| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 6, Number 1, March 2017, pages 17-22
Tubal Serous Borderline Tumor With Strongly Positive p53 Which Developed in a 50-Year-Old Woman
Hiroharu Kobayashia, b, Michiyasu Kawaia
aDepartment of Obstetrics and Gynecology, Toyohashi Municipal Hospital, 50 Aza-hachiken-nishi, Aotake-chou, Toyohashi-shi, Aichi-ken 441-8570, Japan
bCorresponding Author: Hiroharu Kobayashi, Seirei Hamamatsu Hospital Medical Office, 2-12-12 Sumiyoshi Naka-ku Hamamatsu-shi, Shizuoka-ken 430-8558, Japan
Manuscript accepted for publication December 27, 2016
Short title: Tubal Serous Borderline Tumor
doi: https://doi.org/10.14740/jcgo417w
| Abstract | ▴Top |
A 50-year-old woman was referred to our hospital for the treatment of left ovarian tumor. Transvaginal ultrasound examination revealed a unilocular lesion of 4 cm diameter at left adnexa and magnetic resonance imaging showed a solid component with contrast effect at the luminal wall. She underwent laparotomy for the diagnosis of ovarian carcinoma at laparotomy. It revealed globally swelled left fallopian tube and normal left ovary. Histopathological examination of frozen section of left adnexa showed serous borderline tumor of left fallopian tube. Additionally, hysterectomy, right salpingo-oophorectomy and partial omentectomy were performed. Final pathologic diagnosis was serous borderline tumor of left fallopian tube (FIGO stage IA). She had no recurrent sign 1 year after surgery. Borderline tumor of fallopian tube is very rare. There were 21 previously reported cases, 15 cases being serous, five cases mucinous and one case endometrioid.
Keywords: Fallopian tube; Borderline tumor; Serous tumor
| Introduction | ▴Top |
Serous borderline tumors of the fallopian tube are rare malignancies that were first reported by Zheng et al in 1996; there have been 21 reported cases to date. Herein, we report a case of a 50-year-old woman with serous borderline tumor of the fallopian tube.
| Case Report | ▴Top |
The patient was a 50-year-old woman, who was gravida 2, para 1, and premenopausal. She was found to have a left adnexal mass upon gynecological examination and was referred to our hospital. Past history included angina, cholecystectomy, and appendicitis. She had neither vaginal discharge nor abnormal genital bleeding. Papanicolaou smear test results were normal. Transvaginal ultrasonography revealed a left unilocular adnexal mass 4 cm in diameter and magnetic resonance imaging (MRI) showed a solid component with contrast effect at the luminal wall (Figs. 1 and 2). Serum CA125 and CA19-9 were 50.0 and 27.0 U/mL, respectively. No retroperitoneal lymph node swelling or metastasis was detected on chest and abdominal computed tomography. She underwent laparotomy based on a diagnosis of left ovarian carcinoma. There were no ascites or adhesions in the abdominal cavity. The left fallopian tube showed global swelling, while the left ovary was normal; the uterus and right adnexa were also macroscopically normal. Left salpingo-oophorectomy was initially performed, revealing that the left fallopian tube comprised entirely of a unilocular cyst 4 cm in diameter; normal fimbria were observed on its smooth surface (Fig. 3). The lesion contained serous fluid, and a papillary solid component 1 cm in diameter projected on the inner wall. Histopathological examination of the tumor’s frozen section revealed a serous borderline tumor, after which hysterectomy, right salpingo-oophorectomy, and partial omentectomy were performed. No intraperitoneal spreading was detected macroscopically, and peritoneal washing cytology was negative. Final pathological diagnosis was serous borderline tumor of the left fallopian tube, stage IA. The patient did not undergo adjuvant chemotherapy and had no evidence of recurrence 1 year after surgery.
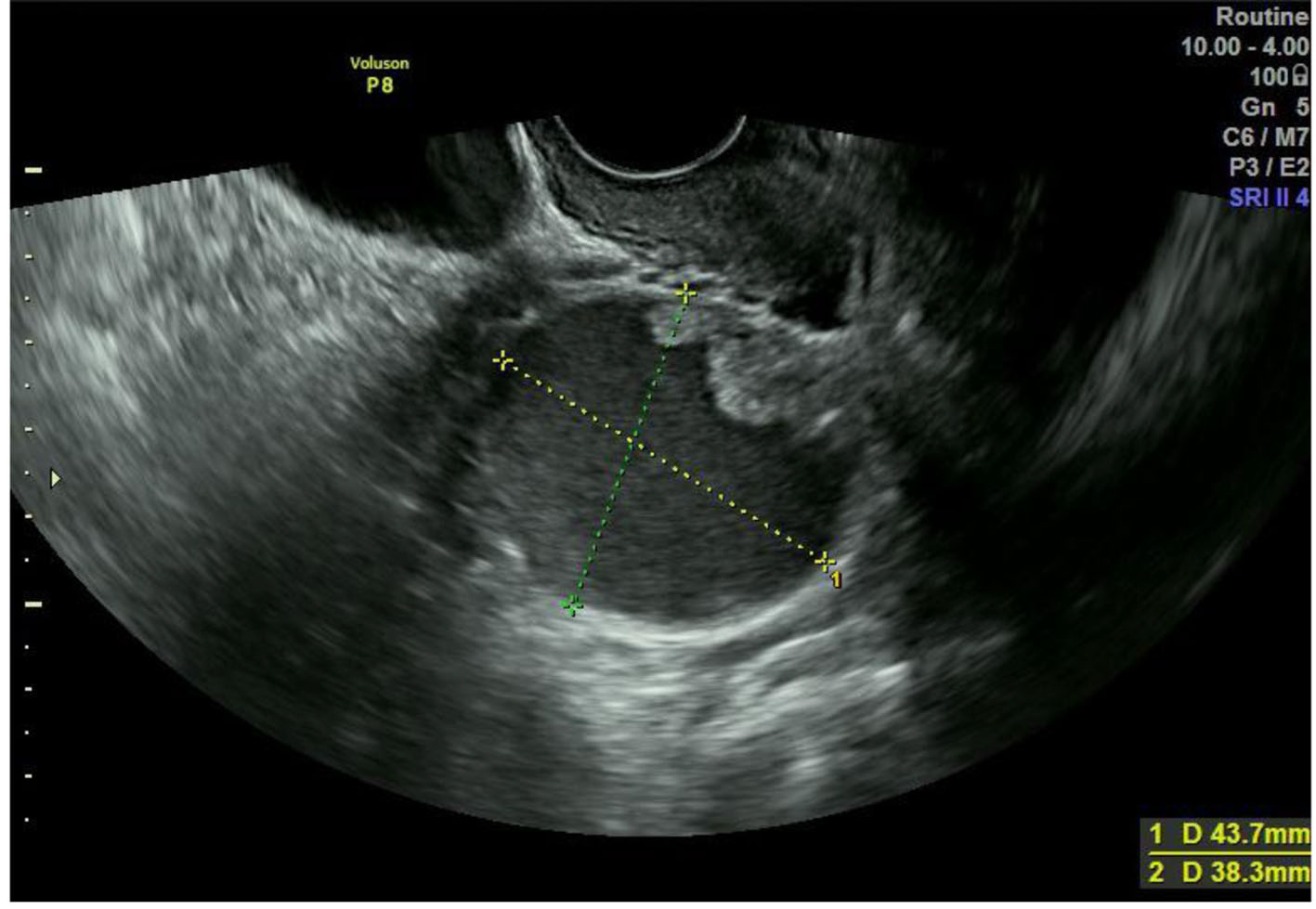 Click for large image | Figure 1. Transvaginal ultrasonography showing a left unilocular adnexal mass. |
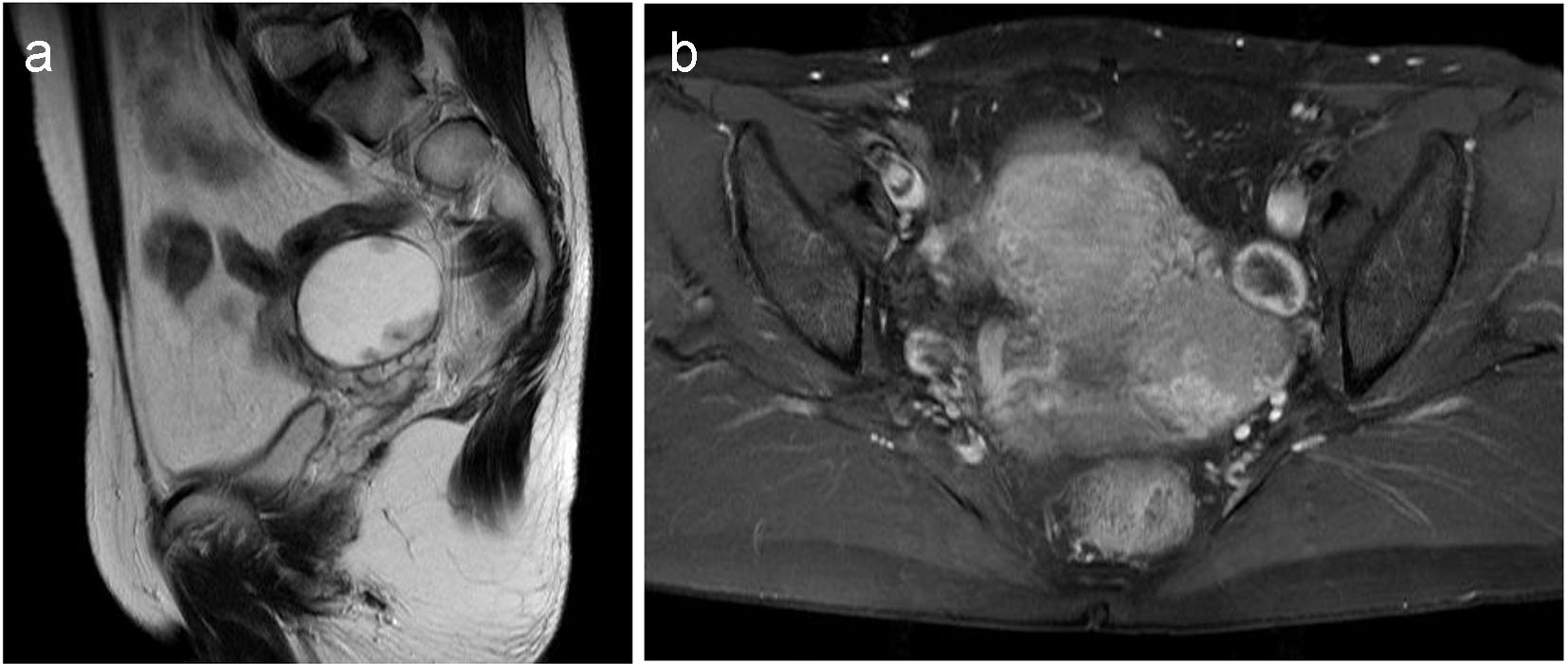 Click for large image | Figure 2. Magnetic resonance imaging of the mass: (a) T2-weighted image; (b) T1-weighted image (contrast-enhanced). |
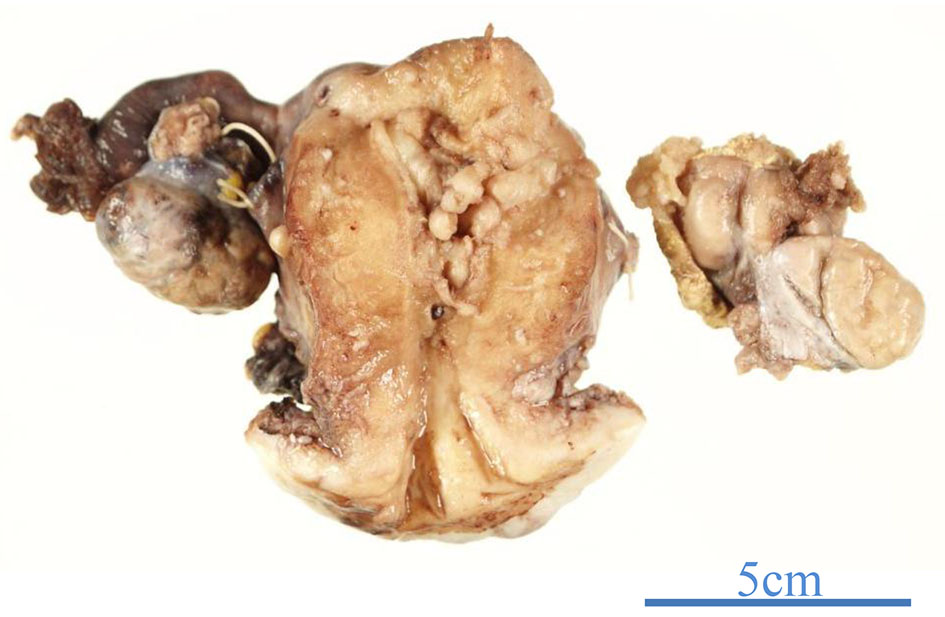 Click for large image | Figure 3. The left fallopian tube was globally swollen, while the left ovary appeard normal. |
Microscopic examination showed multiple papillary projections composed of fibrovascular cores covered with serous epithelium (Fig. 4); papillae had stratification and budding. Nuclear atypia was mild. Some psammoma bodies were observed in the stroma. There was no invasion into the stroma. The case was consistent with serous borderline morphology ascribed to ovarian borderline tumors. Immunohistochemistry of p53 showed moderate-to-strong expression in the nuclei of 60% of the tumor cells (Fig. 5a). Immunohistochemistry of Ki67 showed moderate-to-strong expression in the nuclei of less than 10% tumor cells (Fig. 5b).
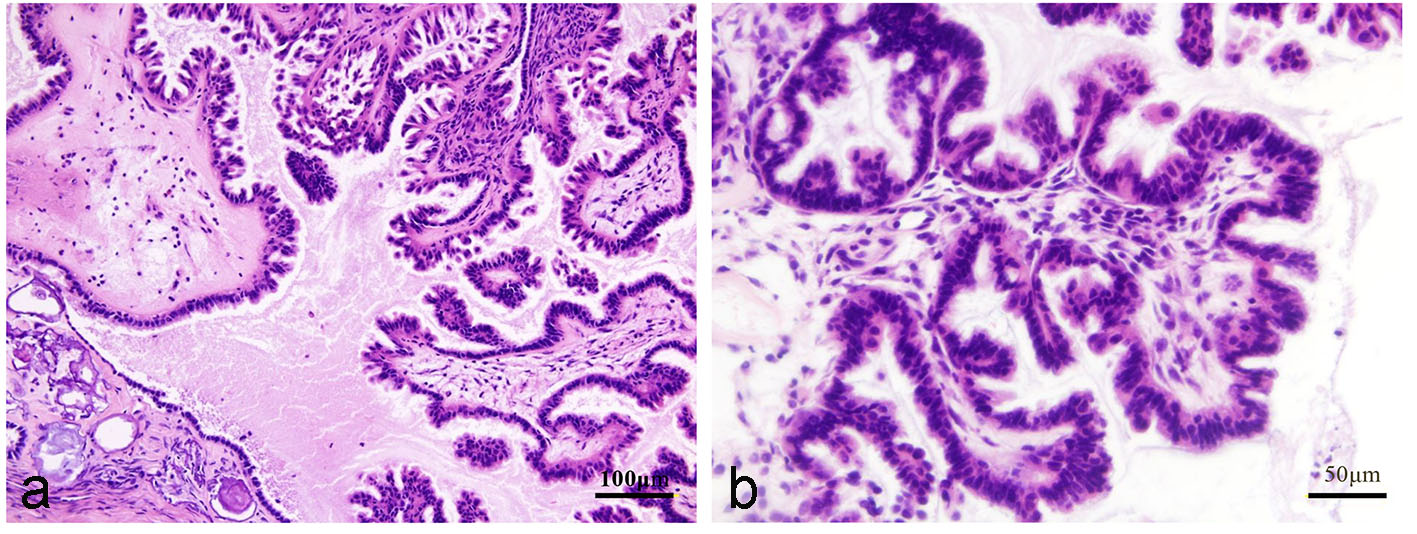 Click for large image | Figure 4. Hematoxylin and eosin staining (a: low-power field; b: high-power field). |
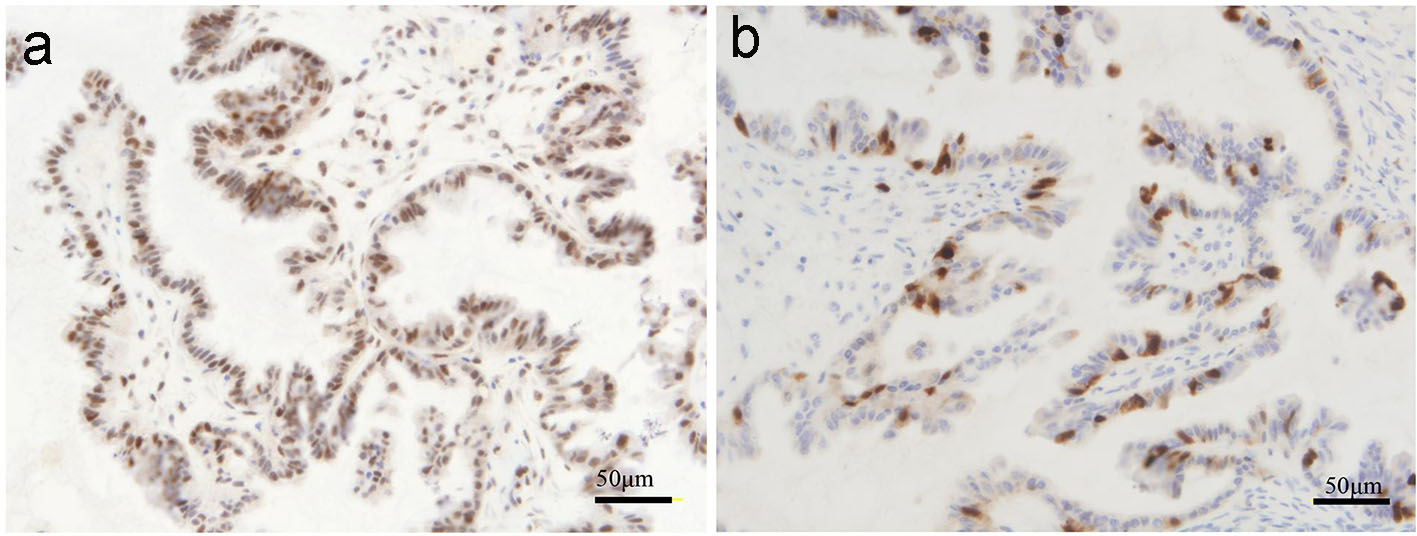 Click for large image | Figure 5. Immunohistochemical staining (a: p53; b: Ki67). |
| Discussion | ▴Top |
Borderline tumors of the fallopian tube are rare. There were 21 previously reported cases, of which 15 were serous, five were mucinous, and one was endometrioid (Table 1 [1-19]). Papillary cystadenocarcinoma reported by Gatto et al in 1986, and borderline cystadenofibroma reported by Valerdiz Casasola et al in 1989, are now considered to be serous borderline tumors [12, 13, 16, 18].
 Click to view | Table 1. Literature Review of Borderline Tumors of Fallopian Tube |
The average age of 16 patients of serous borderline tumor (including the present case) was 30.0 ± 9.0 years old. In 10 of these cases, the preoperative diagnosis was an ovarian or adnexal tumor. Three patients had been preoperatively diagnosed with primary disease of fallopian tube; one of them was 8 weeks pregnant and was suspected to be tubal ectopic pregnancy. The other two patients were found to have a normal-sized ovary adjacent to the cystic mass on transvaginal ultrasonography. When a normal-sized ovary is not identified on ultrasonography or MRI, it is difficult to preoperatively determine whether a tumor arises from the ovary or fallopian tube.
In 14 of 16 serous borderline tumor cases, the tumor formed a cystic mass with a papillary projection at the inner wall. The mean diameter of these cysts was 6.8 ± 5.0 cm. They were localized at the distal tube (fimbriated end or ampullary region) or the entire tube. In the other two cases, the tumor formed approximately 5 cm sized solid mass with cauliflower-like surface that protruded from the tubal fimbria. One case had a non-invasive peritoneal implant in the Douglas’ pouch, for which peritonectomy was performed. These observations suggest that tubal serous borderline tumors arise at the fimbriated end. Serous tubal intra-epithelial carcinoma (STIC) is a lesion that arises at the fimbriated end of fallopian tube and is suspected to be a precursor lesion of high-grade pelvic serous carcinoma [20]. Vang et al proposed that papillary tubal hyperplasia (PTH) in a fallopian tube implant in the ovary develops into a low-grade serous ovarian carcinoma [21]. PTH is found in the ampullary portion of the fallopian tube more frequently than in the infundibulum and isthmus, and the histological appearance of PTH is essentially identical to that of a serous borderline tumor. In our case, there were no atypical lesions in the ovaries. When PTH that arises in the fallopian tube forms a cyst and develops in a closed state, a tubal serous borderline tumor may form.
The 16 cases of tubal serous borderline tumors were unilateral and CA125 was often mildly elevated. Most of these cases were stage IA, and 11 of these patients underwent fertility-sparing surgery (salpingectomy or adnexectomy). Four patients who did not desire fertility underwent hysterectomy; surgical procedure undergone by the 16th patient is unknown. Four patients underwent retroperitoneal lymphadenectomy and were found to have no metastasis. All patients were followed without adjuvant chemotherapy after surgery. The longest follow-up period was 6 years, and no recurrences or deaths were reported.
The optimal treatment for tubal borderline tumors has not been established due to the disease’s rarity. However, it appears appropriate to treat these tumors as ovarian borderline tumors. In many cases, tumors are not preoperatively diagnosed as arising from the fallopian tube. When discovered during surgery, salpingectomy is first performed; however, intraoperative frozen section diagnosis of ovarian borderline tumors has a low sensitivity and a low positive predictive value [22, 23]. Although the surgical management of tubal tumors based on intraoperative diagnosis by frozen section should be performed with caution, the basic surgical procedure for tubal borderline tumors ought to be hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and intraperitoneal inspection. For patients who prefer to preserve their fertility, salpingectomy or adnexectomy of the affected side should be performed with omentectomy and intraperitoneal inspection. When salpingectomy is performed instead of salpingo-oophorectomy, biopsy of the adjacent normal-appearing ovary may be required. However, biopsy of the contralateral normal-appearing adnexa is probably not necessary because it is unnecessary for ovarian counterpart [24]. In ovarian borderline tumors, cystectomy increases the pregnancy rate but shortens the time to first recurrence compared to adnexectomy [24]. Tubal tumor cystectomy will not contribute to fertility preservation unless the tumor is paratubal (paraovarian). Morice et al reported that invasive peritoneal implants and residual disease after surgery were the two factors predicting invasive recurrence in ovarian borderline tumor patients who underwent fertility-sparing surgery [25]. In 16 cases of tubal serous borderline tumors, only one had a peritoneal implant (although it was non-invasive). No case had residual disease after surgery. However, intraperitoneal inspection should be carefully performed, especially after fertility-sparing surgery. Systemic lymphadenectomy is probably not necessary for a tubal borderline tumor because it is unnecessary for ovarian counterpart. Lymph node involvement does not affect the prognosis of ovarian serous borderline tumors [26].
The average age of five patients of mucinous borderline tumor was 59.0 ± 11.0 years. This is in contrast to serous borderline tumors, in which the patients were much younger. Among these five cases, three were complicated by peritoneal pseudomyxoma. Today ovarian tumor complicated by pseudomyxoma peritonei is considered to be metastasis from a perforated appendiceal mucinous tumor [27].
p53 is a tumor suppressor protein, and its mutation is known to be associated with the development of many tumor types. p53 mutation often prolongs its half-life; therefore, it appears to be overexpressed on immunohistochemistry [28]. In ovarian tumors, immunopositivity for p53 is diffuse and strong in high-grade serous carcinomas, while it is focal and isolated in low-grade counterpart. Benign tumors have no positive p53 staining. In ovarian serous borderline tumors, less than 10% of cases were positive for p53, and the level of staining in those cases that were positive was weak [29]. There have been two previous reports on tubal serous borderline tumors; one showed mild to moderate expression in a limited area of mild atypia and the other showed no nuclear immunoreactivity [4, 13]. Our study showed moderate-to-strong nuclear expression in 60% of the tumor cell; this appears to be exceptionally high for borderline tumors.
Ki67 protein is associated with cell proliferation, and its expression positively correlates with tumor malignancy. In ovarian tumors, high-grade serous carcinoma showed strong and diffuse nuclear expression in 75% of the tumor cells [29]. Low-grade serous carcinomas and borderline tumors showed heterogeneous and isolated expression in 29% and 21% of the tumor cells, respectively [29]. In tubal serous borderline tumors, Ondic et al reported that Ki67 was expressed in the nuclei of less than 5% of tumor cells [4]. Our study showed moderate-to-strong nuclear expression in less than 10% of the tumor cells; this was consistent with borderline tumor staining.
The tubal serous borderline tumor reported by Seamon et al consisted of a cyst attached to the tube, and was independent of the lumen [6]. Therefore, it was considered a paratubal lesion. Fifty-three such paraovarian (paratubal) tumors of borderline malignancy have been reported to date [30]. There is no histological difference between tubal and paratubal serous borderline tumor lesions, and tubal serous borderline tumors are likely to arise from fallopian fimbria. However, it is not known whether paratubal serous borderline tumors arise from the fallopian fimbria or the peritoneal mesothelium.
In conclusion, we experienced a rare case of a tubal serous borderline tumor. The patient was older than in any of the other cases reported to date; moreover, p53 expression was exceptionally high for a borderline tumor. Additional reports on these tumor types are required, not only to establish standard treatments but to also elucidate the pathogenesis of ovarian tumors themselves.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
| References | ▴Top |
- Choi SM, Choi MY, Kang WD, Choi HS, Kim SM. Serous borderline tumor of the fallopian tube. Obstet Gynecol Sci. 2014;57(4):334-337.
doi pubmed - Liu F, Wei J, Shen D, Liu J. Mucinous borderline tumor involving fallopian tube: case report and review of the literature. Int J Clin Exp Pathol. 2013;6(5):962-965.
pubmed - Kishi H, Nakata T, Hayashi H. Case report of 16 year-old woman with bordeline tumor of fallopian tube [Japanese]. Rinsho Sanfujinka. 2013;67:1077-1080.
- Ondic O, Kalis V, Sima R. Borderline papillary serous tumor of the fimbriated end of the fallopian tube with peritoneal implants. J Obstet Gynaecol Res. 2011;37(11):1702-1705.
doi pubmed - Abreu R, Dick M, Simoes Silva T, Mota F, Bettencourt E. Serous borderline tumor of the fallopian tube presented as an adnexal mass. Arch Gynecol Obstet. 2011;283(2):349-352.
doi pubmed - Seamon LG, Holt CN, Suarez A, Richardson DL, Carlson MJ, O'Malley DM. Paratubal borderline serous tumors. Gynecol Oncol. 2009;113(1):83-85.
doi pubmed - Noguchi S, Matsumoto K, Shimura R. Case report of serous borderline tumor of fallopian tube [Japanese]. Nihon sankafujinka kantourengou chihoubukai kaishi. 2008;45:287-287
- Villella JA, Pauli SA, Wang J, Intengan M, Lele S. Tumors of low malignant potential arising in the fallopian tube: case reports. Eur J Gynaecol Oncol. 2005;26(3):327-329.
pubmed - Krasevic M, Stankovic T, Petrovic O, Smiljan-Severinski N. Serous borderline tumor of the fallopian tube presented as hematosalpinx: a case report. BMC Cancer. 2005;5:129.
doi pubmed - Haratz-Rubinstein N, Fromberg E, Lederman S. Sonographic diagnosis of a serous tumor of low malignant potential of the fallopian tube. J Ultrasound Med. 2004;23(6):869-872.
pubmed - Kayaalp E, Heller DS, Majmudar B. Serous tumor of low malignant potential of the fallopian tube. Int J Gynecol Pathol. 2000;19(4):398-400.
doi pubmed - Alvarado-Cabrero I, Navani SS, Young RH, Scully RE. Tumors of the fimbriated end of the fallopian tube: a clinicopathologic analysis of 20 cases, including nine carcinomas. Int J Gynecol Pathol. 1997;16(3):189-196.
doi pubmed - Zheng W, Wolf S, Kramer EE, Cox KA, Hoda SA. Borderline papillary serous tumour of the fallopian tube. Am J Surg Pathol. 1996;20(1):30-35.
doi pubmed - Seidman JD. Mucinous lesions of the fallopian tube. A report of seven cases. Am J Surg Pathol. 1994;18(12):1205-1212.
doi pubmed - Friedmann W, Minguillon C, Wessel J, Lichtenegger W, Pickel H. [Pseudomyxoma peritonei caused by proliferating mucinous adenoma of the fimbria mucosa]. Geburtshilfe Frauenheilkd. 1990;50(7):579-580.
doi pubmed - Valerdiz Casasola S, Pardo Mindan J. Cystadenofibroma of fallopian tube. Appl Pathol. 1989;7(4):256-259.
pubmed - McCarthy JH, Aga R. A fallopian tube lesion of borderline malignancy associated with pseudo-myxoma peritonei. Histopathology. 1988;13(2):223-225.
doi - Gatto V, Selim MA, Lankerani M. Primary carcinoma of the fallopian tube in an adolescent. J Surg Oncol. 1986;33(3):212-214.
doi pubmed - Jones DH. Pseudomyxoma peritonei. Br J Clin Pract. 1965;19(12):675-679.
pubmed - Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007;211(1):26-35.
doi pubmed - Vang R, Shih Ie M, Kurman RJ. Fallopian tube precursors of ovarian low- and high-grade serous neoplasms. Histopathology. 2013;62(1):44-58.
doi pubmed - Morice P. Borderline tumours of the ovary and fertility. Eur J Cancer. 2006;42(2):149-158.
doi pubmed - Tempfer CB, Polterauer S, Bentz EK, Reinthaller A, Hefler LA. Accuracy of intraoperative frozen section analysis in borderline tumors of the ovary: a retrospective analysis of 96 cases and review of the literature. Gynecol Oncol. 2007;107(2):248-252.
doi pubmed - Darai E, Fauvet R, Uzan C, Gouy S, Duvillard P, Morice P. Fertility and borderline ovarian tumor: a systematic review of conservative management, risk of recurrence and alternative options. Hum Reprod Update. 2013;19(2):151-166.
doi pubmed - Morice P, Uzan C, Fauvet R, Gouy S, Duvillard P, Darai E. Borderline ovarian tumour: pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. Lancet Oncol. 2012;13(3):e103-115.
doi - Lesieur B, Kane A, Duvillard P, Gouy S, Pautier P, Lhomme C, Morice P, et al. Prognostic value of lymph node involvement in ovarian serous borderline tumors. Am J Obstet Gynecol. 2011;204(5):438 e431-437.
- Bevan KE, Mohamed F, Moran BJ. Pseudomyxoma peritonei. World J Gastrointest Oncol. 2010;2(1):44-50.
doi pubmed - Lepelley P, Preudhomme C, Vanrumbeke M, Quesnel B, Cosson A, Fenaux P. Detection of p53 mutations in hematological malignancies: comparison between immunocytochemistry and DNA analysis. Leukemia. 1994;8(8):1342-1349.
pubmed - Giurgea LN, Ungureanu C, Mihailovici MS. The immunohistochemical expression of p53 and Ki67 in ovarian epithelial borderline tumors. Correlation with clinicopathological factors. Rom J Morphol Embryol. 2012;53(4):967-973.
pubmed - Suzuki S, Furukawa S, Kyozuka H, Watanabe T, Takahashi H, Fujimori K. Two cases of paraovarian tumor of borderline malignancy. J Obstet Gynaecol Res. 2013;39(1):437-441.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
