| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 1, Number 4-5, October 2012, pages 71-75
Value of Serum β-hCG in Pathogenesis of Pre-Eclampsia
Kanika Mandi Choudhurya, Munmun Dasb, Sulekha Ghosh (Sarkar)c, Debasis Bhattacharyad, Tapan Kumar Ghoshe, f
aDepartment of Biochemistry, Burdwan Medical college, Burdwan, India & Govt of West Bengal,WBMES, Kolkata, India
bDepartment of Microbiology, Burdwan Medical College, Burdwan, India
cDepartment of Pathology, Calcutta National Medical College, Kolkata, India
dSagar Dutta Medical College, Panihati, India
eDepartment of Pathology, Burdwan Medical College, Burdwan, India
fCorresponding author: Tapan Kumar Ghosh, 40, Kotalhat, Burdwan-2, Burdwan, West Bengal, India
Manuscript accepted for publication October 4, 2012
Short title: Value of Serum β-hCG
doi: https://doi.org/10.4021/jcgo57w
| Abstract | ▴Top |
Background: Pre-eclampsia is a pregnancy specific disorder responsible for maternal and foetal morbidity and mortality. The aim of the present study was to find out the association of serum level of maternal β-hCG in normal pregnancy and pre-eclampsia and its role in development of pre-eclampsia.
Methods: The prospective randomized controlled study was conducted on 50 cases of pre-eclampsia with singleton foetus and 50 normotensive singleton mothers in the third trimester of pregnancy. The paired samples of serum samples were estimated for β-hCG and results were analyzed statistically using SPSS 17.
Result: The serum level of maternal β-hCG was markedly raised in preeclampsia (18,087.42 ± 2,014.17 mIU/mL) in comparison to controlled (8,391.06 ± 1,909.64 mIU/mL) and parallel with the severity of pre-eclampsia.
Conclusion: The maternal serum level of β-hCG plays one of the important role in pathogenesis of pre-eclampsia and its severity.
Keywords: Pre-eclampsia; β-hCG
| Introduction | ▴Top |
Pre-eclampsia is a pregnancy specific disorder characterized by newly onset of blood pressure more than 140/90 mmHg ,in at least two consecutive occasion and proteinuria (> 300 mg per 24 hours collection) in third trimester of pregnancy [1].
It is responsible 25% of all fetal growth retardation and 15% preterm birth in developed countries [2]. The incidence of pre-eclampsia in India is about 8-10 % [3] and maternal mortality due to be reported 8% [4]. Pre-eclampsia is common below 25 years of age [5]. The human chorionic gonadotropin (hCG) is a glycoprotein composed of two non covalently linked subunits, α and β, and is produced by syncytiotrophoblast cells of the placenta. Maternal serum hCG peaks at 8 - 10 wk of gestation and then declines to reach a plateau at 18 - 20 wk of gestation. The free β-subunit can derive from three sources, namely, direct trophoblast cell production, dissociation of hCG into free α- and free β-subunits, and by macrophage or neutrophil enzymes nicking the hCG molecule [6]. The free β-hCG circulating in maternal serum corresponds to only about 0.3-4% of the total hCG [7, 8].
In pre-eclampsia histological examination reveal focal cellular necrosis in the syncytiotrophoblast and increased mitotic activity with cellular proliferation in the cytotrophoblast [9]. In addition the proliferating trophoblast in severe preeclampsia is rapidly transformed into syncytiotrophoblast within 72 hours. The normal placenta differentiates during pregnancy with the cytotrophoblast dominant in early gestation and the syncytotrophoblast dominant in late pregnancy. Placental vascular damage leading to decreased oxygen supply might result in increased hCG production by hyperplasic cytotrophoblastic cells [10].
There is a strict relationship between PIH and elevated serum β-HCG levels, indicating that there should be an abnormal placental secretary function in patients with severe pre eclampsia. The aim of this present study was to find out the role of β-hCG in pathogenesis of pre-eclampsia and its association with severity of pre-eclampsia.
| Material and Methods | ▴Top |
The present study was conducted at the Department of Obstetrics and gynecology, Department of Pathology and Department of Microbiology of Burdwan Medical College, Burdwan after taking approval from ethical committee from 2007 to December 2010. The prospective randomized study was conducted on 50 healthy pregnant mothers(Group-A) between 28 - 36 weeks of without any cardiovascular illness, chronic hypertension, symptomatic infectious disease, periodontitis, obesity, premature rupture of membrane, clinical chorioamniotitis, mothers taking corticosteroid < 7 days and in labour, 50 preeclampsia mothers between 28 -36 weeks of gestation were included in this study (Group-B). Out of these 50 preeclampsia mothers 20 were diagnosed as severe preeclampsia and 30 were mild preeclampsia. Three mothers developed eclampsia during later stage of pregnancy. The mothers were diagnosed as pre-eclampsia when the systolic blood pressure were persistently ≥ 140 mmHg and diastolic blood pressure ≥ 90 mmHg ,on two occasion each 6 hours apart ,accompanied by proteinuria at least 1+ on dip stick testing at third trimester of gestation. Severe preeclampsia was considered having blood pressure ≥ 160/110 mmHg and proteinuria at least 3+ on dip stick. Eclampsia diagnosed when the PIH mothers developed convulsion. The serum β-hCG was measured between 28 - 36 weeks of gestation and after 72 hours of delivery both normal and pre-eclampsia mothers by quantitative determination of hCG by OptiCoat ™ by Sandwich enzyme immunoassay (Biotecx Laboratories INC, USA).
All the data were analyzed in SPSS version17.
| Result | ▴Top |
The clinical parameters and serum β-hCG level of enrolled mothers were tabulated and compared in both groups (Control n = 50, Pre-eclampsia n = 50) (Table 1). No significant correlation of ages was observed among both groups. The systolic and diastolic blood pressure of both groups were 115.60 ± 7.93 mIU/mL vs161.68 ± 18.25 mIU/mL (P < 0.001) and 73.08 ± 6.77 mIU/mL vs103.20 ± 9.57 mIU/mL (P < 0.001) respectively. High level of serum β-hCG was noted in preeclampsia group B mothers (18,087.42 ± 2,014.71 mIU/mL) than control group A mothers (8,391.06 ± 1,909.64 mIU/mL) (P < 0.001) during pregnancy where no significant changes were observed after delivery in both groups (3,491.12 ± 382.63 mIU/mL vs 3,377.06 ± 382.63 mIU/mL, P < 0.057) (Table 1).
 Click to view | Table 1. Clinical/Parameters of Both Control and PIH |
Out of 50 pre-eclampsia mothers 30 were mild pre-eclampsia and 20 were severe pre-eclampsia. The clinical results of mild and severe pre-eclampsia mothers were compared (Table 2).The level of Serum β-hCG markedly raised in severe preeclampsia (19,793.40 ± 950 mIU/mL) mothers than mild preeclampsia mothers (16,950.00 ± 1,709.22 mIU/mL) (P < 0.001) (Table 2, Fig. 1).
 Click to view | Table 2. Clinical/Biochemical’s Parameters of Both Mild PIH and Severe PIH Mothers |
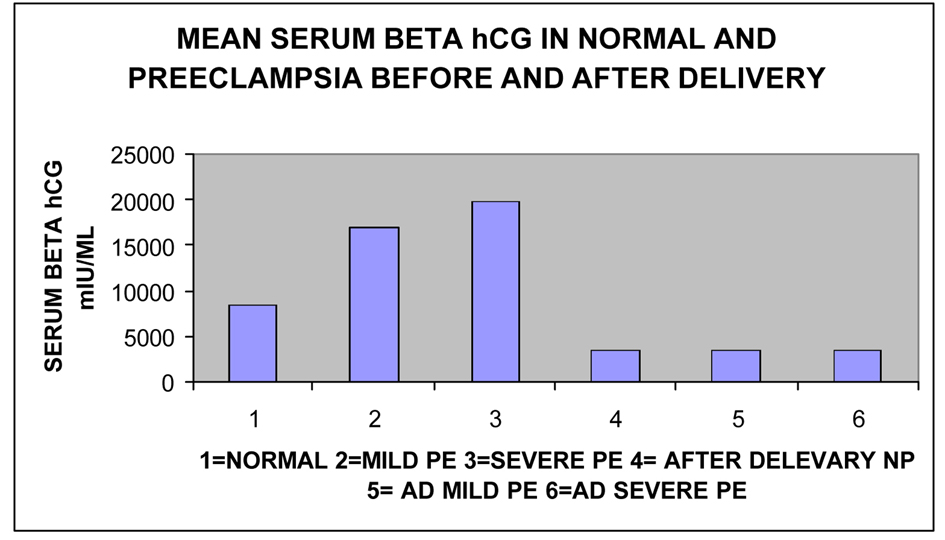 Click for large image | Figure 1. Showing mean Serum β-hCG in normal pregnant mother, Mild and Severe Pre-eclampsia before and after delivery. |
| Discussion | ▴Top |
In pre-eclampsia the rise of blood pressure is due to vasoconstriction and impaired angiogenesis leading to hypoxia and hyperplasia of trophoblastic cells which causes hyper secretion of placental hormone ultimately leading to high level of circulating β-hCG (Fig. 2).
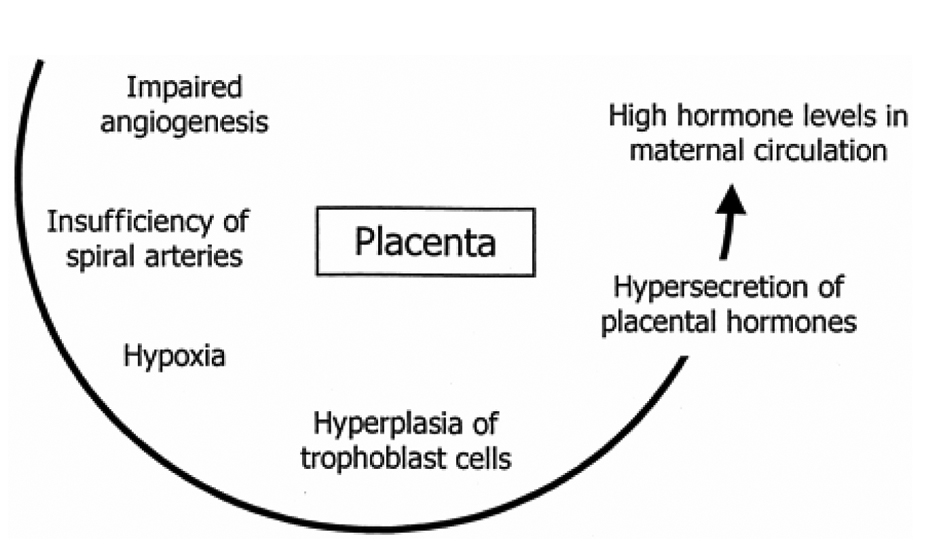 Click for large image | Figure 2. Showing mechanism of rise of hCG. |
Human chorionic gonadotropin, a glycoprotein hormone is produced in excess by normal and neoplastic trophoblastic conditions like twin and molar pregnancies. High-level of circulating β-hCG are found in pre-eclampsia. As pre-eclampsia is probably a trophoblastic disorder, elevated β-hCG is thought to reflect early placental damage or dysfunction. Therefore, the study of pathologic changes and secretary reaction of the placenta may prove essential for understanding this disease. There is general agreement that the placenta remains the main source of hCG in patients with pre-eclampsia, whether the cause of the high circulating levels of the hormone by placenta is still debated. Some advocate that hCG secretion may be increased as a consequence of abnormal placental invasion or placental immaturity. It may also be linked to the trophoblast response to hypoxia with the development of a hyper secretary state compared with normal pregnancies. It is well known that the cytotrophoblast is an undifferentiated stem cell, predominantly found in late trimester of pregnancy. The syncytotrophoblast is a differentiated trophoblast found in early gestational period transformed from the cytotrophoblast. Although the mechanism of regulation of gestational hCG remains largely unknown, it is generally accepted that hCG, are only secreted by syncytotrophoblasts. In pre eclampsia the cytotrophoblast transformed into syncytottrophoblast. Human placenta synthesizes steroid, protein, and glycoprotein hormones throughout gestation [11]. The production of hCG by the placenta in early pregnancy is critical for implantation and maintenance of the blastocyst. Since it is postulated that preeclampsia is likely a trophoblastic disorder [12].
In the present study like all other investigators the serum hCG level was found significantly increased in pre-eclampsia than control mothers (P ≤ 0.0001) with remarkably raised in severe preeclampsia than mild (P ≤ 0.0001) and no significant serum level were found after delivery.
So to understand the disease, it may be essential to investigate the pathologic and secretary reaction of the placenta. Twin pregnancies [13] and molar pregnancies [14] produce higher levels of hCG and they are associated with a higher incidence of preeclampsia than uncomplicated singleton pregnancies. An association was reported between preeclampsia and elevated third trimester hCG levels [15], whereas early experience with second trimester levels suggests a link between increased hCG and other adverse pregnancy outcomes [16, 17].
Considerable evidence suggests that there is an association between serum hCG levels and pre-clampsia.
Remzi Gokdeniz et al found a strict relationship between severe pre-eclampsia and elevated serum β-hCG levels, indicating that there should be an abnormal placental secretary function in patients with severe pre-eclampsia [18].
Conclusion
Hyper secretion of human chorionic gonadotropin hormone by placenta reflecting high level of serum circulating β-hCG level in pre-eclampsia disorder and its severity. So in pre-eclampsia a trophoblastic disease association with the circulating β-hCG may have a pathogenic role.
| References | ▴Top |
- Helske S, Vuorela P, Carpen O, Hornig C, Weich H, Halmesmaki E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol Hum Reprod. 2001;7(2):205-210.
doi pubmed - Powers RW, Bodnar LM, Ness RB, Cooper KM, Gallaher MJ, Frank MP, Daftary AR, et al. Uric acid concentrations in early pregnancy among preeclamptic women with gestational hyperuricemia at delivery. Am J Obstet Gynecol. 2006;194(1):160.
doi pubmed - Krishna Menon MK and Palaniappam B. Hypertensive disorder of pregnancy. In Mudaliar Menon (ed) Clinical Obstetrics. 9th edn. Orient Longman 1994; 133-154.
- Jain K, Kavi V, Raghuveer CV, Sinha R. Placental pathology in pregnancy-induced hypertension (PIH) with or without intrauterine growth retardation. Indian J Pathol Microbiol. 2007;50(3):533-537.
pubmed - Shruti S Dubnashi, Wani RJ, Priti Chikhal, Hegde CV. PIH – Confounding Situations, Management Dilemmas andSevere Consequences : Does Antenatal Care have a role. Bombay Hospital Journal; vol 50, 2008; 45-49.
- Cole LA, Kardana A, Park SY, Braunstein GD. The deactivation of hCG by nicking and dissociation. J Clin Endocrinol Metab. 1993;76(3):704-710.
doi pubmed - Spencer K. Evaluation of an assay of the free beta-subunit of choriogonadotropin and its potential value in screening for Down's syndrome. Clin Chem. 1991;37(6):809-814.
pubmed - Fuhrmann W, Altland K, Jovanovic V, Holzgreve W, Miny P, Wenger D, Rauskolb R. First-trimester alpha-fetoprotein screening for Down syndrome. Prenat Diagn. 1993;13(3):215-218.
doi pubmed - Jones CJ, Fox H. An ultrastructural and ultrahistochemical study of the human placenta in maternal pre-eclampsia. Placenta. 1980;1(1):61-76.
doi - Majumdar s, Dasgupta H, Bhattacharya K, Bhattacharya A. A study of Placenta In Normal and Hypertensive Pregnancies. J Ant Soc India. 2005; 54(2):1-9.
- Shima DT, Mailhos C. Vascular developmental biology: getting nervous. Curr Opin Genet Dev. 2000;10(5):536-542.
doi - Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114(Pt 5):853-865.
pubmed - Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90(22):10705-10709.
doi - Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker RT. Hydatidiform mole: diagnosis, management, and long-term followup of 347 patients. Obstet Gynecol. 1975;45(1):1-8.
pubmed - Hsu CD, Chan DW, Iriye B, Johnson TR, Hong SF, Repke JT. Elevated serum human chorionic gonadotropin as evidence of secretory response in severe preeclampsia. Am J Obstet Gynecol. 1994;170(4):1135-1138.
pubmed - Wenstrom KD, Owen J, Boots LR, DuBard MB. Elevated second-trimester human chorionic gonadotropin levels in association with poor pregnancy outcome. Am J Obstet Gynecol. 1994;171(4):1038-1041.
pubmed - Onderoglu LS, Kabukcu A. Elevated second trimester human chorionic gonadotropin level associated with adverse pregnancy outcome. Int J Gynaecol Obstet. 1997;56(3):245-249.
doi - Remzi G, Erdal A, Nursel B, Ozcan B. Elevated Serum -hCG Levels in Severe Preeclampsia. Turk J Med Sci. 2000; 43–45.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
