| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 5, Number 4, December 2016, pages 101-105
Serum Levels of Soluble Urokinase Plasminogen Activator Receptor Are Elevated in Severe Pre-Eclampsia
Ville Jalkanena, e, Kati Tihtonenb, Kati Jalkanenb, Aaro J. Jalkanenc, Jukka Uotilab, d, Sari Karlssona
aDepartment of Intensive Care Medicine, Tampere University Hospital, Tampere, Finland
bDepartment of Obstetrics and Gynecology, Tampere University Hospital, Tampere, Finland
cSchool of Pharmacy, Faculty of Health Sciences, University of Eastern Finland, Kuopio, Finland
dSchool of Medicine, Tampere University, Tampere, Finland
eCorresponding Author: Ville Jalkanen, Department of Intensive Care Medicine, Tampere University Hospital, Teiskontie 35, PO Box 2000, 33521 Tampere, Finland
Manuscript accepted for publication November 29, 2016
Short title: Serum suPAR Elevated in Severe Pre-Eclampsia
doi: https://doi.org/10.14740/jcgo418w
| Abstract | ▴Top |
Background: Soluble urokinase plasminogen activator receptor (suPAR) is a novel marker for systemic inflammation both in infectious and in inflammatory diseases. Since pathophysiology of pre-eclampsia has been found to include impairment of maternal immune system, we wanted to explore serum level of suPAR in women with severe pre-eclampsia and whether it differs from healthy pregnancies and non-pregnant women.
Methods: Serum samples of suPAR concentrations were analyzed from 10 patients with severe pre-eclampsia, 10 patients with uncomplicated pregnancies and 10 non-pregnant women. SuPAR concentrations were analyzed with enzyme-linked immune-sorbent assay (ELISA).
Results: SuPAR levels were significantly higher in pre-eclampsia 3.5 ng/mL (2.4 - 4.8 ng/mL) compared to healthy pregnancy 2.0 ng/mL (1.7 - 2.6 ng/mL) (P =0.008) or non-pregnant women 2.3 ng/mL (1.9 - 2.9 ng/mL) (P = 0.015). Meanwhile, suPAR concentrations in healthy pregnant patients did not differ from non-pregnant women (P = 0.211).
Conclusions: Serum suPAR levels are elevated in patients with severe pre-eclampsia implying that the systemic inflammatory response is enhanced in severe pre-eclampsia.
Keywords: Soluble urokinase plasminogen activator receptor; Pre-eclampsia; Gestational hypertension
| Introduction | ▴Top |
Pre-eclampsia (PE) is one of the leading causes for maternal and perinatal morbidity and mortality, affecting 5-11% of all pregnancies [1, 2]. Despite extensive research, the etiology and detailed pathophysiology of PE have remained unclear. An abnormal invasion of placental cytotrophoblasts into the superficial layers of placental myometrium and the aberrant transformation of maternal spiral arteries in myometrium are thought to lead to placental ischemia and hypoperfusion. Ischemia evokes endothelial damage as well as the clinical manifestations of PE. Several factors may be involved in the endothelial dysfunction, e.g. free radicals, an imbalance of angiogenic and anti-angiogenic factors and the presence of inflammatory cytokines [3].
Normal pregnancy (NP) is characterized by a mild but significant inflammatory activity. This inflammatory activity is believed as a physiological consequence to the presence of the “foreign” fetus and it is considered to be important in the maintenance of pregnancy [4]. However, excessive inflammatory reactions are thought to be associated with pregnancy complications such as PE, intrauterine fetal growth restriction and fetal loss [5-7].
Urokinase plasminogen activator receptor (uPAR) is a glycoprotein that is localized not only on the outer surface of the plasma membrane of different leucocyte cell surfaces, but also in numerous other cell types such as endothelial cells, tumor cells and smooth muscle cells [8]. UPAR is involved in numerous physiological and pathological pathways, like plasminogen activation, modulation of cell adhesion and regulation of proteolysis [9]. Interestingly, a high uPAR expression has been detected in placental trophoblasts and the myometrium in NP [10, 11]. There are several proteases capable of cleaving uPAR from the cell surface to produce the soluble form, suPAR [9, 12]. The overall systemic inflammation and immunological activation elevate serum suPAR levels in humans [13, 14] and elevated levels of suPAR are known to predict mortality in infectious diseases [15-17], critical illness [18, 19] and cancer [20].
The aim of the present preliminary study was to investigate whether serum suPAR levels would be elevated in PE in comparison to NP or the non-pregnant state. In order to explore this hypothesis, we determined serum suPAR levels in patients with severe PE and compared them with those from women enjoying an NP and non-pregnant female controls.
| Materials and Methods | ▴Top |
In this prospective study, 10 pregnant patients with severe PE, 10 gestational age-matched patients with NP and 10 non-pregnant fertile-aged women were recruited into our study according to the predefined inclusion criteria. The data were collected between November 2012 and January 2014. One patient in the NP group was later excluded because she developed clinical PE before childbirth.
The patients were considered eligible in NP group, if they were aged 18 years or more, previously healthy, nulliparous and singleton uncomplicated pregnancy in gestational weeks 24 - 34. In addition, in the severe PE group, patients had at least one of the following findings: new-onset hypertension (defined as blood pressure over 160/110 mm Hg), daily urinary protein (dU-prot) more than 5 g, oliguria less than 500 mL/day, thrombocytopenia less than 100 × 109/L or subjective symptoms (severe headache, visual disturbances, and upper gastric pain). The HELLP syndrome was considered as severe PE and was defined as hemolysis with increased lactate dehydrogenase ≥ 600 U/L, alanine aminotransferase ≥ 70 U/L and thrombocytopenia/platelet count ≤ 100 × 109/L. A fetus small for gestational age (SGA) was defined as the estimated weight < 10th percentile. The non-pregnant controls were females aged 18 years or more, previously healthy and nulliparous. The exclusion criteria in all groups were infection at the time of sampling or not meeting inclusion criteria. The pregnant patients were recruited, when admitted to Tampere University Hospital. The voluntary non-pregnant controls were recruited from the hospital staff.
The study protocol was approved by the Ethical Committee of Tampere University Hospital (reference number R11174/2011). When the inclusion criteria were met, verbal and written information was given to the patients. After the patient provided informed consent, blood samples were drawn. Blood was allowed to clot at room temperature for 90 min and serum was separated by centrifugation (2,000 × g, 10 min). Serum samples were frozen to -80 °C until thawed and analyzed.
SuPAR concentrations were analyzed with a commercially available enzyme-linked immune-sorbent assay (ELISA) kit adhering to the manufacturer’s instructions (SuPARnostic®, ViroGates, Birkeroed, Denmark). All samples and standards were analyzed in duplicate and the mean of the absorbance values was used in the calculations. The analysis passed the manufacturer’s pre-set criteria for accuracy and precision.
In pregnant patients, the basic characteristics of pregnancy and childbirth were compiled in an electrical case report form from the institutional database. In the PE group, hemoglobin (g/L), hematocrit (%), platelet count (× 109/L), alanine aminotransferase (U/L) and uric acid levels (μmol/L) were analyzed in order to diagnose PE-related organ complications. In pregnant women, blood pressure and heart rate were obtained with a digital sphygmomanometer (OMRON M6 HEM 7211E, Omron Corporation®) with the subject in the sitting position after 15 min of rest.
Statistics were calculated by IBM SPSS 22 software. The data were analyzed in three groups: PE, NP and non-pregnant controls. Descriptive data were carried out using median and interquartile range (IQR). Non-parametric data were compared with Mann-Whitney U-test and categorical with Fisher’s exact test or Chi-square test. The Spearman correlation test was used to detect the correlation between suPAR and other laboratory findings. We considered a P-value of < 0.05 as statistically significant in all tests.
| Results | ▴Top |
The basic characteristics of the pregnant patients and suPAR levels are shown in Table 1. The median age and gestational weeks at sampling were comparable between the study groups. Pre-eclamptic women delivered in earlier gestational weeks and the birthweight of their newborns was smaller compared to normal pregnancies. Ninety percent of the newborns were SGA after pre-eclamptic pregnancy and none after NP. The severity of PE is described in Table 2. One patient in the PE group was diagnosed with deep venous thrombosis in the lower extremitas after delivery and one patient in NP group suffered from puerperal endometritis. Four patients in NP group suffered a perineal laceration during labor. No other significant maternal complications were noticed in either study groups.
 Click to view | Table 1. Basic Characteristics of Patients With Pre-Eclampsia or Having a Normal Pregnancy |
 Click to view | Table 2. Severity of Pre-Eclampsia in the Pre-Eclampsia Group (Median and Interquartile Range (IQR)) |
The median suPAR concentration was significantly higher in PE group compared to NP group (P = 0.008) and control group (P = 0.015). SuPAR concentrations did not differ between NP and non-pregnant controls (P = 0.211) (Fig. 1).
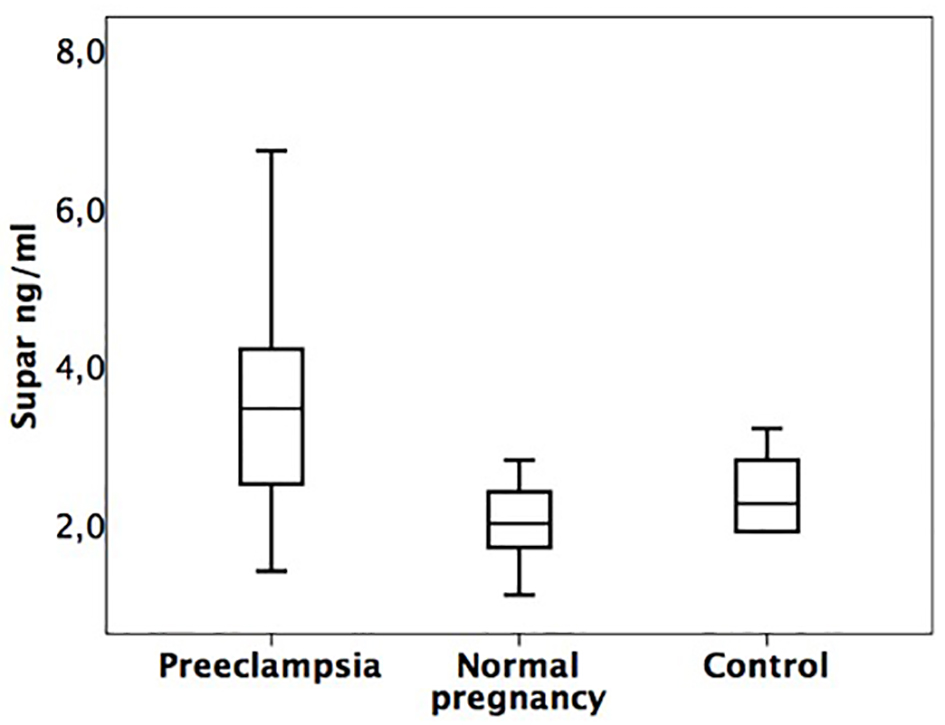 Click for large image | Figure 1. Box plot of serum suPAR concentrations (interquartile ranges) subdivided into the three study groups. PE: 3.5 ng/mL (2.4 - 4.8 ng/mL). NP: 2.0 ng/mL (1.7 - 2.6 ng/mL). Control group: 2.3 ng/mL (1.9 - 2.9 ng/mL). |
The correlation was evaluated in PE group between suPAR levels and blood pressure, age and laboratory findings (hemoglobin, hematocrit, leucocytes, platelet count, alanine transferase, daily proteinuria and uric acid). Negative significant correlations were found between systolic blood pressure (r = -0.70, P = 0.023) and diastolic blood pressure (r = -0.64, P = 0.045) in PE group. There was also a tendency towards a positive correlation between the concentrations of suPAR and uric acid (r = 0.63, P = 0.05). Furthermore, a positive correlation between maternal suPAR and birthweight (r = 0.70, P = 0.024) was found. No association between the suPAR level and gestational age or other laboratory findings or how urgently cesarean section was done due to the worsening of PE was found within the PE group.
| Discussion | ▴Top |
Our results indicate that the serum levels of the biomarker suPAR are significantly elevated in severe PE compared to both NP and non-pregnant controls. There is accumulating evidence that PE is associated with an enhanced systemic inflammatory reaction. It has also been proposed that the endothelial dysfunction responsible for many of the symptoms of PE is accompanied by elevated levels of inflammatory markers [21, 22]. In our study, there were no differences in suPAR levels between women with an NP and non-pregnant controls. This finding together with low levels and narrow range of suPAR in NP supports the view that in an NP, the inflammatory response is mild and physiological. Since serum suPAR levels are considered to represent the degree of inflammation [14], the significant difference noted in serum suPAR levels between PE and NP indicates that the activation of the inflammatory process is more pronounced in PE and furthermore that suPAR may serve as a potential marker of this inflammatory response [23]. In studies involving patients in the first and early second trimester before the clinical signs of PE are present, the assessment of suPAR concentrations was not found to predict the development of PE in late pregnancy [24, 25]. This finding could imply that the inflammatory process in PE is not more pronounced until clinical signs of PE.
In our material, the patients represented a rather homogenous form of severe PE; it was early-onset, patients were nulliparous and previously healthy and furthermore, cesarean section had to be performed quite urgently within days after recruitment to the study because of the worsening clinical status. The wide interquartile range in suPAR concentrations in PE implies that there may be greater individual variations in the inflammatory response in severe PE than in healthy pregnancies. Our finding of the level of serum concentration of suPAR in pre-eclamptic women or in NPs is in consistency with previous study by Toldi et al [23].
In other studies, very high serum suPAR levels have been detected in critically ill patients with high mortality rates [19, 26, 27]. However, mortality is very rare even in severely pre-eclamptic women, for example in our material, all of the patients recovered from PE after delivery without any major life-threatening complications. Furthermore, the levels of suPAR remained lower in PE than described in critically ill patients with poor prognosis in other studies [14, 18].
We did not find significant positive correlations between clinical status (blood pressure or interval from study entry to cesarean section due to worsening PE) and suPAR nor laboratory values describing organ dysfunction associated with PE. However, a tendency towards a positive correlation with the serum levels of uric acid and suPAR was observed. In critically ill patients, elevated suPAR and uric acid levels have been associated with acute kidney injury [18, 28]. Interestingly, there are data indicating that elevated suPAR levels may be prognostic for chronic kidney disease [29], while the risk for kidney disease after a hypertensive disorder in pregnancy has been established previously [30]. On the other hand, it is not quite clear why uric acid is elevated in PE besides of maternal renal dysfunction, it could also be due to increased production or tissue ischemia and acidosis [31]. Nonetheless, these relationships should be viewed with caution because of the small number of patients in our study and the potential for causality should be further investigated. Our data do not allow comprehensive interpretation of the relationship between suPAR levels and complications in PE.
Elevated suPAR concentrations have also been associated with low-grade inflammation leading to cardiovascular diseases and diabetes mellitus [32]. It is interesting that women who have experienced a pregnancy complicated by PE are known to have a higher risk for ischemic heart disease and chronic hypertension PE [33]. The mechanism behind this relationship has remained unknown, but there are some studies that support the view of persisting endothelial dysfunction after PE [34].
In conclusion, we detected significantly higher serum suPAR levels in women with severe PE in comparison to NPs. This might be a reflection of an elevated systemic inflammatory response in patients with severe PE.
Acknowledgments
We thank our study nurses Atte Kukkurainen and Simo Varila for their help in collecting and storage of the samples. We also acknowledge Jaana Leskinen for her technical assistance in the ELISA analyses, and Dr. Ewen MacDonald for linguistic advice. This study was supported by the grants from Finnish Medical Foundation Duodecim and from the Clinical Chemistry Research Foundation.
Financial Disclosures
Dr. Jalkanen has received a speaker’s fee from Orion Pharma. Dr. Jalkanen declares that this fee had no influence on the contents of this article.
Conflicts of Interest
Authors have no conflicts of interest to report.
| References | ▴Top |
- Kaaja R, Kinnunen T, Luoto R. Regional differences in the prevalence of pre-eclampsia in relation to the risk factors for coronary artery disease in women in Finland. Eur Heart J. 2005;26(1):44-50.
doi pubmed - Ghulmiyyah L, Sibai B. Maternal mortality from preeclampsia/eclampsia. Semin Perinatol. 2012;36(1):56-59.
doi pubmed - Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785-799.
doi - Hsu P, Nanan RK. Innate and adaptive immune interactions at the fetal-maternal interface in healthy human pregnancy and pre-eclampsia. Front Immunol. 2014;5:125.
doi pubmed - Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499-506.
doi - Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, Clark P, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44(5):708-714.
doi pubmed - Borzychowski AM, Sargent IL, Redman CW. Inflammation and pre-eclampsia. Semin Fetal Neonatal Med. 2006;11(5):309-316.
doi pubmed - Mazar AP. The urokinase plasminogen activator receptor (uPAR) as a target for the diagnosis and therapy of cancer. Anticancer Drugs. 2001;12(5):387-400.
doi pubmed - Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27(3):157-172.
doi pubmed - Uszynski M, Perlik M, Uszynski W, Zekanowska E. Urokinase plasminogen activator (uPA) and its receptor (uPAR) in gestational tissues; Measurements and clinical implications. Eur J Obstet Gynecol Reprod Biol. 2004;114(1):54-58.
doi pubmed - Feng Q, Liu Y, Liu K, Byrne S, Liu G, Wang X, Li Z, et al. Expression of urokinase, plasminogen activator inhibitors and urokinase receptor in pregnant rhesus monkey uterus during early placentation. Placenta. 2000;21(2-3):184-193.
doi pubmed - Beaufort N, Leduc D, Rousselle JC, Magdolen V, Luther T, Namane A, Chignard M, et al. Proteolytic regulation of the urokinase receptor/CD87 on monocytic cells by neutrophil elastase and cathepsin G. J Immunol. 2004;172(1):540-549.
doi pubmed - Smith HW, Marshall CJ. Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol. 2010;11(1):23-36.
doi pubmed - Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ, Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38(9):1418-1428.
doi pubmed - Huttunen R, Syrjanen J, Vuento R, Hurme M, Huhtala H, Laine J, Pessi T, et al. Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med. 2011;270(1):32-40.
doi pubmed - Wittenhagen P, Kronborg G, Weis N, Nielsen H, Obel N, Pedersen SS, Eugen-Olsen J. The plasma level of soluble urokinase receptor is elevated in patients with Streptococcus pneumoniae bacteraemia and predicts mortality. Clin Microbiol Infect. 2004;10(5):409-415.
doi pubmed - Molkanen T, Ruotsalainen E, Thorball CW, Jarvinen A. Elevated soluble urokinase plasminogen activator receptor (suPAR) predicts mortality in Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2011;30(11):1417-1424.
doi pubmed - Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A, Yagmur E, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15(1):R63.
doi pubmed - Jalkanen V, Yang R, Linko R, Huhtala H, Okkonen M, Varpula T, Pettila V, et al. SuPAR and PAI-1 in critically ill, mechanically ventilated patients. Intensive Care Med. 2013;39(3):489-496.
doi pubmed - Fidan E, Mentese A, Ozdemir F, Deger O, Kavgaci H, Caner Karahan S, Aydin F. Diagnostic and prognostic significance of CA IX and suPAR in gastric cancer. Med Oncol. 2013;30(2):540.
doi pubmed - Guven MA, Coskun A, Ertas IE, Aral M, Zencirci B, Oksuz H. Association of maternal serum CRP, IL-6, TNF-alpha, homocysteine, folic acid and vitamin B12 levels with the severity of preeclampsia and fetal birth weight. Hypertens Pregnancy. 2009;28(2):190-200.
doi pubmed - Mihu D, Razvan C, Malutan A, Mihaela C. Evaluation of maternal systemic inflammatory response in preeclampsia. Taiwan J Obstet Gynecol. 2015;54(2):160-166.
doi pubmed - Toldi G, Biro E, Szalay B, Stenczer B, Molvarec A, Rigo J, Vasarhelyi B, et al. Soluble urokinase plasminogen activator receptor (suPAR) levels in healthy pregnancy and preeclampsia. Clin Chem Lab Med. 2011;49(11):1873-1876.
doi pubmed - Odden N, Henriksen T, Morkrid L. Serum soluble urokinase plasminogen activator receptor (suPAR) in early pregnancy prior to clinical onset of preeclampsia. Acta Obstet Gynecol Scand. 2012;91(10):1226-1232.
doi pubmed - Haedersdal S, Salvig JD, Aabye M, Thorball CW, Ruhwald M, Ladelund S, Eugen-Olsen J, et al. Inflammatory markers in the second trimester prior to clinical onset of preeclampsia, intrauterine growth restriction, and spontaneous preterm birth. Inflammation. 2013;36(4):907-913.
doi pubmed - Haupt TH, Petersen J, Ellekilde G, Klausen HH, Thorball CW, Eugen-Olsen J, Andersen O. Plasma suPAR levels are associated with mortality, admission time, and Charlson Comorbidity Index in the acutely admitted medical patient: a prospective observational study. Crit Care. 2012;16(4):R130.
doi pubmed - Uusitalo-Seppala R, Huttunen R, Tarkka M, Aittoniemi J, Koskinen P, Leino A, Vahlberg T, et al. Soluble urokinase-type plasminogen activator receptor in patients with suspected infection in the emergency room: a prospective cohort study. J Intern Med. 2012;272(3):247-256.
doi pubmed - Otomo K, Horino T, Miki T, Kataoka H, Hatakeyama Y, Matsumoto T, Hamada-Ode K, et al. Serum uric acid level as a risk factor for acute kidney injury in hospitalized patients: a retrospective database analysis using the integrated medical information system at Kochi Medical School hospital. Clin Exp Nephrol. 2016;20(2):235-243.
doi pubmed - Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, et al. Soluble Urokinase Receptor and Chronic Kidney Disease. N Engl J Med. 2015;373(20):1916-1925.
doi pubmed - Wu CC, Chen SH, Ho CH, Liang FW, Chu CC, Wang HY, Lu YH. End-stage renal disease after hypertensive disorders in pregnancy. Am J Obstet Gynecol. 2014;210(2):147 e141-148.
- Hawkins TL, Roberts JM, Mangos GJ, Davis GK, Roberts LM, Brown MA. Plasma uric acid remains a marker of poor outcome in hypertensive pregnancy: a retrospective cohort study. BJOG. 2012;119(4):484-492.
doi pubmed - Eugen-Olsen J, Andersen O, Linneberg A, Ladelund S, Hansen TW, Langkilde A, Petersen J, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268(3):296-308.
doi pubmed - Wikstrom AK, Haglund B, Olovsson M, Lindeberg SN. The risk of maternal ischaemic heart disease after gestational hypertensive disease. BJOG. 2005;112(11):1486-1491.
doi pubmed - Amaral LM, Cunningham MW, Jr., Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag. 2015;11:403-415.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
