| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 9, Number 1-2, June 2020, pages 25-28
Uterine Artery Embolization Prior to Induced Second-Trimester Abortion With Placenta Previa: Report of a Series of Cases
Patricia Perez-Moneo Pereza, c, Nerea Ruiz Sacedona, Belen Aparicio Navarroa, Jorge Gomez Valdesb, Reyes Balanza Chancosaa
aDepartment of Obstetrics and Gynaecology at Hospital Doctor Peset, Valencia, Spain
bDepartment of Radiology at Hospital Doctor Peset, Valencia, Spain
cCorresponding Author: Patricia Perez Perez-Moneo, Department of Obstetrics and Gynaecology at Hospital Doctor Peset, Avd. Gaspar Aguilar 90, Valencia, Spain
Manuscript submitted January 30, 2020, accepted February 26, 2020, published online June 5, 2020
Short title: Embolization in Abortion With Placenta Previa
doi: https://doi.org/10.14740/jcgo511
| Abstract | ▴Top |
Due to the higher prevalence of placenta previa in induced second-trimester abortions, prophylactic uterine artery embolization prior to induction of labor is a safe and effective technique to reduce maternal blood loss and morbidity.
Keywords: Placenta previa; Uterine artery embolization; Abortion
| Introduction | ▴Top |
During the second trimester of pregnancy, a large number of placenta previa cases are frequently recorded. Placenta previa is a condition where the placenta is attached in the lower third of the uterus, totally or partially covering the internal cervical os or being very close to it [1]. This is because uterine growth occurs gradually in the first month of pregnancy, resulting in an exponential growth by the third trimester. The so-called “placental migration” toward the uterine fundus occurs from the second trimester onwards [1], and is due firstly to the elongation of the upper uterine segment followed by that of the lower uterine segment, and finally by a lateral expansion with rotation of the uterus [2]. Therefore, 90% of placenta previa cases in week 20 will not be placenta previa at the end of pregnancy [2].
Late abortions or second-trimester terminations of pregnancy are a common practice in many hospitals due to several reasons, both maternal and fetal. Induced vaginal delivery with drug treatment is the technique of choice to reduce maternal morbidity. With an unfavorable Bishop (< 6), induction of labor is performed firstly by administering prostaglandins to improve cervical condition, and subsequently when necessary, oxytocin to expel the fetus and placenta. Following our protocol, the dose of prostaglandin administered will depend on the gestational age. Should cervical condition be favorable, intravenous oxytocin could be administered alone [3].
However, in second-trimester pregnancies, the existence of placenta previa can hinder vaginal delivery of the fetus, and complicate it by increasing maternal morbidity due to more severe bleeding, an increasing need for blood transfusion or longer hospital stay.
Therefore, we present a series of cases where it is noted that selective uterine artery embolization prior to induction of labor could be a prophylactic method to decrease maternal morbidity.
| Case Reports | ▴Top |
Case 1
This is a 37-year-old patient, 26 weeks and 6 days pregnant, gravida 4 with three previous eutocic deliveries, with a previous diagnosis of growth retardation type IV, an estimated fetal weight less than 400 g and complete placenta previa. After being approved by the Ethics Committee, a legal pregnancy termination was performed with prior informed consent of the patient.
Previous blood tests results showed: hemoglobin (Hb) 13.2 g/dL and hematocrit (Hct) 39.9%. After feticide, a prophylactic selective bilateral uterine artery embolization was performed with Espongostan® before induction of labor (Fig. 1a, b). After 12 h, epidural anesthesia was administered and the standard for cervical ripening with misoprostol was initiated according to our protocol, with 400 µg/4 h up to five doses, followed by intravenous oxytocin (20 IU/500 mL, glucose at 5%) without delivery of fetus and placenta, which led to a manual delivery in the operating room. Subsequent blood test results showed: Hb 12.6 g/dL without excessive bleeding at any time. This patient developed a subsequent infection and a hysterectomy was required.
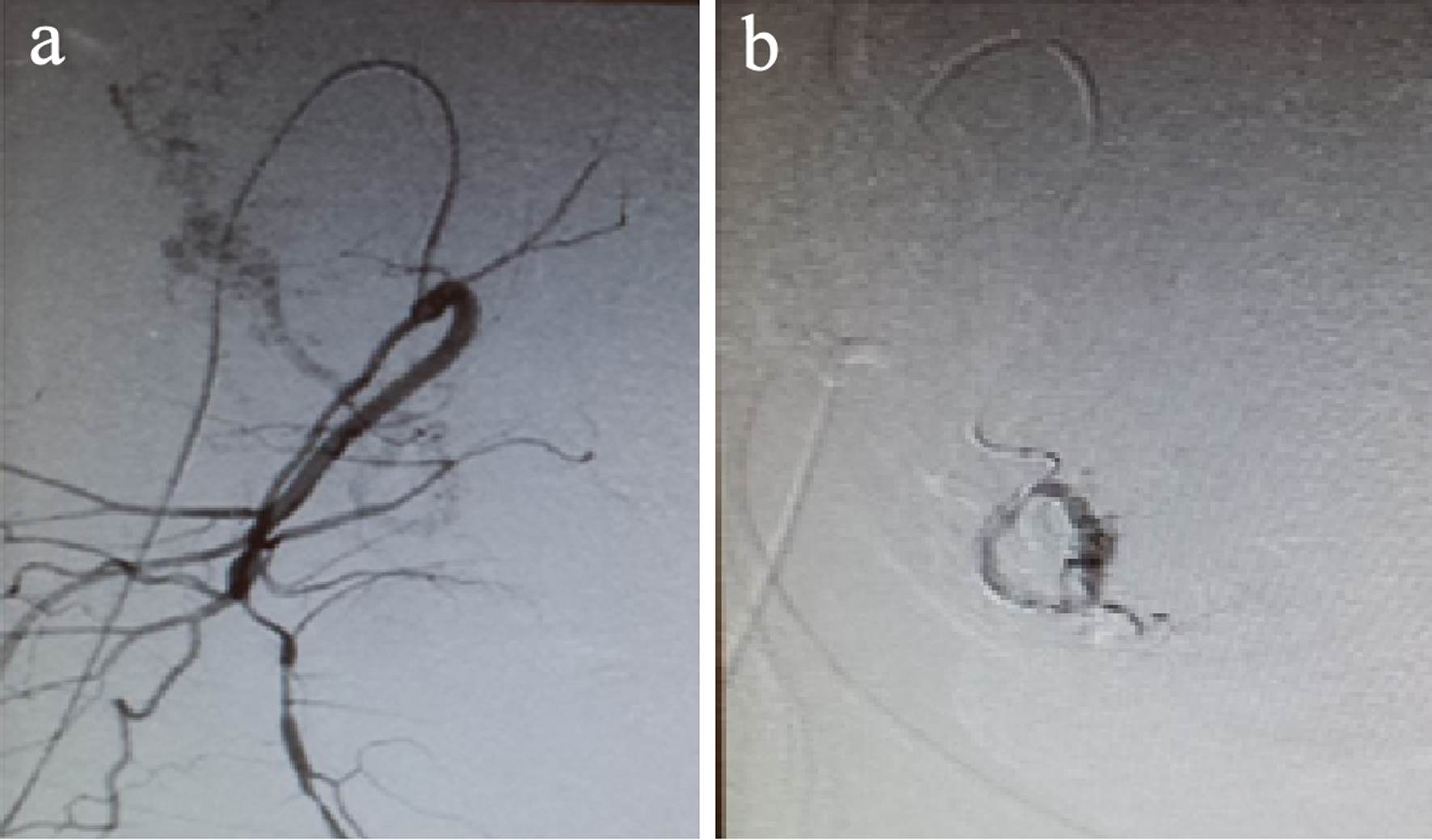 Click for large image | Figure 1. (a) Uterine artery prior to embolization. (b) Uterine artery after embolization. |
Case 2
A 35-year-old patient, 19 weeks and 3 days pregnant, gravida 2 with a previous spontaneous delivery came to the Gynecology Emergency Department of our hospital with bleeding similar to menstruation.
Ultrasound revealed singleton pregnancy, transverse presentation, positive heartbeat, anhydramnios and low-lying, partial placenta previa. AmniSure® test result was positive. Blood test results showed 20,300 leukocytes/mm3 with neutrophilia, high C-reactive protein (CRP) of 76 mg/L, Hb 12.7 g/dL and Hct 37.1%. Following these findings, pregnancy was decided to be terminated and uterine artery embolization was performed. Selective bilateral uterine artery embolization was carried out with Espongostan® via right femoral artery approach. After 12 h, induced abortion was performed with misoprostol 400 µg/4 h up to three doses resulting in the delivery of the fetus and placenta. Subsequent ultrasound showed empty uterine cavity. Massive bleeding was not observed at any time, with the subsequent Hb 12.7 g/dL and Hct 37.6%. The following year, the patient was pregnant again, with healthy newborn by vaginal delivery at week 34.
Case 3
This is a 34-year-old patient, 20 weeks pregnant, and primigravida, diagnosed with fetal trisomy 21, who requested legal termination of pregnancy. Upon induction of labor, low-lying complete placenta previa was confirmed (Fig. 2). Previous blood test results showed: Hb 12.4 g/dL. Mifepristone 600 mg was administered 48 h prior to bilateral uterine artery embolization with Gelfoam®. After 12 h, misoprostol 400 µg/4 h up to five doses was administered vaginally followed by administration of oxytocin (20 IU/500 mL in glucose at 5%) until delivery of the fetus and placenta. Subsequent endocervical curettage was performed to remove placental remnants. Massive bleeding was not observed at any time. Subsequent blood test results showed: Hb 11.3 g/dL.
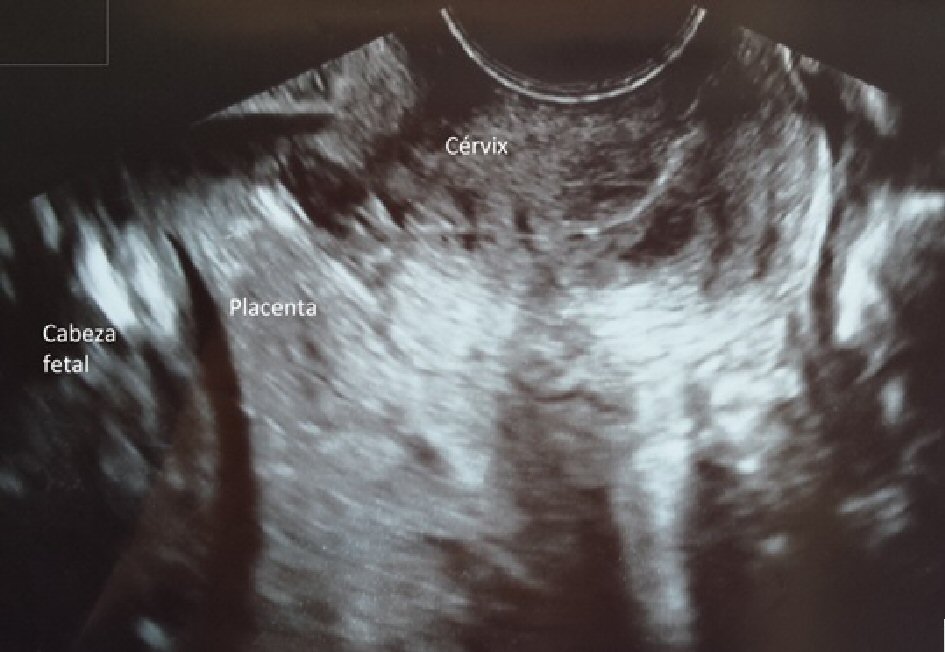 Click for large image | Figure 2. Complete placenta previa. |
Case 4
This is a 41-year-old patient, 19 weeks pregnant, gravida 5 with history of cesarean section, an antepartum stillbirth and two late abortions. She was diagnosed with antiphospholipid syndrome and treated with Clexane 30 mg and aspirin 100 mg during the current pregnancy. Trisomy 21 was detected and induction of second-trimester abortion was planned. Ultrasound confirmed marginal complete placenta previa. Previous blood test results showed: Hb 10.6 g/dL. Mifepristone 600 mg was administered 48 h before bilateral uterine artery embolization with Gelfoam®. After 12 h, misoprostol (400 µg/4 h) up to five doses was administered vaginally with epidural anesthesia, followed by oxytocin (20 IU/500 mL glucose at 5%) until delivery of the fetus in the breech position.
Removal of the placenta with Foerster sponge forceps and subsequent endocervical aspiration were needed. Massive bleeding was not observed at any time. Final blood tests results showed: Hb 10.3 g/dL. Afterwards, she was pregnant again and the term newborn was delivered by cesarean section.
Case 5
A 24-year-old patient, 23 weeks pregnant was referred to our hospital with preterm amniorrhexis and suspected chorioamnionitis with placenta previa. The patient had normal vital signs, fetid vaginal discharge and scant vaginal bleeding. No hypertonus or abdominal pain was recorded. Ultrasound showed singleton pregnancy, positive heart rate, anhydramnios and low-lying complete placenta previa. Blood test revealed that 21,500 leukocytes/mL, high CRP (24.2 mg/L), Hb 11.3 g/dL and Hct 32.8%.
Bilateral uterine artery embolization with Spongostan® was performed, and after epidural anesthesia, the protocol for induction of labor was followed with two doses of misoprostol at 400 µg/4 h and subsequent oxytocin at 10 IU/500 mL glucose-saline perfusion/8 h until delivery of the fetus and placenta. Because of persistence of placental remnants, endocervical aspiration curettage was carried out. Subsequent blood test results showed Hb 10.6 g/dL and Hct 31.7%.
| Discussion | ▴Top |
Induced second- and third-trimester abortions are a common practice in many hospitals, but the presence of placenta previa at these gestational ages can complicate them by increasing maternal morbidity. According to some studies [4, 5], the existence of placenta previa is related to a higher maternal blood loss during termination of pregnancy in second trimester.
Despite that, vaginal delivery continues to be the method of choice, so strategies must be found to perform it with the least possible morbidity.
With that aim, we presented a series of cases showing that uterine artery embolization prior to induced abortion is a beneficial and safe technique in induced second- and early third-trimester abortions with placenta previa resulting in a more effective control of uterine bleeding, that leads to a decrease in maternal morbidity. Although one of the patients developed a subsequent infection, its origin was unclear and could not be directly attributable to embolization, so further studies are necessary to evaluate the possible complications of this technique.
The results obtained in our series of cases were similar to those in the existing studies [6]. In this series of patients, tests results after the procedure showed a fall of Hb to levels between 0 and 0.9 g/dL. None of the patients presented severe bleeding, nor required blood transfusion.
Moreover, resorbable materials such as Spongostan® and Gelfoam® were used in this procedure, with a 3 - 15 days duration of action, which usually allows future pregnancies.
In summary, prophylactic uterine artery embolization prior to induced abortions with placenta previa is an effective technique to reduce maternal blood loss, and has been added to the protocol for those abortions.
Acknowledgments
None to declare.
Financial Disclosure
The authors do not have any financial interest to declare.
Conflict of Interest
None to declare.
Informed Consent
All of the patients gave their consents for publication.
Author Contributions
P. Perez-Moneo, N. Ruiz and R. Balanza researched literature and conceived the study. P. Perez-Moneo, B. Aparicio and J. Gomez were involved in protocol development, patient recruitment and medical records review. P. Perez-Moneo wrote the first draft of the manuscript. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Llusia JB. La placenta. Fisiologia y patologia. Ediciones Diaz de Santos. 1992.
- Cabro L, Saldivar D, Cabrillo E. Obstetricia y Medicina Materno-Fetal. Ed. Panamericana. Cap. 77.
- Protocolo SEGO. Muerte fetal anteparto, Junio 2008. Available from: http://www.prosego.com/categoria-guia-asistencia/medicina-perinatal/page/5/.
- Matsuzaki S, Matsuzaki S, Ueda Y, Tanaka Y, Kakuda M, Kanagawa T, Kimura T. A case report and literature review of midtrimester termination of pregnancy complicated by placenta previa and placenta accreta. AJP Rep. 2015;5(1):e6-e11.
- Thomas AG, Alvarez M, Friedman F, Jr., Brodman ML, Kim J, Lockwood C. The effect of placenta previa on blood loss in second-trimester pregnancy termination. Obstet Gynecol. 1994;84(1):58-60.
- Huang L, Awale R, Tang H, Zeng Z, Li F, Chen Y. Uterine artery embolization, not cesarean section, as an option for termination of pregnancy in placenta previa. Taiwan J Obstet Gynecol. 2015;54(2):191-193.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
