| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 2, June 2016, pages 59-63
Comparison of Oral Versus Vaginal Misoprostol for Legal Abortion in Iranian Women
Fariba Farhadifara, b, Shole Shahgheibia, Ghobad Moradib, Faezeh Malekmohammadi Memara, c
aDepartment of Obstetrics & Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
bDepartment of Epidemiology, Kurdistan Research Center for Social Determinants of Health, Kurdistan University of Medical Sciences, Sanandaj, Iran
cCorresponding Author: Faezeh Malekmohammadi Memar, Department of Obstetrics & Gynecology, Faculty of Medicine, Kurdistan University of Medical Sciences, Sanandaj, Iran
Manuscript accepted for publication April 20, 2016
Short title: Oral Versus Vaginal Misoprostol for Abortion
doi: http://dx.doi.org/10.14740/jcgo406e
| Abstract | ▴Top |
Background: Considering the different available results on effectiveness of various doses of misoprostol and different methods of administration, as well as legal issues of abortion in Iran, the aim of this study was to compare oral and vaginal misoprostol for legal abortion in pregnant women.
Methods: This randomized double-blind clinical trial study was performed on 70 pregnant women applying for abortion referring to Besat Hospital in Sanandaj in 2014 - 2015. Pregnant women were divided randomly into two oral misoprostol and vaginal misoprostol groups by simple sampling. In both groups, misoprostol 200 μg every 6 hours up to six times (36 hours) was used. To make the study double-blind, placebo was used. The data collection instrument was a questionnaire. The effectiveness of misoprostol (the excretion of gestation products) and its side effects (bleeding, fever, etc.) were studied in two groups. Data were analyzed using SPSS Ver.18 software, t-test, Chi-square test and Fisher exact test.
Results: The results showed that there were no significant differences statistically between oral (82.3%) and vaginal (80%) misoprostol groups in terms of response to treatment (the excretion of gestation products). Although in our study, the need for curettage in the vaginal group (42.8) was higher than oral group (34.3), the difference was not statistically significant. Intervals of consuming oral misoprostol pills to the excretion of gestation products in the oral and vaginal groups were 4.09 ± 1.56 and 3.67 ± 1.4 hours, respectively (P > 0.05). In terms of complications, only two cases of oral misoprostol group experienced complications.
Conclusions: Although the risk of complications in oral method and the need for curettage in vaginal group is high, effectiveness of oral and vaginal misoprostol for induction of legal abortion is similar.
Keywords: Abortion; Legal; Misoprostol; Adverse effects
| Introduction | ▴Top |
According to the World Health Organization, abortion is defined as the termination of pregnancy before 20 weeks of gestation or fetus less than 500 g of weight [1]. Nowadays, with the use of new technologies, early detection of certain diseases that would jeopardize the life of the mother before the birth is possible. Abortion is done according to the laws of any country [2]. Legally, therapeutic abortion means ending a pregnancy by a physician due to illness of the mother or the fetus [3].
Approximately 42 million abortions are performed annually [4]. About 205 million pregnancies occur each year in the world, of which more than a third of them are unwanted and about one-fifth terminate intentionally [5]. The incidence of abortion in the developed world is 24 per 1,000 women and in developing countries is 29 abortions per 1,000 women aged 15 - 44 years. Since 2003, the number of abortions fell by 600,000 in the developed world but increased by 2.8 million in the developing world [6]. In Iran, out of six married women, one had at least an intentional abortion in her lifetime [7].
Abortion is performed usually by medical therapy, surgical therapy and expectant management [8]. Induced abortion means to eliminate pregnancy medically or surgically before fetus viability due to fetal and maternal reasons. Some of the maternal reasons for abortion include severe cardiovascular disease and invasive cervical cancer, and some fetal reasons include preventing babies being born with anatomical defects and disabilities [1].
Surgical procedures to terminate pregnancy include dilatation and curettage, aspiration and evacuation which have complications such as cervical rupture, uterine perforation, and sometimes even damage to the abdominal viscera [9]. Sometimes, it causes cervical insufficiency in subsequent pregnancies due to cervical dilatators; anesthesia complications are another complication of surgical methods of abortion [10].
In recent decades, medical techniques became affordable alternative to termination of pregnancy in the first trimester [11]. It is estimated that each year 26 million women around the world attempt to medical abortion. Because of cost-effectiveness and appropriate response, medical abortion has most commonly used than surgical procedures [12].
In medical treatment, different medications can be used to induce abortion. One of these medications is prostaglandin E1 analogue, misoprostol, which can be used orally, vaginally, rectally and sublingually, but mainly it is used vaginally and orally. Misoprostol has little side effects such as nausea, vomiting, diarrhea, chills, fever and pelvic pain [13].
Several studies have been conducted to compare oral and vaginal misoprostol success rate and complications to end pregnancies in the second trimester of pregnancy. In some of these studies, the success of vaginal [14] and in some studies oral success rate was high [15]. There are some studies that show the same effect of oral and vaginal methods [16].
Like many other nations, Iranians have been trying to reduce the rate of abortion by using different instruments including legal means, but the issue of abortion is still a problem in Iranian society [17]. Some studies estimated that total abortion rate in Iran is 0.26 abortions per married women, and generally 7.5 abortions per 1,000 married women in childbearing ages in each year [18-20]. The rate of abortions has been reported as 29 per 1,000 pregnancies in Southeast Iran [21]. Majlessi in a study reported that 21% of all pregnancies in Iran were unwanted and illegally induced abortion was performed for 21% of them [22]. After passing “Therapeutic Abortion Act” in 2005 by Iranian parliament, more official data of legal abortions are available but the rate of spontaneous and criminal abortions is still undetermined [23].
Considering the different available results on effectiveness of various doses of misoprostol and different methods of administration, as well as legal issues of abortion in Iran, the aim of this study was to compare oral and vaginal misoprostol for legal abortion in pregnant women.
| Materials and Methods | ▴Top |
This randomized double-blind clinical trial study was performed on 70 pregnant women applying for abortion referring to Besat Hospital in Sanandaj in 2014 - 2015. Inclusion criteria included pregnant women who were candidate for termination of pregnancy with legal medicine permission. Exclusion criteria were lactation, severe liver disease, chronic lung disease, mitral valve stenosis, inflammatory bowel disease and a history of allergy to prostaglandins, asthma, glaucoma, hypertension and severe bleeding.
Because obtaining legal permission for abortion is long and time-consuming, therefore the sample size of 35 cases in each group was determined. Pregnant women using four block sampling methods were randomly divided into two oral misoprostol and vaginal misoprostol groups (Fig. 1).
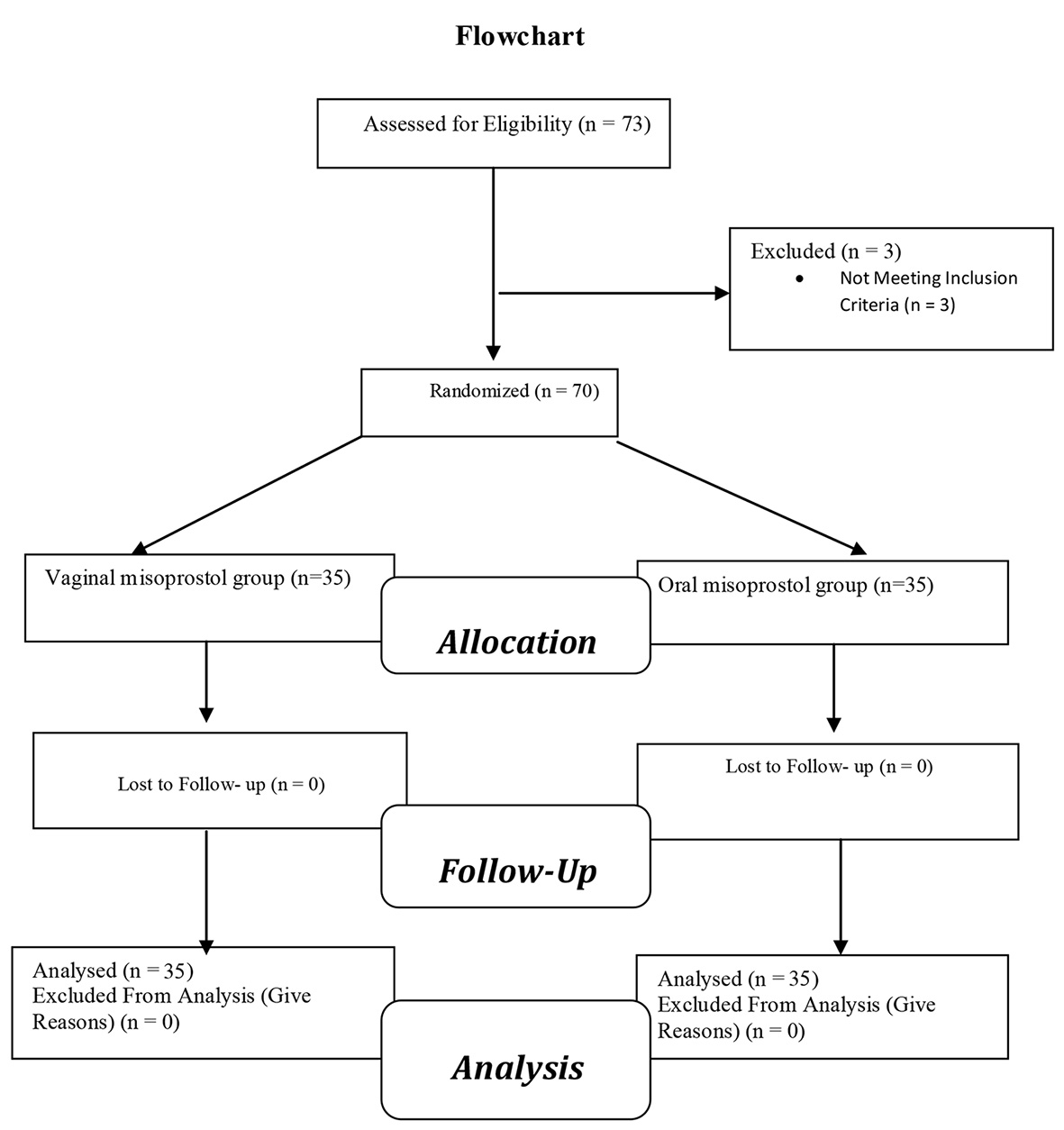 Click for large image | Figure 1. Flow diagram of the progress through the phases of a two-group parallel randomized trial. |
First, women were justified about vaginal and oral misoprostol for termination of pregnancy and also potential complications, and then written informed consent was obtained from all of them.
In oral group, misoprostol 200 μg every 6 h up to six times (36 h) plus a vaginal placebo was administered and in the vaginal group misoprostol 200 μg every 6 h up to six times plus placebo was administered orally. Given that in the oral group vaginal placebo and in vaginal group oral placebo was used simultaneously, so researcher and participants in the study were not aware about the medication and placebo.
The subjects were evaluated in terms of the excretion of gestation products and side effects (fever, chills, blood pressure, etc.) during the study and, if necessary they were treated. Also in case of serious problems and severe complications such as severe bleeding and hemodynamic imbalance and incomplete abortion, necessary measures were taken.
The data collection instrument was a questionnaire developed by the researcher and approved by the supervisor. Demographic information, information on previous and current pregnancies, the misoprostol treatment process and clinical complaints during treatment with misoprostol and side effects were recorded.
At the end of the study, the effectiveness (the excretion of gestation products) and side effects (bleeding, fever, etc.) in the two study groups were measured.
Data were analyzed using SPSS Ver.18 software, t-test, Chi-square test and Fisher exact test.
This study has been approved by Ethics Committee of Kurdistan University of Medical Sciences and also has been registered in Iranian Registry for Clinical Trials under the code of IRCT2014110812789N9.
| Results | ▴Top |
The mean ages in the oral group and vaginal group were 30.7 and 28.8 years and also the gestational ages in oral and vaginal groups were 14.3 and 14.5 weeks, respectively.
In terms of the number of pregnancies, history of abortion, delivery mode and the number of used misoprostol pills, there was no statistically significant difference between the two groups (P > 0.05) (Table 1).
 Click to view | Table 1. The Comparison of Variables in Two Oral and Vaginal Misoprostol Groups |
Intervals of consuming oral misoprostol pills to excretion of gestation products in the oral and vaginal groups were 4.09 ± 1.56 and 3.67 ± 1.4 h, respectively (P = 0.28).
Considering Chi-square test in terms of response to treatment, there was no significant difference in both oral and vaginal misoprostol groups statistically. Also in terms of need to curettage and complications, there was no significant difference statistically between two groups (P > 0.05) (Table 2).
 Click to view | Table 2. Comparison of the Frequency of Treatment Response to Misoprostol, the Need for Curettage and Complications in Two Groups |
| Discussion | ▴Top |
Misoprostol is a stable and synthetic prostaglandin E1 analogue [24], and currently its highest consumption is in obstetrics and gynecology. The drug absorption rate varies depending on the methods of application and dosage. With the use of misoprostol, 80-90% of cases lead to complete abortion [25]. It has been shown that misoprostol is an effective agent for cervical ripening and labor induction, but there have been concerns about hyperstimulation associated with its use [26].
In this study, in terms of the response to treatment (the excretion of gestation products), there were no significant differences statistically between oral (82.3%) and vaginal (80%) misoprostol groups. In a study by Behrashi et al [7], response to treatment in vaginal group was 86.7% and in oral group was 43.3%. In a study by Tale et al, response to the treatment in the oral misoprostol group was 71.1% [27] and in Hassanzadeh’s study, response to treatment was 83% in the vaginal misoprostol group [3]. In a study by Ayati et al, in vaginal misoprostol group 75.6% and in oral misoprostol group 84.6% had excreted gestation products [28]. Mirmohammadi et al demonstrated that in vaginal misoprostol, 44% had excreted gestation products completely and 56% had incomplete excretion [29]. Ganguly et al have shown that complete abortion in sublingual misoprostol group was more than the oral group (P = 0.0338) and vaginal group (P = 0.562) [30].
Madhusudan study results showed that in terms of cervix dilation, there was no significant difference between vaginal and oral groups (P > 0.05). Eighty-eight percent of women were satisfied taking oral misoprostol, while this ratio was 74% for the vaginal group [31]. In Kaur et al study, sublingual misoprostol group had faster and longer cervical dilatation compared to vaginal group (P = 0.0001) [32]. The differences in various studies may be related to population of study, the time of pregnancy, number of pregnancies, age and different doses of misoprostol.
Although in our study, the need for curettage in the vaginal group (42.8) was higher than oral group (34.3), the difference was not statistically significant. In a study by Hassanzadeh et al, 17% [3] in Jahangir et al study 12.5% [33] and in Ayatir et al study [28], 24.4% of women in misoprostol vaginal group had a need for curettage. Also in Ganguly et al study, the need for curettage was lower in the misoprostol sublingual group [30].
In our study, the time intervals from misoprostol administration to gestation products excretion in oral and vaginal groups were 4.09 ± 1.56 and 3.67 ± 1.40 h, respectively (P > 0.05). In a study by Kaur, the abortion time after the use of misoprostol in oral group (2.62 ± 0.64) was lower than vaginal group (3.17 ± 0.17) and the difference was statistically significant (P = 0.0001) [32]. Ganguly et al showed that the time interval in sublingual group was lower than the oral group (P < 0.0001) and vaginal (P < 0.001) [30]. In a study by Parveen et al, the mean time taken for cervical ripening was less in sublingual administration (3.7 ± 1.2 h) as compared to the vaginal (4.9 ± 2.6 h) and oral (11.7 ± 1.9 h) routes [34]. The differences in misoprostol administration time to abortion may be due to various doses prescribed in various studies.
In terms of complications including fever and bleeding, only two cases in the oral misoprostol group experienced complications and serious complications such as uterine rupture, and severe bleeding and abnormal coagulation disorder was not observed in any patient. In a study by Jahangir et al, the most common complications in vaginal misoprostol group were lower abdominal pain and fever [33]. Mirmohammadi reported clinical complaints in vaginal group [29]. In a study by Madhusudan, women in vaginal group experienced more vaginal bleeding [31]. In Kaur et al study in sublingual group vaginal bleeding was less compared to vaginal group, but this finding was not statistically significant (P = 0.286) [32]. In a study by Parveen et al, the average blood loss during surgery was higher in sublingual group compared to vaginal and oral group [34]. On the use of misoprostol, the risk of uterine rupture in women with a history of uterine scar is about 6-12% [35].
Madhusudan showed that oral misoprostol for abortion in the first 3 months is better than vaginal misoprostol [31] and Kaur showed that sublingual misoprostol is more effective than vaginal method in first trimester abortions [32]. Gunguly also reported that sublingual misoprostol had better results than oral and vaginal administration [30].
In conclusion, although the risk of complications in oral method and the need for curettage in vaginal group is more, effectiveness of oral and vaginal misoprostol for induction of legal abortion in pregnant women is similar. Therefore, because women are satisfied taking oral misoprostol than vaginal, it can be said that oral method is an alternative for vaginal method.
Acknowledgments
This study has been extracted from Obstetrics and Gynecology residency thesis. Authors would like to thank Vice Chancellor for Research of Kurdistan University of Medical Sciences to support the study financially.
| References | ▴Top |
- Cunningham FG, Leveno KJ, Bloom SL, Hauth JC, Rouse DJ, Spong CY. Williams obstetrics, 24th ed. New York: McGraw-Hill; 2015. p. 316-347.
- Honkanen H, Piaggio G, Hertzen H, Bartfai G, Erdenetungalag R, Gemzell-Danielsson K, Gopalan S, et al. WHO multinational study of three misoprostol regimens after mifepristone for early medical abortion. BJOG. 2004;111(7):715-725.
doi pubmed - Hasanzadeh M, VahidRoudsari F, Naghedi F, Ayati S, Shakeri MT. Medical abortion at first trimester of pregnancy with misoprostol. Iran J Obstet Gynecol Infertil. 2009;12(3):45-49. [Article in Persian].
- Shah I, Ahman E. Unsafe abortion: global and regional incidence, trends, consequences, and challenges. J Obstet Gynaecol Can. 2009;31(12):1149-1158.
doi - Cheng L. Surgical versus medical methods for second-trimester induced abortion: RHL commentary (last revised: 1 November 2008). The WHO Reproductive Health Library; Geneva: World Health Organization.
- Shulman LP. Induced abortion: incidence and trends worldwide from 1995 to 2008. Yearbook of Obstetrics, Gynecology and Women's Health. 2012:271-272.
- Behrashi M, Mahdian M. Vaginal versus oral misoprostol for second-trimester pregnancy termination: a randomized trial. Pak J Biol Sci. 2008;11(21):2505-2508.
doi pubmed - Blum J, Winikoff B, Gemzell-Danielsson K, Ho PC, Schiavon R, Weeks A. Treatment of incomplete abortion and miscarriage with misoprostol. Int J Gynaecol Obstet. 2007;99(Suppl 2):S186-189.
doi pubmed - Muffley PE, Stitely ML, Gherman RB. Early intrauterine pregnancy failure: a randomized trial of medical versus surgical treatment. Am J Obstet Gynecol. 2002;187(2):321-325; discussion 325-326.
doi pubmed - Speroff Leon, A Fritz Marc. Clinical Gyncology infertility, 7th ed. Lippincott Williams & willkins. 2005; Chap 28: p. 1069-1102.
- Beucher G, Baume S, Bekkari Y, Legrand-Horras M, Herlicoviez M, Dreyfus M. [Medical treatment of early spontaneous miscarriages: a prospective study of outpatient management using misoprostol]. J Gynecol Obstet Biol Reprod (Paris). 2004;33(5):401-406.
doi - Berer M. Medical abortion: a fact sheet. Reprod Health Matters. 2005;13(26):20-24.
doi - Burns M, Seattle P. Misoprostol for obstetric and gynecologic uses. Path & Engeder Health. 2001;67:438-45.
- Gilbert A, Reid R. A randomised trial of oral versus vaginal administration of misoprostol for the purpose of mid-trimester termination of pregnancy. Aust N Z J Obstet Gynaecol. 2001;41(4):407-410.
doi pubmed - Saha S, Bal R, Ghosh S, Krishnamurthy P. Medical abortion in late second trimester - a comparative study with misoprostol through vaginal versus oral followed by vaginal route. J Indian Med Assoc. 2006;104(2):81-82, 84.
pubmed - Feldman DM, Borgida AF, Rodis JF, Leo MV, Campbell WA. A randomized comparison of two regimens of misoprostol for second-trimester pregnancy termination. Am J Obstet Gynecol. 2003;189(3):710-713.
doi - Abbasi M, Shamsi Gooshki E, Allahbedashti N. Abortion in Iranian legal system: a review. Iran J Allergy Asthma Immunol. 2014;13(1):71-84.
pubmed - Aramesh K. Iran's experience on religious bioethics: an overview. Asian Bioethics Review. 2009;1(4):318-328.
- Ghotbi N, Tsukatani T. Evaluation of the national health policy of thalassaemia screening in the Islamic Republic of Iran. East Mediterr Health J. 2005;11(3):308-318.
pubmed - Ranji A. Induced abortion in Iran: prevalence, reasons, and consequences. J Midwifery Womens Health. 2012;57(5):482-488.
doi pubmed - Erfani A, McQuillan K. Rates of induced abortion in Iran: the roles of contraceptive use and religiosity. Stud Fam Plann. 2008;39(2):112.
doi - Majlessi F, Forooshani AR, Shariat M. Prevalence of induced abortion and associated complications in women attending hospitals in Isfahan. East Mediterr Health J. 2008;14(1):103-109.
pubmed - Sadr S. Shahabodin, Abedi Mohamad hasan, Ghadyani Mohamad hasan, Abedi Mariam. A survey on permits of therapeutic abortion in Iran by Legal Medicine Organization within one year from Jan. to Dec. 2003. Journal of Legal Medicine Organization of Islamic Republic of Iran. 2006;11:199.
- Silverstein FE. Improving the gastrointestinal safety of NSAIDs: the development of misoprostol - from hypothesis to clinical practice. Dig Dis Sci. 1998;43(3):447-458.
doi pubmed - von Hertzen H, Piaggio G, Wojdyla D, Marions L, My Huong NT, Tang OS, Fang AH, et al. Two mifepristone doses and two intervals of misoprostol administration for termination of early pregnancy: a randomised factorial controlled equivalence trial. BJOG. 2009;116(3):381-389.
doi pubmed - Kundodyiwa TW, Alfirevic Z, Weeks AD. Low-dose oral misoprostol for induction of labor: a systematic review. Obstet Gynecol. 2009;113(2 Pt 1):374-383.
doi pubmed - Tale M, Kashanian M, Dehghani Zadeh A. Evaluation of Oral Misoprostol Effect on the Treatment of Spontaneous First Trimester Incomplete Abortion. RJMS. 2004;11(41):441-447.
- Sedigheh Ayati, Fatemeh Vahid Roudsari , Maliheh Banavi , Mohammad Taghi Shakeri, Azhar Berahmat. Comparison between Rectal Misoprostol and Vaginal Misoprostol for First Trimester Termination of Pregnancy in Patients with Previous Uterus Surgery. The Iranian Journal of Obstetrics, Gynecology And Infertility. 1391;15(42):1-6.
- Mir Mohammadi Meibodi R ,Tabatabaei SM , Karim Zadeh Meibodi MA, Faraj Khoda T, Dehghani Firooz Abadi R, SM Tabatabaei. The efficacy of vaginal Misoprostol in treatment of missed abortions. Journal of Shahid Sadoughi University of Medical Sciences and Health Services. 1384;13(3):31-38.
- Ganguly RP, Saha SP, Mukhopadhyay S, Bhattacharjee N, Bhattacharyya SK, Patra KK. A comparative study on sublingual versus oral and vaginal administration of misoprostol for late first and early second trimester abortion. J Indian Med Assoc. 2010;108(5):283-284, 286.
pubmed - Dey M. Oral misoprostol is an effective and acceptable alternative to vaginal administration for cervical priming before first trimester pregnancy termination. Med J Armed Forces India. 2013;69(1):27-30.
doi pubmed - Kaur P, Kaur M, Kaur B, Kaur MM; Kaur K, Jindal P. Comparative Study of Sublingual versus Vaginal Misoprostol on Preoperative Cervical Priming in First Trimester Abortion. Indian Journal of Clinical Practice. 2013; 23(9).
- Jahangir M , Behrashi M , Fazel MR , Arbabi M.Efficacy of vaginal Misoprostol for terminating missed abortion.Feyz, Kashan University of Medical Sciences & Health Services. 1384;9(34):1-5.
- Parveen S, Khateeb ZA, Mufti SM, Shah MA, Tandon VR, Hakak S, Singh Z, et al. Comparison of sublingual, vaginal, and oral misoprostol in cervical ripening for first trimester abortion. Indian J Pharmacol. 2011;43(2):172-175.
doi pubmed - Plaut MM, Schwartz ML, Lubarsky SL. Uterine rupture associated with the use of misoprostol in the gravid patient with a previous cesarean section. Am J Obstet Gynecol. 1999;180(6 Pt 1):1535-1542.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
