severity of emesis and the severity of dysmenorrhea in adolescent and adult (Fig. 1, 2).
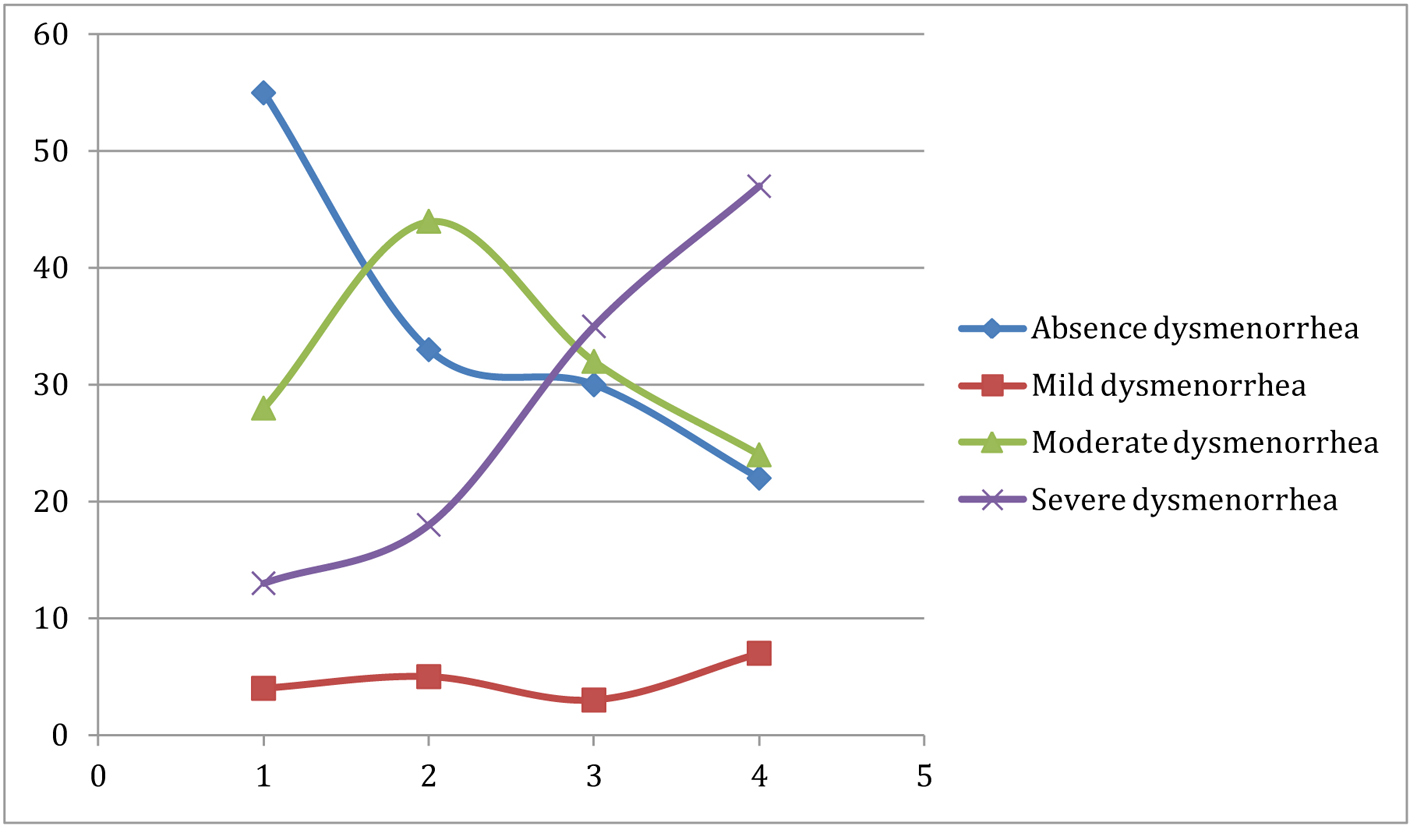 Click for large image | Figure 1. The relationship between intensity of adolescent dysmenorrhea and severity of nausea and vomiting. |
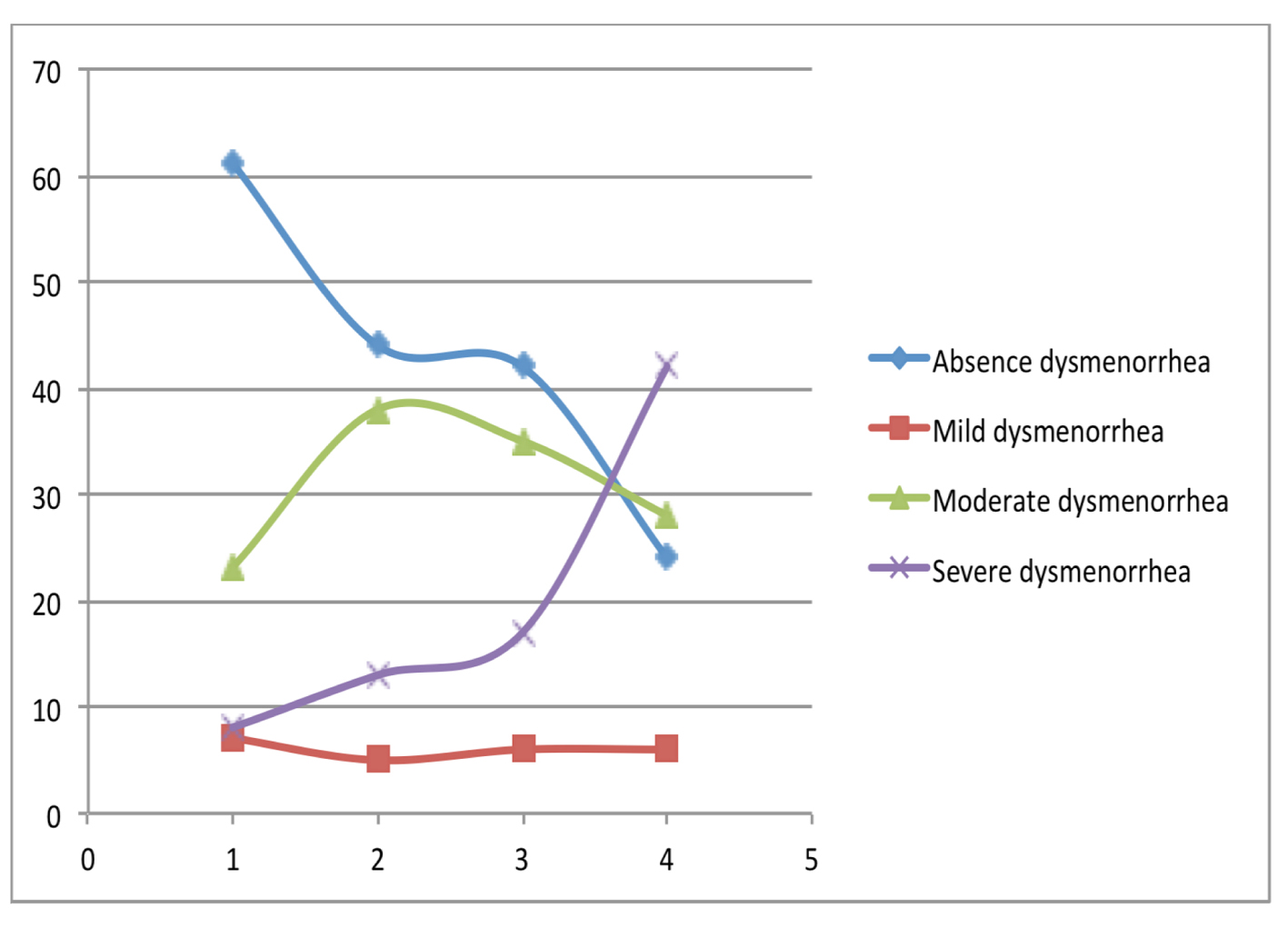 Click for large image | Figure 2. The relationship between intensity of adult dysmenorrhea and severity of nausea and vomiting. |
Dysmenorrhea and hyperemesis are two disease entities that the overall etiologies are both unknown. The positive correlation between these diseases in our study may be due to an increase in prostaglandins, hormones and other factors [29]. The association between HG and increase level of hCG still remains debatable. An isoform of hCG that has genetic connotation has been proposed [2, 30, 31]. However, conditions that are associated with high level of hCG such as chorio-carcinoma, do not typically result in nausea and vomiting, and many pregnant women with high hCG levels do not suffer from HG. In addition, there are a significant number of women with HG whose symptoms go beyond first trimester when hCG levels are falling. The use of hCG as luteal support or to trigger oocyte maturation has not been found to cause symptoms of HG. Hence, other hormones and factors that have been linked to HG include progesterone, estrogen, thyroid hormones, leptin, adrenal cortex hormones, prolactin, growth hormone, placenta serum markers, immunology, gastro-intestinal infection and intestinal motility, change in lower esophageal sphincter pressure, increased fluid secretion in the gastro-intestinal tract, liver enzymes, amylase, vitamin 6 and trace element deficiency [32].
Many hormones, hormone releasing factors, cytokines and prostaglandins have been found to stimulate the synthesis and release of hCG from syncytiotrophoblast cells in early pregnancy. Of all these factors, only prostaglandin E2 (PGE2) and prostaglandin F2α (PGF2α) are known to cause nausea and vomiting of pregnancy when these prostaglandins are given to procure legal termination of early pregnancy. PGE2 is produced by the syncytiotrophoblast cells of human trophoblast at 7 - 11 weeks of gestation. The production of PGE2 is stimulated by several hormones and cytokines such as interleukin-1 and hCG itself. The production and release of PGE2 is controlled by prostaglandin dehydrogenase (PGDH) which is found in early trophoblast and convert PGE2 to its inactive metabolite 5-ketoprostaglandin E2 [33]. The syncytiotrophoblasts which are in direct contact with maternal blood are devoid of PGDH but it is present in cytotrophoblast and the decidual cells. This results in increased maternal serum level of PGE2 disproportionate to the decidual tissues and small blood vessels [34]. The activities of PGDH are regulated by progesterone, and increased production of progesterone is directly proportional to synthesis of PGDH especially in the decidual tissues which prevent miscarriage. The natural reduction of progesterone between 5 and 9 weeks of pregnancy results in low activities of PGDH which invariably increase maternal level of PGE2 [35]. Increased maternal serum level of PGE2 causes nausea and vomiting of pregnancy.
After reviewing PubMed, Cochrane library, Medline using MeSH search, there is no study till date that shows that there is an association between dysmenorrhea and HG. The major link between the two disease entities is prostaglandin as proposed by Cunningham and Muneyyirci-Delale in their hypothesis [29]. It is established that prostaglandin is the main etiology of primary dysmenorrhea in both adolescents and adults [25, 26]. Our study shows that women with history of adolescent and adult dysmenorrhea have five-fold increased risk of developing HG. This risk further increased by about 10-fold when they have severe dysmenorrhea. There is a modest increased risk of HG with moderately severe adolescent and adult dysmenorrhea. Mild adolescent and adult dysmenorrhea was not found to increase the risk of nausea and vomiting of pregnancy and HG in this study.
Conclusion
While it is possible to ameliorate the effects of HG, the scourge of intractable condition remained a huge challenge. Many of the prior interventions such as thalidomide and diethylstilbestrol (DES) ended up causing more harm than good. The reduction in prostaglandin and leukotriene synthesis can both have synergistic effects in reducing the syntheses and release of hCG which will invariably decrease the occurrence and severity of nausea and vomiting in pregnancy. There is speculation that pre-pregnancy aggressive treatment of dysmenorrhea with NSAID to reduce the level and effect of prostaglandin until pregnancy may reduce the severity of HG. Corticosteroids such as methylprednisolone or dexamethasone which inhibit synthesis of prostaglandin and leukotrienes are used for treatment of refractory HG [36]. Early treatment of severe nausea and vomiting with corticosteroids in patients with history of severe dysmenorrhea may reduce the morbidity associated with HG. However, corticosteroids especially dexamethasone which crosses the placenta should be used with precautions because of its potential teratogenic effects. When required, they should be used after 8 - 10 weeks gestation when lip fusion has been completed. However, further research is needed to establish causal relationship between dysmenorrhea and HG.
Support
No support was received for this work.
Conflict of Interest
The authors declare no conflict of interest.
| References | ▴Top |
- Jewell D, Young G. Interventions for nausea and vomiting in early pregnancy (Cochrane Review). In: The Cochrane Library, issue 4, 2003, Chichester, UK: John Wiley and Sons Ltd (Meta-analysis).
- Klebanoff MA, Koslowe PA, Kaslow R, Rhoads GG. Epidemiology of vomiting in early pregnancy. Obstet Gynecol. 1985;66(5):612-616.
pubmed - Gadsby R, Barnie-Adshead AM, Jagger C. Pregnancy nausea related to women's obstetric and personal histories. Gynecol Obstet Invest. 1997;43(2):108-111.
doi pubmed - Goodwin TM, Montoro M, Mestman JH. Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am J Obstet Gynecol. 1992;167(3):648-652.
doi - Simpson SW, Goodwin TM, Robins SB, Rizzo AA, Howes RA, Buckwalter DK, Buckwalter JG. Psychological factors and hyperemesis gravidarum. J Womens Health Gend Based Med. 2001;10(5):471-477.
doi pubmed - Flaxman SM, Sherman PW. Morning sickness: a mechanism for protecting mother and embryo. Q Rev Biol. 2000;75(2):113-148.
doi - Bernstein L, Pike MC, Lobo RA, Depue RH, Ross RK, Henderson BE. Cigarette smoking in pregnancy results in marked decrease in maternal hCG and oestradiol levels. Br J Obstet Gynaecol. 1989;96(1):92-96.
doi pubmed - Depue RH, Bernstein L, Ross RK, Judd HL, Henderson BE. Hyperemesis gravidarum in relation to estradiol levels, pregnancy outcome, and other maternal factors: a seroepidemiologic study. Am J Obstet Gynecol. 1987;156(5):1137-1141.
doi - Borgeat A, Fathi M, Valiton A. Hyperemesis gravidarum: is serotonin implicated? Am J Obstet Gynecol. 1997;176(2):476-477.
doi - Jarnfelt-Samsioe A. Nausea and vomiting in pregnancy: a review. Obstet Gynecol Surv. 1987;42(7):422-427.
doi pubmed - Yoneyama Y, Suzuki S, Sawa R, Yoneyama K, Doi D, Otsubo Y, Araki T. The T-helper 1/T-helper 2 balance in peripheral blood of women with hyperemesis gravidarum. Am J Obstet Gynecol. 2002;187(6):1631-1635.
doi pubmed - Fairweather DV. Nausea and vomiting during pregnancy. Obstet Gynecol Annu. 1978;7:91-105.
pubmed - Fairweather DV. Nausea and vomiting in pregnancy. Am J Obstet Gynecol. 1968;102(1):135-175.
pubmed - Whitehead SA, Andrews PL, Chamberlain GV. Characterization of nausea and vomiting in early pregnancy; a survey of 1,000 women. J ObstetGynecol. 1992;12:364-369.
doi - Basso O, Olsen J. Sex ratio and twinning in women with hyperemesis or pre-eclampsia. Epidemiology. 2001;12(6):747-749.
doi pubmed - Shoeneck FJ, Syracuse NY. Gonadotrophic hormone concentration in emesis gravidarum. Am J Onstet Gynecol. 1942;43:308-311.
- Masson GM, Anthony F, Chau E. Serum chorionic gonadotrophin and Schwangerschaft's protein, progesterone and estradiol levels in patients with nausea and vomiting in early pregnancy. Brit J Obstet Gynaecol. 1985;92:211-215.
doi - Jarnfelt-Samsioe A, Bremme K, Eneroth P. Non-steroid hormones and tissue polypeptide antigen (TPA) in emetic and non-emetic pregnancy. Acta Obstet Gynecol Scand. 1986;65(7):745-751.
doi pubmed - Kauppila A, Huhtaniemi I, Ylikorkala O. Raised serum human chorionic gonadotrophin concentrations in hyperemesis gravidarum. Br Med J. 1979;1(6179):1670-1671.
doi pubmed - Soules MR, Hughes CL, Jr., Garcia JA, Livengood CH, Prystowsky MR, Alexander E, 3rd. Nausea and vomiting of pregnancy: role of human chorionic gonadotropin and 17-hydroxyprogesterone. Obstet Gynecol. 1980;55(6):696-700.
pubmed - Shi QJ, Lei ZM, Rao CV, Lin J. Novel role of human chorionic gonadotropin in differentiation of human cytotrophoblasts. Endocrinology. 1993;132(3):1387-1395.
pubmed - Klein JR, Litt IF. Epidemiology of adolescent dysmenorrhea. Pediatrics. 1981;68(5):661-664.
pubmed - Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154(12):1226-1229.
doi pubmed - Davis AR, Westhoff CL. Primary dysmenorrhea in adolescent girls and treatment with oral contraceptives. J Pediatr Adolesc Gynecol. 2001;14(1):3-8.
doi - Chan WY, Hill JC. Determination of menstrual prostaglandin levels in non-dysmenorrheic and dysmenorrheic subjects. Prostaglandins. 1978;15(2):365-375.
doi - Lundstrom V, Green K. Endogenous levels of prostaglandin F2alpha and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. Am J Obstet Gynecol. 1978;130(6):640-646.
pubmed - Dawood YM, Khan-Dawood FS. Differential suppression of menstrual fluid prostaglandin F2α, prostaglandin E2, 6-keto prostaglandin F1A, and thromboxane B2 with Ibuprofen in women with primary dysmenorrhea. Prostaglandins Lipid Mediators. 2007;83:146 - 153.
doi pubmed - Karim SM, Filshie GM. Use of prostaglandin E2 for therapeutic abortion. Br Med J. 1970;3(5716):198-200.
doi - Cunningham AS, Muneyyirci-Delale O. The association between primary dysmenorrhea and hyperemesis gravidarum. Med Hypotheses. 2009;73(1):90-91.
doi pubmed - Jordan V, MacDonald J, Crichton S, Stone P, Ford H. The incidence of hyperemesis gravidarum is increased among Pacific Islanders living in Wellington. N Z Med J. 1995;108(1006):342-344.
- Jordan V, Grebe SK, Cooke RR, Ford HC, Larsen PD, Stone PR, Salmond CE. Acidic isoforms of chorionic gonadotrophin in European and Samoan women are associated with hyperemesis gravidarum and may be thyrotrophic. Clin Endocrinol (Oxf). 1999;50(5):619-627.
doi - Fatum M, Abramob Y. [Hyperemesis gravidarum: an updated review]. Harefuah. 2003;142(1):61-65, 77.
pubmed - Jarabak J. Early steps in prostaglandin metabolism in the human placenta. Am J Obstet Gynecol. 1980;138(5):534-540.
doi - Cheng L, Kelly RW, Thong KJ, Hume R, Baird DT. The effects of mifepristone (RU486) on prostaglandin dehydrogenase in decidual and chorionic tissue in early pregnancy. Hum Reprod. 1993;8(5):705-709.
pubmed - Falkay G, Sas M. Correlation between the concentrations of prostaglandin dehydrogenae and progesterone in the early human placenta. J Endocrinol. 1978;76(1):173-174.
doi pubmed - Nausea and vomiting of pregnancy. ACOG Practice Bulletin. 2004;52.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
