| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 7, Number 2, June 2018, pages 57-61
Vaginal Mucosal Implantation After Total Laparoscopic Hysterectomy for an Early Stage Endometrial Cancer
Koji Hisamotoa, Miho Kitaib, Ayuko Otoshia, Chikako Tsukaharaa, Yukihiro Nishioa, Takashi Miyatakea, c
aDepartment of Obstetrics and Gynecology, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 5430035, Osaka, Japan
bDepartment of Gynecologic Oncology, Hyogo Cancer Center, 13-70 Kitaoji-cho, Akashi, Hyogo 6738558, Japan
cCorresponding Author: Takashi Miyatake, Department of Obstetrics and Gynecology, Osaka Police Hospital, 10-31 Kitayama-cho, Tennoji-ku, Osaka 5430035, Japan
Manuscript submitted May 1, 2018, accepted May 25, 2018
Short title: Vaginal Mucosal Implantation After Laparoscopic Hysterectomy
doi: https://doi.org/10.14740/jcgo495w
| Abstract | ▴Top |
We present an extremely rare case of vaginal implantation of endometrial cancer, which is presumed to have been caused by transvaginal uterine removal during laparoscopic hysterectomy. A 48-year-old woman, nulligravida, had had a bilateral salpingo-oophorectomy due to benign mature cystic teratoma at 46 years old. She presented abnormal genital bleeding and had a total laparoscopic hysterectomy, due to stage Ia endometrial cancer. Postoperative pathology revealed an endometrioid adenocarcinoma G1, showing myometrial invasion, not exceeding the half of the muscular layer. The vascular space invasion and lymphatic space invasion were markedly recognized. Three cycles of combination chemotherapy with paclitaxel and carboplatin, with an interval of 3 weeks, have been postoperatively executed. Four months after the completion of adjuvant chemotherapy, a gross tumor of 6 mm in diameter was found at left vaginal mucosa near hymen. Vaginal biopsy of the lesion revealed a serous adenocarcinoma, and review of the primary endometrial tumor concluded that the primary endometrial carcinoma is corrected to a diagnosis of serous adenocarcinoma. It is considered that the vaginal lesion have been implanted through transvaginal uterine removal during the laparoscopic surgery. The vaginal lesion was surgically resected, however, during the follow-up examination, the other undifferentiated sarcoma was detected at right lung and the treatment was focused on to the sarcoma. And the patient died of the sarcoma, without the further recurrence of endometrial cancer.
Keywords: Vaginal implantation; Endometrial cancer; Laparoscopic hysterectomy
| Introduction | ▴Top |
Laparoscopic surgery has been widely spread in gynecology region for both benign and malignant disease, because of its minimal invasiveness. In Japan, laparoscopic surgery for early stage endometrial cancer was approved as the coverage of medical insurance at April 2014, and routinely implemented nationwide afterward [1]. Endometrial cancer is the most common gynecological malignancy and approximately 70% of the disease is diagnosed as stage I [2]. And most frequent recurrent endometrial cancer occurs as local invasion within 2 or 3 years from surgery [3]. The vaginal implantation of endometrial cancer is quite rare and we would present a case of vaginal implantation of endometrial cancer, which is presumed to be caused through transvaginal uterine removal during laparoscopic hysterectomy. Documented informed consent has been obtained from the patient.
| Case Report | ▴Top |
A 48-year-old woman, nulligravida, had undergone a bilateral salpingo-oophorectomy due to benign mature cystic teratomas at 46 years old. Bilateral ovarian tumors did not show any pathological malignancy. She had not had a history of hormone replacement therapy after the oophorectomy. At 48 years old, she presented abnormal genital bleeding, and was diagnosed as endometrial adenocarcinoma G1, with endometrial biopsy. She consulted to our hospital for the treatment of endometrial cancer. At the consultation, bimanual examination revealed an atrophic uterus and the bilateral adnexa were free. Pelvic magnetic resonance imaging (MRI) showed a low signal endometrial mass of 19 mm in diameter on T2-weighted sagittal section. The endometrial mass was enhanced in contrast study (Fig. 1). Myometrial invasion or uterine cervical invasion of the mass was not proven in MRI. No observable metastatic lesion was detected in positron emission tomography-computed tomography (PET-CT). The serum tumor markers were CA 125: 6 U/mL, CA 19-9: 8 U/mL, and CEA: 1.8 ng/mL, and they were below the cut-off value. Estimated preoperative diagnosis of the endometrial cancer was endometrioid adenocarcinoma G1, clinical stage Ia. For the primary treatment of early stage endometrial cancer, we operated total laparoscopic hysterectomy (TLH). In surgical procedure, a 12 mm trocar was inserted through the umbilicus and via the trocar the abdominal cavity was explored. Other three trocars of 5 mm were inserted into lower abdomen with diamond style [4, 5]. In the abdominal cavity, a slight adhesion was observed between the abdominal wall and the omentum. There was no ascites or dissemination in the peritoneal cavity. Uterine manipulator was used for uterine support and a vaginal pipe was used at vaginal incision. After the vaginal incision, the resected uterus was transvaginally removed, without intraabdominal division and without a use of specimen retrieval bag. The total operation time was 173 min, and the blood loss in the hysterectomy was 50 mL. Postoperative diagnosis was endometrial adenocarcinoma grade 1, pT1aN0M0, peritoneal washing cytology was negative, and the tumor had invaded into the myometrium, but remained within half of the muscular layer. There were findings of vascular space invasion and lymphatic vascular space invasion (Fig. 2). The primary endometrial cancer was considered as to be intermediate risk group [6, 7], and we administered three cycles of adjuvant combination chemotherapy with paclitaxel and carboplatin, with 3-week interval [6-8].
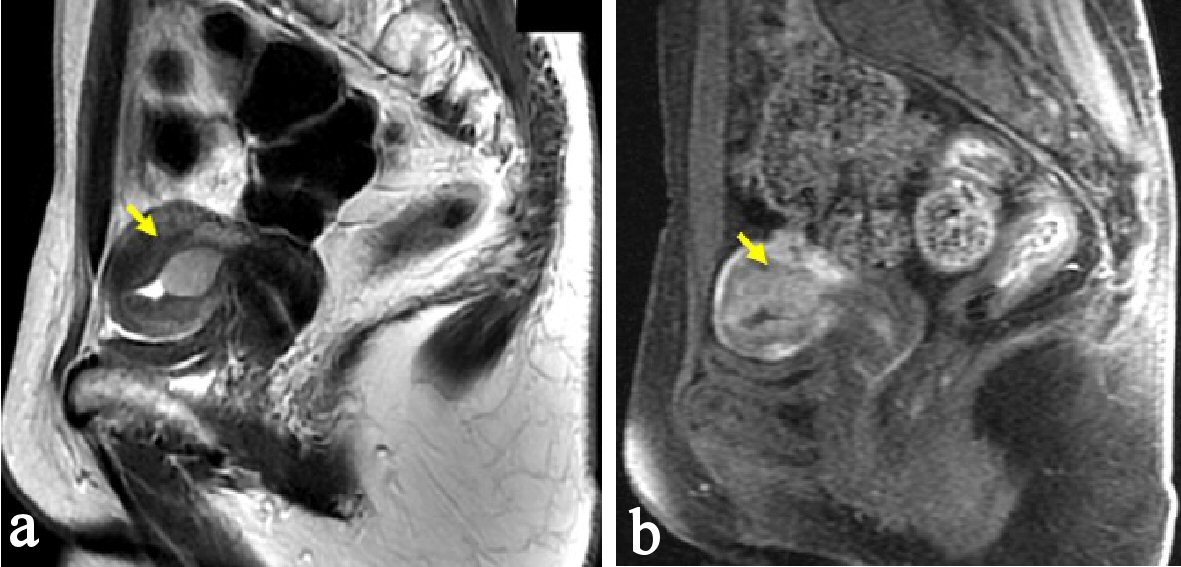 Click for large image | Figure 1. Pelvic MRI, sagittal section at the first consultation. (a) There is a low signal endometrial mass of 19 mm in diameter on T2-weighted sagittal section (arrow). (b) The endometrial mass was enhanced in contrast study (arrow). Myometrial invasion or uterine cervical invasion of the mass was not proven in MRI. |
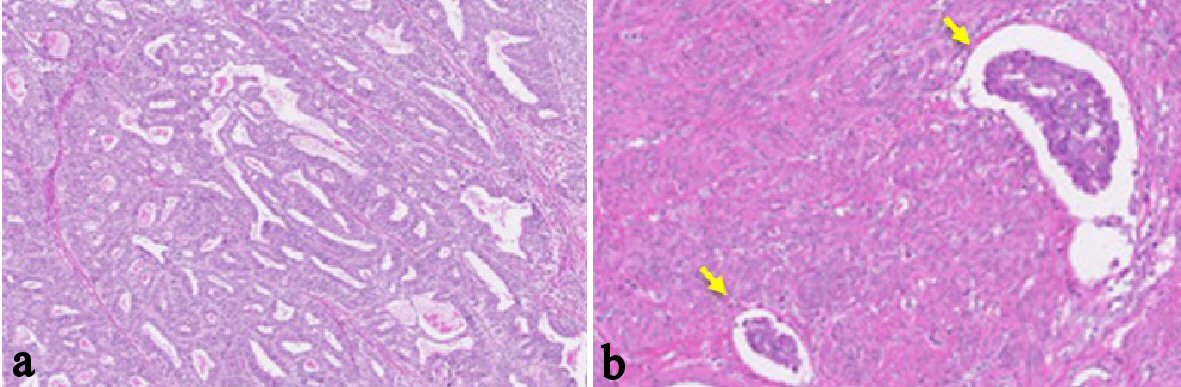 Click for large image | Figure 2. Histological findings of the endometrial tumor of surgically resected uterus (H&E stain; the original magnification is × 40). (a-b) Atypical glands are proliferating with tubular and cribriform pattern. There is no finding of papillary proliferation. The tumor had invaded into the myometrium within half of the muscular layer. There were findings of vascular space invasion and lymphatic vascular space invasion (b), (arrows). Postoperative diagnosis was endometrial adenocarcinoma grade 1, pT1aN0M0. |
Four months after the completion of chemotherapy (8 months after initial surgery), a symptom of genital bleeding occurred and internal examination have found a protruding lesion on the left wall of vaginal mucosa near hymen. The biopsy of the vaginal tumor proved a serous adenocarcinoma. The atypical cancer cells were proliferating with papillary appearance, with forming multiple layers. At H&E staining appearance, the findings of vaginal tumor was not similar with the primary endometrioid adenocarcinoma, which had not formed papillary proliferation.
For the investigation of the vaginal tumor, the vaginal tumor was surgically resected. And for the investigation of other lesions, thoracic CT scan revealed a nodular mass of 6 mm in diameter at right lung S3, which was also suspected as metastatic lesion.
Histological finding of the resected vaginal tumor revealed the same serous adenocarcinoma as that found in former vaginal biopsy. And immunostaining results of the vaginal tumor were positive for p53, strongly positive for p16, and negative for ER (Fig. 3). And additionally, exactly same immunostaining results were obtained from the primary endometrial cancer, which was diagnosed as endometrioid adenocarcinoma (Figures not shown). The histological and immunostaining results indicated that the origin of the primary endometrial cancer and the vaginal serous adenocarcinoma were same, and immunohistochemistry assumed that the both cancers are serous adenocarcinoma. We concluded the vaginal tumor as a vaginal recurrence of primary serous adenocarcinoma of endometrium, pTa1N0M0. It is considered that the vaginal lesion has been implanted through transvaginal uterine removal during the laparoscopic surgery.
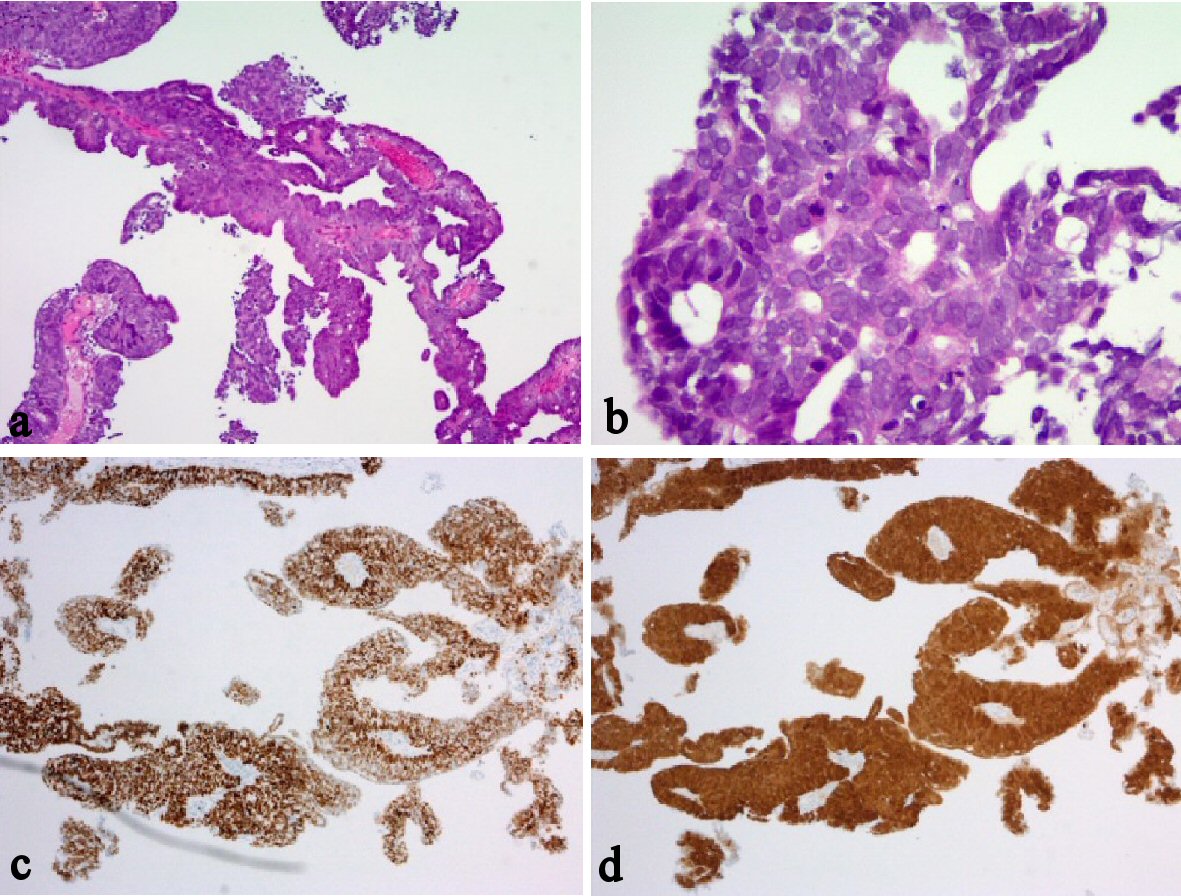 Click for large image | Figure 3. Histological and immunohistochemical findings of vaginal tumor. (a-b) H&E stain. (c) P53 immunohistochemistry. (d) P16 immunohistochemistry. The atypical cancer cells were proliferating with papillary appearance, with forming multiple layers (a-b); the original magnifications are × 40 and × 100, respectively. Immunostaining results of the vaginal tumor were positive for p53 (c), the original magnification is × 40; strongly positive for p16 (d), the original magnification is × 40; and negative for ER. Additionally, exactly same immunostaining results were obtained from the primary endometrial cancer, which was diagnosed as endometrioid adenocarcinoma (Figures not shown). |
Adjuvant chemotherapy for the vaginal recurrence of serous adenocarcinoma had been planned; however, the right lung nodule found at CT scan was subsequently resected with thorascoscopic resection and was histologically probed to be other undifferentiated sarcoma. Histological examination did not clarify the similarity between the sarcoma and the endometrial cancer; and review of the uterine/vaginal cancer lesions does not prove a sarcomatous component. At the follow-up thoracic/abdominal CT after 1month from lung surgery, new lesion was found at the upper lobe of right lung and the treatment for the patient was focused for the sarcoma after that. However, the recurrent sarcoma further progressed and the patient died of sarcoma at 15 months from the right lung nodule resection, without further recurrence of endometrial cancer.
| Discussion | ▴Top |
Laparoscopic surgery for malignant diseases requires not only minimally invasiveness and tolerability, but also achievement of oncologic outcomes equivalent to laparotomy. In a RCT of the Gynecologic Oncology Group (GOG) LAP 2, laparoscopic surgery for endometrial cancer showed the hazard ratio for recurrence of 1.14 (95% CI: 0.92 - 1.46), comparing to laparotomy, and the RCT resulted that there was no difference in the estimated 5-year survival between laparoscopy and laparotomy. In literature, there are also several reports that showed laparoscopic surgery is superior on intraoperative complications, blood loss during the surgery, shorter term of hospitalization, and post-operative psychosomatic and social recovery [9, 10]. So the adaptation of laparoscopic surgery will be expected to expand in the future.
On the other hand, there are some recurrence patterns peculiar to laparoscopic surgery, such as port site recurrence [11-13] and as cancer dissemination with using uterine manipulator [14-16]. According to the use of the uterine manipulator, the laparoscopic surgery with uterine manipulator does not increase the possibility of atypical cytology of peritoneal cavity [17, 18], and does not affect the risk of recurrence [19, 20]. The present case was after the removal of both adnexa, so the bilateral fallopian tube had already been closed. We estimated there was low risk of peritoneal dissemination of cancer, and the use of the uterine manipulator was considered to have been safe. The intraperitoneal cytology was negative, whereas the recurrence occurred after surgery on the vaginal mucosa. We have not used the sample collection bag at the removal of the resected uterus. The report on the vaginal implantation after laparoscopic surgery of endometrial cancer is extremely limited [12]; and there is no report on the relationship between the use of sample collection bag and vaginal implantation of endometrial cancer. However, it is suggested that use of a bag during removal of the uterus through the vagina can limit seeding of malignant cells. Abdullah et al reported the vulvar recurrence of endometrial cancer at 8 months after the robotic surgery; and it is said that such cases are iatrogenic recurrence, which can be avoided by using sample collection bag [21].
Another diagnostic issue of the present case is the histological aggressiveness of the cancer. Serous adenocarcinoma of the uterine endometrium is known as aggressive type of histology and is responsible for 40% of deaths of endometrial cancer [22]. Pathological feature of serous adenocarcinoma is a predominant papillary growth, which is also found in some subtypes of endometrioid adenocarcinoma. Distinction is usually easy when attention is paid to the presence of papillary architecture, however, serous adenocarcinoma may also present a pseudoglandular pattern, and in such cases, differential diagnosis may be problematic with endometrioid adenocarcinoma [22]. The primary uterine lesion of the present case was first diagnosed as endometrioid adenocarcinoma with lack of papillary structure. Immunohistochemistry of p53, p16, IMP2 and IMP3 are reported to be useful for the specific in serous adenocarcinomas [22, 23] and we reached the diagnosis of serous adenocarcinoma after the investigation of vaginal lesion. The vaginal implantation of the present case was highly due to aggressive feature of serous adenocarcinoma, although, the primary lesion of endometrium has showed relatively low-grade endometrioid histology. In the adaptation of laparoscopic surgery for endometrial cancer, as well as laparotomy, we should take care for the diagnostic problem on the pathology of endometrial cancer. If we had recognized the serous pathology at the primary surgery, we should have done more extended surgery including lymphadenectomy and have done more thorough adjuvant therapy.
Conclusions
We experienced a quite rare case of vaginal implantation of endometrial cancer, which is caused by transvaginal uterine removal during laparoscopic hysterectomy. And there also seems to be difficulty in the pathological diagnosis of endometrial cancer. With the coverage of medical insurance, it is expected that increasing number of institutions conduct laparoscopic surgery for endometrial cancer in Japan. In order to achieve oncologic outcomes comparable to laparotomy, it is important to carefully establish the safe procedures preventing from anticipated surgical complications and from cancer recurrence.
Conflict of Interest
The authors declare no conflict of interest regarding this paper.
Financial Support
There was no funding source for this research.
| References | ▴Top |
- Isaka K, Kato R, Ito H. [Uterine cancer]. Gan To Kagaku Ryoho. 2014;41(11):1354-1357.
pubmed - Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
doi pubmed - Tejerizo-Garcia A, Alvarez-Conejo C, Munoz-Hernando L, Guillen-Gamez C, Seoane-Ruiz JM, Perez-Sagaseta C, Jimenez-Lopez JS. Tumor recurrence and tumor-related mortality in endometrial cancer: Analysis in 276 patients. Indian J Cancer. 2015;52(4):682-684.
doi pubmed - Reich H, Johns DA, Davis G, Diamond MP. Laparoscopic oophorectomy. J Reprod Med. 1993;38(7):497-501.
pubmed - Johns DA, Diamond MP. Laparoscopically assisted vaginal hysterectomy. J Reprod Med. 1994;39(6):424-428.
pubmed - DiSaia PJ, Creasman WT. Clinical Gynecologic Oncology E-Book. 8th ed. St. Louis: Elsevier Health Sciences. 2012:851, 1 online resource
pubmed - Berek JS, Novak E. Berek & Novak's gynecology. 15th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012:xix, 1539.
- Fujiwara K, Egawa-Takata T, Ueda Y, Kimura T, Yoshino K, Fujita M, Miyatake T, et al. Investigating the relative efficacies of combination chemotherapy of paclitaxel/carboplatin, with or without anthracycline, for endometrial carcinoma. Arch Gynecol Obstet. 2012;285(5):1447-1453.
doi pubmed - Mourits MJ, Bijen CB, Arts HJ, ter Brugge HG, van der Sijde R, Paulsen L, Wijma J, et al. Safety of laparoscopy versus laparotomy in early-stage endometrial cancer: a randomised trial. Lancet Oncol. 2010;11(8):763-771.
doi - Janda M, Gebski V, Brand A, Hogg R, Jobling TW, Land R, Manolitsas T, et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol. 2010;11(8):772-780.
doi - Kadar N. Port-site recurrences following laparoscopic operations for gynaecological malignancies. Br J Obstet Gynaecol. 1997;104(11):1308-1313.
doi pubmed - Wang Y, Du J, Lv S, Sui Y, Xue X, Sun C, Zou J, et al. Vaginal implantation metastasis of endometrial carcinoma: A case report. Oncol Lett. 2016;12(1):513-515.
doi pubmed - Sanjuan A, Hernandez S, Pahisa J, Ayuso JR, Torne A, Martinez Roman S, Lejarcegui JA, et al. Port-site metastasis after laparoscopic surgery for endometrial carcinoma: two case reports. Gynecol Oncol. 2005;96(2):539-542.
doi pubmed - Krizova A, Clarke BA, Bernardini MQ, James S, Kalloger SE, Boerner SL, Mulligan AM. Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review. Am J Surg Pathol. 2011;35(1):115-126.
doi pubmed - Jan H, Ghai V, Thakar R. Simplified laparoscopic sacrohysteropexy. J Minim Invasive Gynecol. 2018.
doi - Lim S, Kim HS, Lee KB, Yoo CW, Park SY, Seo SS. Does the use of a uterine manipulator with an intrauterine balloon in total laparoscopic hysterectomy facilitate tumor cell spillage into the peritoneal cavity in patients with endometrial cancer? Int J Gynecol Cancer. 2008;18(5):1145-1149.
doi pubmed - Zhang C, Havrilesky LJ, Broadwater G, Di Santo N, Ehrisman JA, Lee PS, Berchuck A, et al. Relationship between minimally invasive hysterectomy, pelvic cytology, and lymph vascular space invasion: a single institution study of 458 patients. Gynecol Oncol. 2014;133(2):211-215.
doi pubmed - Lee M, Kim YT, Kim SW, Kim S, Kim JH, Nam EJ. Effects of uterine manipulation on surgical outcomes in laparoscopic management of endometrial cancer: a prospective randomized clinical trial. Int J Gynecol Cancer. 2013;23(2):372-379.
doi pubmed - Uccella S, Bonzini M, Malzoni M, Fanfani F, Palomba S, Aletti G, Corrado G, et al. The effect of a uterine manipulator on the recurrence and mortality of endometrial cancer: a multi-centric study by the Italian Society of Gynecological Endoscopy. Am J Obstet Gynecol. 2017;216(6):592 e591-592 e511.
- Tinelli R, Cicinelli E, Tinelli A, Bettocchi S, Angioni S, Litta P. Laparoscopic treatment of early-stage endometrial cancer with and without uterine manipulator: Our experience and review of literature. Surg Oncol. 2016;25(2):98-103.
doi pubmed - Abdullah A, Seagle BL, Bautista E, Hansra BS, Samuelson R, Shahabi S. Vulvar metastasis of an early-stage well-differentiated endometrial cancer after minimally invasive surgery. J Minim Invasive Gynecol. 2014;21(4):708-711.
doi pubmed - Gatius S, Matias-Guiu X. Practical issues in the diagnosis of serous carcinoma of the endometrium. Mod Pathol. 2016;(Suppl 1):S45-58.
doi pubmed - Chen W, Husain A, Nelson GS, Rambau PF, Liu S, Lee CH, Lee S, et al. Immunohistochemical profiling of endometrial serous carcinoma. Int J Gynecol Pathol. 2017;36(2):128-139.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
