| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 5, Number 3, September 2016, pages 92-96
Combined Surgery for Uterine and Renal Cell Cancers in Obese Patients: A Case Series
Ross Harrisona, d, Laura B. Huffmanb, E. Jason Abelc, Stephen Roseb
aDepartment of Obstetrics & Gynecology, University of Wisconsin, Madison, WI, USA
bDivision of Gynecologic Oncology, Department of Obstetrics & Gynecology, University of Wisconsin, Madison, WI, USA
cDepartment of Urology, University of Wisconsin, Madison, WI, USA
dCorresponding Author: Ross Harrison, Department of Obstetrics & Gynecology, University of Wisconsin, 202 South Park Street, Meriter Hospital 5-East, Madison, WI 53715, USA
Manuscript accepted for publication August 15, 2016
Short title: Combined Surgery for Uterine and Renal Cancer
doi: http://dx.doi.org/10.14740/jcgo412w
| Abstract | ▴Top |
We describe our experience with concomitant surgery for synchronously diagnosed uterine and renal cell cancer, two obesity-linked malignancies, to better identify the challenges posed by such patients. Our institution’s tumor registry and renal cell cancer database were queried for patients with both coincident cancers who were treated from 2000 to present day. The medical records of these patients were systematically reviewed. Six patients were synchronously diagnosed with both uterine and renal cell cancer and underwent combined surgical management. Five of these were performed by an open approach and one by using a minimally invasive surgery (MIS) technique. The majority of patients who had open surgery experienced significant operative or perioperative morbidity. One of these five patients died on postoperative day 3 due to complications from surgery in the setting of significant medical comorbidities. Two other patients with open surgery required splenectomy due to iatrogenic injury at the time of nephrectomy. An MIS technique was utilized for the patient with the largest body mass index (61 kg/m2). This patient recovered without complications. Our experience with combined surgery for coincident uterine and renal cell cancer suggests caution when planning such a procedure. An open approach carries with it significant risk for morbidity, especially in comorbid patients. An MIS approach should be considered when feasible. Synchronous diagnoses of these cancers are rare, but may become more common with the increasing prevalence of obesity.
Keywords: Uterine cancer; Renal cell cancer; Obesity; Surgery; Preoperative outcomes; Combined surgery
| Introduction | ▴Top |
More than one in three adults in the United States are obese [1]. Obesity is a well-recognized risk factor for the development of chronic medical conditions [2]. The risk of all-cause mortality exhibits a direct relationship to increasing body mass index (BMI) [3]. Obesity is a risk factor for multiple cancers and increases the likelihood for cancer-related mortality among those diagnosed [4, 5]. The relationship between obesity and type I endometrial cancer has been well described, and obesity has also been associated with type II endometrial cancer and non-epithelial uterine cancer [6, 7]. Additionally, obesity is a risk factor for the development of renal cell cancer [4, 8]. A recent meta-analysis showed a strong association between each 5 kg/m2 incremental increase in BMI and the risk of endometrial (RR: 1.59) and renal (RR: 1.24) cancer [9]. While it is theorized that peripheral aromatization of steroid precursors explains the increased risk of type I endometrial cancer, the obesity-related pathophysiology for renal cancer is less understood. In most patients, surgery is the standard of care and primary treatment for renal cell and uterine cancers. Thus, obesity represents both a shared risk factor for and a technical challenge to the management of these malignancies, especially if concomitant surgery for synchronous tumors is undertaken. We will describe our institutional experience with concomitant surgery for synchronous uterine and renal cancer in an effort to better characterize the challenges posed by such cases.
| Case Reports | ▴Top |
The University of Wisconsin tumor registry and renal cell cancer database were queried for patients with both uterine and renal cell cancers who were treated from 2003 to present day. Fifteen cases were identified. The electronic health record for each patient was systematically reviewed. This project was approved by the University of Wisconsin Institutional Review Board.
To identify other reports of concurrent surgical management of these cancers, standard strategies were utilized to query the online MEDLINE database with the PubMed search interface. The search was done using keywords and Medical Subject Heading (MeSH) terminology. The literature search included the following terms, as well as synonyms and closely related words: “uterine cancer”, “renal cell cancer”, “hysterectomy”, “nephrectomy”, “synchronous”, “metachronous”, “second primary malignancy”, and “combined surgery”. No articles were identified describing combined surgical management of uterine and renal cell cancers. Based on these results, this may be the first detailed description of concomitant surgical management of these cancers.
Fifteen patients with uterine and renal cell cancer were identified. Six patients underwent combined surgery. Patients with uterine papillary serous carcinoma and uterine carcinosarcoma were included, as obesity has been associated with an increased risk for these tumors [6, 7]. Age at diagnosis, BMI, histology, stage, treatment specifics, perioperative outcomes, and current disease status are presented in Tables 1 and 2.
 Click to view | Table 1. Review of Cases With Synchronous Uterine and Renal Tumors, Patient Characteristics, Surgical Management, and Pathology |
 Click to view | Table 2. Review of Cases With Synchronous Uterine and Renal Tumors, Perioperative Outcomes and Disease Status |
Case 1
A 53-year-old was referred for well-differentiated endometrial adenocarcinoma. Her medical history included supermorbid obesity with a BMI of 61 kg/m2. Preoperative imaging identified a 6.6 cm right renal lesion. She was referred to urology and a core biopsy was obtained that demonstrated clear cell renal carcinoma. She was taken to the operating room (OR) for a combined procedure. A hand-assisted laparoscopic right radical nephrectomy was performed first and was followed by a robotic-assisted total laparoscopic hysterectomy and bilateral salpingo-oophorectomy (BSO). Her postoperative course was uncomplicated. Uterine pathology showed stage II, grade 1 endometrioid adenocarcinoma. She received adjuvant radiation therapy. Renal pathology demonstrated stage T2aNX, grade 3 clear cell carcinoma. She was without evidence of recurrent disease 28 months following surgery.
Case 2
A 71-year-old woman was referred for management of grade 1 endometrioid adenocarcinoma. Her medical history was notable for morbid obesity with a BMI of 49 kg/m2, coronary artery disease with a history of stent placement requiring chronic aspirin use, atrial fibrillation requiring chronic anticoagulation, hypertension, and obstructive sleep apnea. Preoperative imaging revealed a right renal mass (Fig. 1). A core-needle biopsy of the renal mass demonstrated clear cell renal cell cancer. She was referred to urology and the decision was made to proceed with a combined procedure using an open approach. Using a midline vertical incision, hysterectomy, BSO and pelvic and para-aortic lymph node dissection (PPLND) was done first, followed by right radical nephrectomy. Cholecystectomy was performed for intraoperatively identified acute-on-chronic cholecystitis. Postoperatively, the patient did poorly. She was admitted to the surgical intensive care unit due to hypotension requiring vasopressor support despite extensive fluid resuscitation. On the first postoperative day, she was taken back to the OR twice for re-exploration due to concerns for ongoing bleeding. During both of these operations, no source of bleeding was identified. It was felt that her blood loss was secondary to coagulopathy from chronic aspirin given to ensure cardiac stent patency. After these surgeries, the patient’s clinical condition deteriorated. She developed acute respiratory distress syndrome, acute renal failure requiring continuous veno-venous hemofiltration, atrial fibrillation and demand myocardial ischemia, and persistent hypotension requiring multiple-agent vasopressor support. A fourth surgery on postoperative day 3 was done for concern for sepsis. A liver laceration was identified and repaired, but no source of infection could be found. Her clinical condition continued to deteriorate and the possibility for recovery was thought to be remote. After consultation with family, care was withdrawn and the patient died on postoperative day 3. Uterine pathology showed stage IA grade 2 endometrioid adenocarcinoma. Renal pathology showed stage T2aN0 clear cell renal cancer, grade 2.
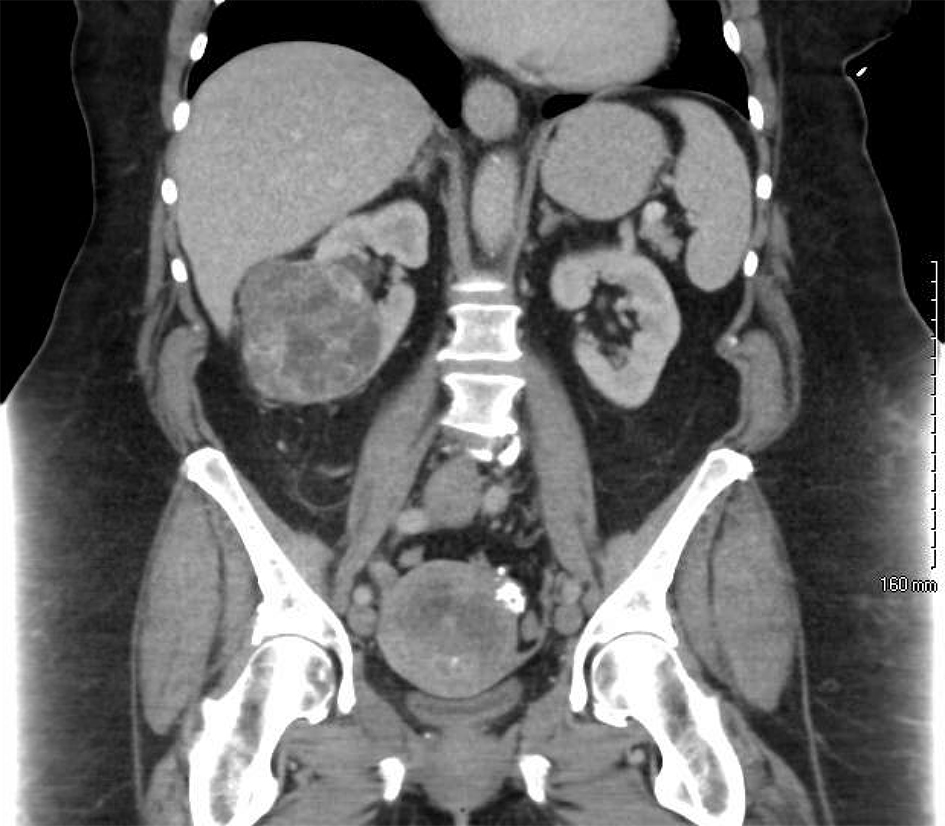 Click for large image | Figure 1. A preoperative CT scan of the abdomen reveals an enlarged, irregular uterus with multiple calcifications as well as an exophytic, heterogeneous mass extending from the inferior pole of the right kidney. |
Case 3
A 74-year-old woman was referred for management of an 18 cm pelvic mass. Her medical history included obesity with a BMI of 32 kg/m2, hypertension, and tobacco use. Preoperative imaging also showed an 8 cm left renal mass. She was referred to urology and a core biopsy of this renal mass showed papillary renal cell cancer. The decision was made to take her to the OR for a combined procedure. An open approach was used through a vertical midline incision. Intraoperatively, the pelvic mass was seen to be of uterine origin. Total abdominal hysterectomy (TAH) and BSO were performed first, followed by total left nephrectomy and left adrenalectomy. An intentional cystotomy was made to facilitate removal of the uterine mass. The left adrenal gland was grossly abnormal, so it was resected. Iatrogenic splenic injury occurred requiring splenectomy. Intraoperative uterine frozen section pathology was consistent with leiomyosarcoma, so lymphadenectomy was not performed. Her postoperative course was complicated by an episode of acute delirium that resolved spontaneously. Final uterine pathology showed stage IVB carcinosarcoma with metastasis to the left adrenal glad. Renal pathology showed stage T2aNx, grade 3 papillary renal cell cancer. Adjuvant chemotherapy was recommended, but the patient declined further treatment. She had no further follow-up, and died 4 months after surgery, presumptively from metastatic uterine carcinosarcoma.
Case 4
A 71-year-old woman was referred for management of grade 3 endometrioid endometrial cancer. Her medical history included obesity with a BMI of 39 kg/m2, hypertension, chronic obstructive pulmonary disease, type II diabetes, coronary artery disease, and sleep apnea. Preoperative imaging revealed a 3 cm exophytic left renal mass. She was referred to urology and it was decided to take her to the OR as a combined case using an open approach. A vertical midline incision was made and TAH, BSO, and PPLND were completed first, followed by a left radical nephrectomy, left adrenalectomy, and additional PPLND. Iatrogenic splenic injury occurred requiring splenectomy. Her postoperative course was complicated by difficulty maintaining adequate oxygen saturations, most likely due to her history of chronic pulmonary disease and obesity. Uterine pathology showed high-grade papillary serous adenocarcinoma, stage IVB with metastatic disease within an oncocytoma within the left kidney. Renal pathology showed papillary renal cell carcinoma, also arising within the oncocytoma, that was stage T1aN0 with no evidence of metastasis outside of the kidney. The patient received six cycles of adjuvant carboplatin and paclitaxel. Shortly following chemotherapy completion, imaging obtained during an unrelated hospital admission demonstrated an osteoblastic lesion in a thoracic vertebra concerning for metastatic disease. This was initially followed by imaging without obvious progression. Her disease was felt to be stable. However, 9 months after surgery, she developed focal neurologic symptoms and imaging revealed multiple foci of intracranial metastatic disease. She recently completed palliative whole brain radiotherapy.
Case 5
A 70-year-old woman was referred for management of carcinosarcoma. Her medical history included obesity with a BMI of 38 kg/m2, hypertension, and type II diabetes. Preoperative imaging showed a 2.5 cm heterogeneous lesion in the inferior pole of the left kidney, which was biopsied showing grade 2 renal cell clear cell carcinoma. She was taken to the OR as a combined case. A vertical midline incision was made and a TAH, BSO, and pelvic lymphadenectomy were completed first, followed by a left partial nephrectomy, and finally para-aortic lymphadenectomy and omentectomy. Her postoperative course was uncomplicated. Uterine pathology showed no residual cancer and no metastatic disease was identified. Renal pathology revealed grade 2, stage T1aN0 renal clear cell carcinoma. The patient received one cycle of adjuvant paclitaxel and carboplatin for uterine carcinosarcoma, which was tolerated very poorly. No further adjuvant chemotherapy was administered as risk was felt to outweigh possible benefit. She was without evidence of recurrent disease 19 months following surgery.
Case 6
A 46-year-old woman was referred for management of endometrial adenocarcinoma and a 34 cm pelvic mass. Her medical history was notable for obesity with a BMI of 45 kg/m2 and chronic lower extremity deep vein thrombosis requiring chronic anticoagulation. Imaging also demonstrated a 5.5 cm right renal mass. She was admitted preoperatively for anticoagulation reversal and placement of an inferior vena cava (IVC) filter was placed. After her anticoagulation was reversed with fresh frozen plasma transfusion, she was taken to the OR. Urology was consulted intraoperatively after frozen section pathology from the renal mass demonstrated renal cell cancer. Using a right paramedian incision, a radial hysterectomy with BSO was performed first, followed by right nephrectomy, and finally PPLND. Extensive venous collateralization secondary to IVC clot and massive uterine fibroids requiring parametrial dissection complicated the surgery. Uterine pathology showed stage IA grade 1 endometrioid adenocarcinoma. Renal pathology showed stage T1bN0 clear cell renal cancer, grade 1. The patient was referred back to her primary gynecologist for post-treatment surveillance.
| Discussion | ▴Top |
This report of six cases of synchronous uterine and renal cell cancers managed concomitantly highlights some inherent challenges with a combined surgical approach. Not surprisingly, the patient who was able to have both cancers managed laparoscopically (case 1) had the least complicated postoperative course. While it is generally considered more difficult to perform laparoscopic procedures in morbidly obese patients, it is interesting to note that the patient in case 1 had the largest BMI (61 kg/m2) of the six patients in our review. This review also highlights the potential for significant morbidity following surgery in women with obesity-related comorbid conditions. The patient described in case 2 reads as a cautionary tale with regard to aggressive surgical management in highly comorbid patients.
Synchronous diagnosis of two cancers in the setting of an obese patient with medical comorbidities represents a unique challenge. It is likely that the incidence of synchronous diagnoses of primary obesity-related cancers may increase with obesity continuing to become more common in the United States. This point is especially salient to gynecologic oncologists given the prevalence of obese patients in their practices. At our institution, imaging is a routine part of the preoperative evaluation of new uterine cancer patients, and this leads to the discovery of second primary tumors in this case series. Clinicians should consider preoperative imaging in obese patients given the risk for a second, obesity-related malignancies.
Our institution’s experience anecdotally suggests that these patients are at high risk of perioperative morbidity and mortality. Obesity is a widely accepted risk factor for poor perioperative outcomes. Two of the patients experienced splenectomy secondary to surgical injury. One patient died in the postoperative period due in part to her obesity-related medical comorbidities. All patients who had open surgery had an estimated operative blood loss of at least 1 L and all received a blood transfusion. The patient who had her surgery done by an MIS technique for both aspects of her disease arguably experienced the best short and long-term outcomes. When considered separately, the literature supports MIS approaches for both uterine and renal cell cancers [10-12]. Given this, it would seem that when concurrent management is undertaken, an MIS approach is optimal when feasible.
Conflicts of Interest
The authors have no conflicts of interest to report.
Disclosures
The authors have no financial disclosures related to the content of this manuscript. All authors reviewed and contributed to the development of this manuscript, and have approved its submission for publication.
| References | ▴Top |
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806-814.
doi pubmed - Field AE, Coakley EH, Must A, Spadano JL, Laird N, Dietz WH, Rimm E, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161(13):1581-1586.
doi pubmed - Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, et al. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N Engl J Med. 2006;355(8):763-778.
doi pubmed - Anderson AS, Key TJ, Norat T, Scoccianti C, Cecchini M, Berrino F, Boutron-Ruault MC, et al. European Code against Cancer 4th Edition: Obesity, body fatness and cancer. Cancer Epidemiol. 2015;39(Suppl 1):S34-45.
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638.
doi pubmed - Schwartz SM, Weiss NS, Daling JR, Gammon MD, Liff JM, Watt J, Lynch CF, et al. Exogenous sex hormone use, correlates of endogenous hormone levels, and the incidence of histologic types of sarcoma of the uterus. Cancer. 1996;77(4):717-724.
doi - Setiawan VW, Yang HP, Pike MC, McCann SE, Yu H, Xiang YB, Wolk A, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol. 2013;31(20):2607-2618.
doi pubmed - Adams KF, Leitzmann MF, Albanes D, Kipnis V, Moore SC, Schatzkin A, Chow WH. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008;168(3):268-277.
doi - Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-578.
doi - Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111(3):407-411.
doi pubmed - Lane BR, Gill IS. 7-year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol. 2010;183(2):473-479.
doi pubmed - Ono Y, Hattori R, Gotoh M, Yoshino Y, Yoshikawa Y, Kamihira O. Laparoscopic radical nephrectomy for renal cell carcinoma: the standard of care already? Curr Opin Urol. 2005;15(2):75-78.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
