| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Review
Volume 8, Number 3, September 2019, pages 63-69
Prenatal Probiotics: The Way Forward in Prevention of Preterm Birth
Karukkupalayam Ramasamy Dhanasekara, Bennur Shilpaa, c, Nachimuthu Gomathyb, Shankar Kundavib
aDepartment of Medical & Scientific Affairs, Tablets India Limited, Chennai, Tamil Nadu, India
bInstitute of Reproductive Medicine & Women’s Health, The Madras Medical Mission, Chennai, Tamil Nadu, India
cCorresponding Author: Bennur Shilpa, Department of Medical & Scientific Affairs, Tablets India Limited, Jhaver Centre, 72 Marshalls Road, Egmore, Chennai, Tamil Nadu 600 008, India
Manuscript submitted July 11, 2019, accepted August 22, 2019
Short title: Prenatal Probiotics
doi: https://doi.org/10.14740/jcgo571
- Abstract
- Introduction
- Vaginal Microbiota in Reproductive Years and Pregnancy
- BV and Preterm Delivery
- Probiotics in PTB
- Choice of Lactobacilli Strain
- Discussion
- Conclusions
- References
| Abstract | ▴Top |
Preterm birth (PTB) has presented a major challenge since decades among the obstetricians. Many premature born individuals have learning disabilities, visual and hearing problems. Abnormal vaginal microbiota and bacterial vaginosis (BV) are important risk factors for PTB and premature rupture of the membranes. In women with BV, there is a dramatic reduction of Lactobacillus and heavy colonization of the pathogenic bacteria. Administration of Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 in pregnant women restores the normal vaginal flora and acidic pH and interrupts the infectious/inflammatory process. Probiotics are preferred over tocolytic therapy to reduce the adverse maternal and fetal outcome.
Keywords: Preterm birth; Bacterial vaginosis; Lactobacillus rhamnosus GR1; Lactobacillus reuteri RC14
| Introduction | ▴Top |
Preterm birth (PTB) is one of the major challenges in modern obstetrics, and it is the leading cause of perinatal mortality, pediatric morbidity and disability [1]. An estimated 15 million babies are born prematurely. Many survivors have lifetime disability, including learning disabilities and visual and hearing problems [2]. Although the etiology of PTB is multifactorial, infection or inflammation contributes to up to 30% of PTBs [3]. A meta-analysis involving 20,232 women has shown that the risk of PTB doubles in women with bacterial vaginosis (BV) at less than 37 weeks of gestational age [4]. Therefore, prevention of BV certainly is expected to reduce the incidence of PTB. In clinical practice, if PTB is encountered, the decision to prolong the gestation is taken if the benefit of tocolysis outweighs the risk associated with it. However, the onset of labor can only be delayed up to 72 h using tocolytic therapy. In addition, the routinely used tocolytics, such as β sympathomimetics, magnesium sulfate, and calcium channel blockers, are associated with adverse effects like arrhythmias, tachycardia and myocardial infarction and premature closure of ductus arteriosus and intraventricular hemorrhage in fetus [5]. Newer tocolytic, such as atosiban (oxytocin inhibitor), is known to have rapid onset of action. Although it delays labor by 48 h, it has adverse effects like hypotension, chest pain, and palpitations. Also the cost of atosiban precludes its use in developing countries [6]. In a recent meta-analysis, although tocolytics caused delay in delivery compared with placebo, tocolytics did have maternal side effects without any clinically significant improvement in perinatal outcome [7]. Therefore, the newer strategy should aim at eliminating the etiological factors that cause PTB. Naturally, the top in the list will be a counter strategy against BV and prevention of recurrence. In this review article, we will discuss the usage of probiotic bacteria as an adjuvant in the management of BV and its clinical implication in prevention of PTB. The objective of this review article is to review the available scientific literature from the relevant clinical trials that assessed the efficacy and safety of probiotics in pregnant women with urogenital infection.
| Vaginal Microbiota in Reproductive Years and Pregnancy | ▴Top |
The composition of normal vaginal microbiota is complex and dynamic. Lactobacilli form the integral part of the vaginal microbiota. The healthy human vagina is dominated by Lactobacilli during reproductive years and pregnancy due to rising estrogen levels [8, 9]. They help to maintain the pH of the vaginal tract and provide effective immunity to the host by producing lactic acid and hydrogen peroxide. Women who have hydrogen-peroxide-producing strains of Lactobacilli have 4% prevalence rate of BV compared with 32% in women colonized by non-hydrogen-peroxide-producing strains and 56% in those without Lactobacilli [9]. Romero et al studied the composition and stability of the vaginal microbiota of normal pregnant women and found that the pathogens such as Prevotella, Sneathia, and Gardnerella which are associated with BV were rarely found in women who delivered at term. Vaginal microbiome was more stable in pregnant women compared to non-pregnant women and there was only shift from one Lactobacillus spp to another Lactobacillus spp in pregnancy [10]. Therefore for the best outcome during pregnancy, normal vaginal microbiome has to be maintained with predominant Lactobacilli.
| BV and Preterm Delivery | ▴Top |
BV is a polymicrobial dysbiosis that is characterized by an alteration in the endogenous vaginal microbiome. BV is characterized by a reduction of beneficial Lactobacilli and heavy colonization of anerobic bacteria, including Gardnerella vaginalis, Atopobium vaginae, Mobiluncus spp, Bacteroides spp and Prevotella spp [11]. These pathogenic bacteria in BV cause localized inflammation in the endometrium and make the intrauterine environment incompatible for embryo implantation and placental development. As mentioned earlier, BV is an important and independent risk factor for PTB [12]. Pathogens ascending through vagina and cervix to uterus release bacterial proteolytic enzymes like phopholipase A2 and collagenase. This initiates the formation of arachidonic acid and increases the level of prostaglandins. Elevated prostaglandin levels induce collagen remodelling in the fetal membranes. This will eventually lead to uterine contraction and preterm labor [13] (Fig. 1).
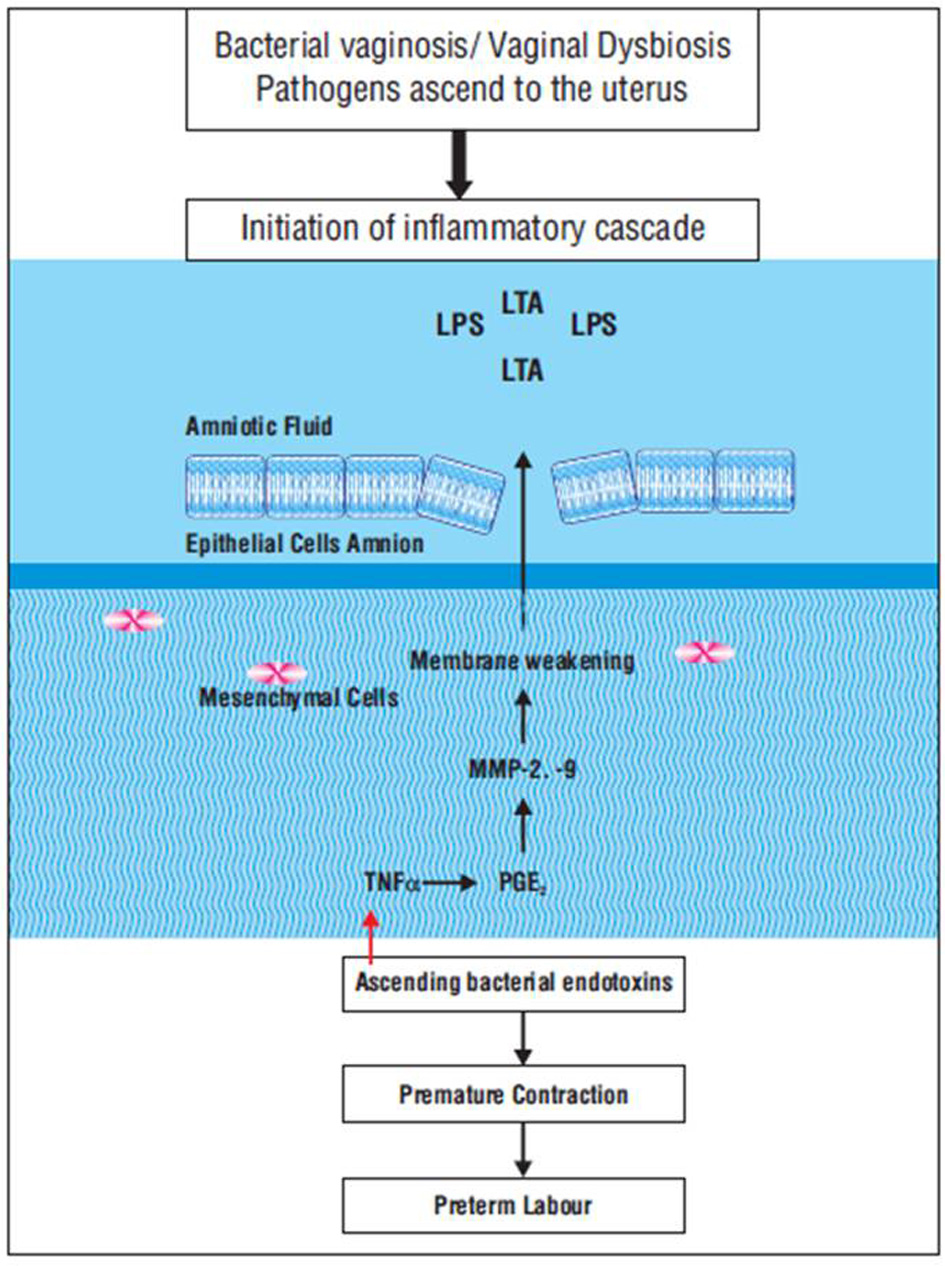 Click for large image | Figure 1. Pathogenesis of preterm birth. In bacterial vaginosis, pathogens ascending through vagina and cervix to uterus release bacterial proteolytic enzymes like phopholipase A2 and collagenase. This initiates the formation of arachidonic acid and increases the level of prostaglandins. Elevated prostaglandin levels induce collagen remodelling in the fetal membranes. This will eventually lead to uterine contraction and preterm labor. |
Another mechanism by which BV increases the risk of PTB is through endotoxins secreted by pathogens, such as lipopolysaccharides (LPSs). These endotoxins specifically bind to toll-like receptor 4 (TLR4) and activate the nuclear factor k light-chain-enhancer of activated B cells (NFkB) pathway to induce pro-inflammatory cytokine and chemokine in the intrauterine tissues. Pro-inflammatory cytokines like tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) as well as IL-6 amplify the inflammatory response [14, 15]. They also stimulate prostaglandin production in the amnion. Prostaglandins which normally initiate the onset of parturition promote premature labor triggered by an infection [16] (Fig. 1).
Once BV is diagnosed in pregnancy, antibiotics remain the treatment of choice, which is unchanged for many decades, and most of the times ineffective in preventing relapse. Metronidazole and clindamycin are the most commonly used antibiotics. Although they are effective in treating an episode of BV, they do not restore the endogenous Lactobacilli that can lead to relapse. Notably, prolonged uses of antibiotics promote the development of drug resistance [17], and, in some cases have increased the incidence of PTB [18]. Therefore in this era of multidrug-resistant bacteria, it is important to look for an alternative and effective method for the prevention of PTB.
| Probiotics in PTB | ▴Top |
Probiotics, particularly Lactobacilli, play a beneficial role in maintenance of healthy urinary and reproductive tracts. A number of clinical trials with probiotics have confirmed that probiotics are both safe and effective for the treatment and/or prevention of numerous infectious and/or inflammatory diseases in both pregnant and non-pregnant women. They also reduce the recurrence of BV by increasing the colonization of Lactobacilli in the vagina.
In a study by Bodean et al in 2013, it was reported that oral administration of Lactobacilli was found to be more effective in treating BV than vaginal route [19]. Orally 109 - 1010 colony forming unit (CFU) is the standard dose believed to be required for passage through the intestine and to reach vagina and displace pathogens. Vaginal suppository containing 109 CFU Lactobacilli has to be administered once weekly to treat and prevent urogenital tract infections [20]. After administration, colonization of Lactobacilli in the vagina is influenced by a number of factors like glycogen levels, use of antibiotics, ability of Lactobacilli to produce hydrogen per oxide and substances used for vaginal douching.
| Choice of Lactobacilli Strain | ▴Top |
The effect of Lactobacilli on the immune system and their vaginal colonization ability are species- and strain-specific. Among many strains of Lactobacilli, Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 are found to have excellent colonizing capability and are the preferred Lactobacilli strain for the treatment of urogenital tract infections. Whereas some strains like Lactobacillus rhamnosus GG and Lactobacillus acidophilus are not well suited to colonizing the vagina and also they do not produce H2O2, explaining why both these strains failed to prevent recurrence of urogenital infections. The strains Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 are granted “Qualified Presumption of Safety” status by the European Food and Safety Authority. They persist up to 19 days in the human vagina following intravaginal administration. Both the strains survive at low pH [21]. They are highly adherent to uroepithelial and vaginal cells and thus prevent the adhesion of pathogenic bacteria. Both the strains are integral part of female genital tract and they are able to colonize in the vagina when administered orally. It may also help to prevent viruses, such as HIV, from infecting the host. The anti-inflammatory property of Lactobacilli is important in control of mucosal and systemic inflammation [22].
Lactobacilli produce bacteriocins, collagen binding proteins and hydrogen per oxide, which are antagonistic to the endotoxins produced by the pathogenic bacteria and thereby inhibit growth and adhesion of urogenital pathogens [23]. Lactobacilli maintain the vaginal pH < 4.5 by metabolizing glycogen secreted by vaginal mucosal epithelia and producing lactic acid, which is a potent microbicide against potential urogenital pathogens [24]. Lactobacillus rhamnosus GR1 enhances IL-10 and colony stimulating factor 3 (CSF3) productions in mouse macrophages. In primary human placental trophoblast cells, GR1 increases IL-10 and CSF3 production via Janus kinase/signal transducers and activators of transcription (JAK/STAT) and mitogen-activated protein kinase (MAPK) pathways, and down-regulates LPS-induced TNFα output through c-Jun-N-terminal kinases (JNKs) inhibition (Fig. 2).
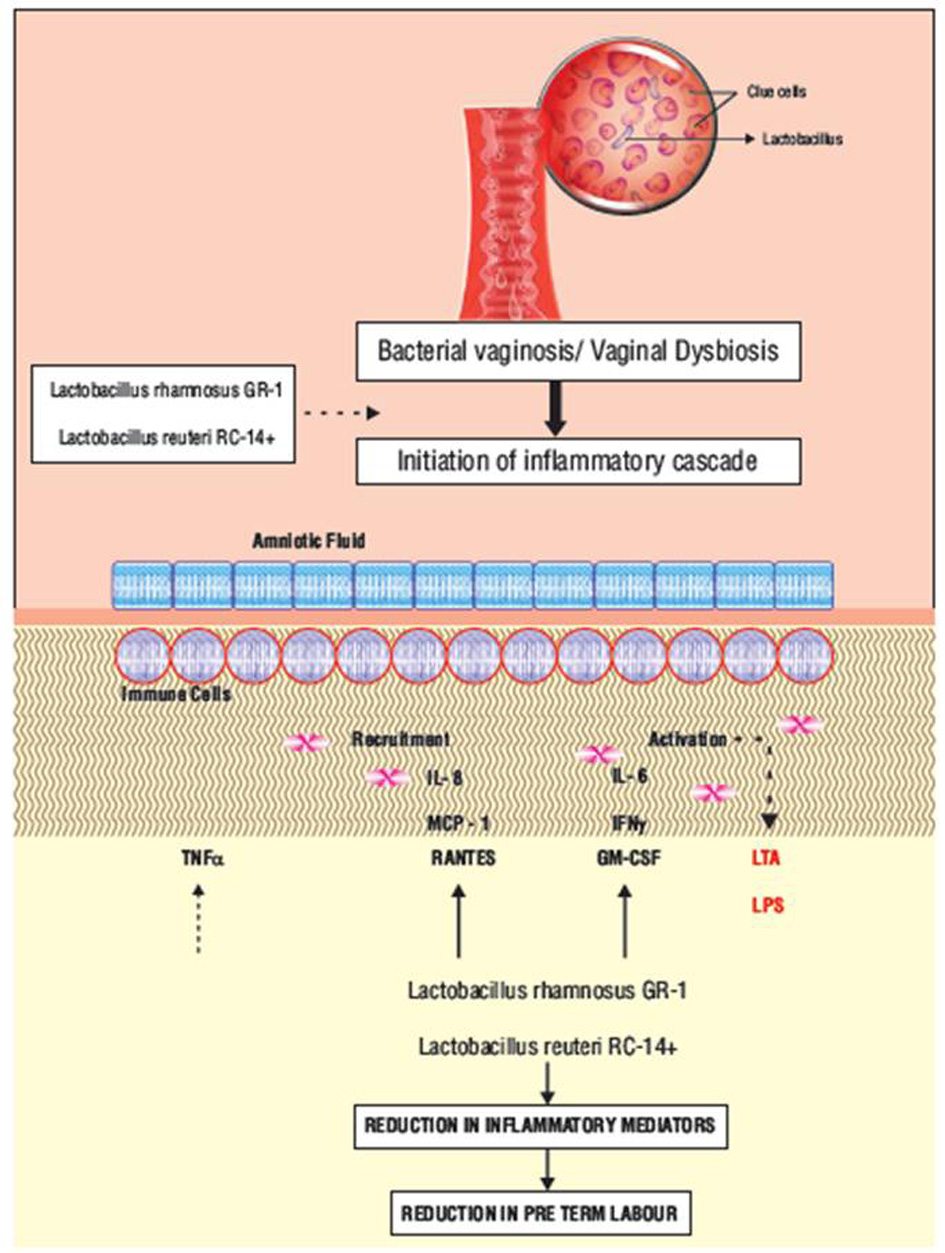 Click for large image | Figure 2. Mechanism of action of Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14+ in preventing preterm birth. Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14+ enhance IL-10 and CSF3 production in human placental cells via JAK/STAT and MAPK pathways, and down-regulate LPS-induced TNFα output through JNK inhibition. Lactobacilli also increase the expression of prostaglandin-metabolizing enzymes and reduce prostaglandin levels therefore preventing preterm birth. CSF3: colony stimulating factor 3; JNK: c-Jun-N-terminal kinase; IL: interleukin; JAK/STAT: Janus kinase/signal transducers and activators of transcription; MAPK: mitogen-activated protein kinase; LPS: lipopolysaccharide; TNFα: tumor necrosis factor α. |
Lactobacillus species have an immunomodulatory role in monocytic cell lines and human placental trophoblasts which promote an anti-inflammatory cytokine profile and increase chemokine production and therefore block the inflammatory cascade initiated by the pathogens in urogenital infections. Proinflammatory cytokines produced by pathogen also stimulate prostaglandin production in the amnion. This is blocked by Lactobacillus species that increase the expression of prostaglandin-metabolizing enzymes and reduce prostaglandin levels [25]. Lactobacilli with the above possible mechanism help in preventing PTB by restoring the vaginal microbiota and by exerting antipathogenic properties (Fig. 2).
| Discussion | ▴Top |
A number of studies have proved Lactobacilli to have beneficial perinatal outcome on prenatal administration. Donders et al’s study published in British Journal of Obstetrics and Gynecology evaluated 759 pregnant women in their first trimester. Results of this study showed that only 1.35% of women with normal vaginal flora had a severe PTB whereas 8.5% of women with abnormal vaginal flora and 4.6% of women with BV had severe PTB (25 to 34 weeks). This study concluded that women with BV and abnormal vaginal flora are at a risk of preterm delivery and late miscarriage [26]. Similar observations were found in other studies which demonstrated a statistically significant association between an abnormal genital tract flora and adverse pregnancy outcome. Riduan et al showed that the detection of abnormal flora in early pregnancy has a greater risk of PTB [27]. Another study by Helen et al evaluated 996 pregnant women and found that women with an increased risk for PTB have two types of abnormal vaginal flora, one consisting of predominantly BV flora, and the other of aerobic microorganisms, such as Klebsiella and Escherichia coli [28]. These studies were well designed with a large sample size and the results prove significant relationship between abnormal vaginal flora and its associated complications in pregnancy [26]. Therefore newer treatment strategy should be aimed at restoring the abnormal vaginal flora to prevent PTB.
In a study conducted by Krauss-Silva et al, pregnant women attending public prenatal care services less than 20 weeks’ gestation with no indication of elective preterm delivery were randomized to receive either placebo or probiotics (Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14), two capsules a day, each capsule containing more than one million bacilli of each strain, for 6 - 12 weeks, up to the 24th - 25th week of gestation. Rate of spontaneous preterm deliveries (sPTDs) < 34 weeks was estimated to be 6% in the placebo group and close to 3% in the intervention group (i.e. efficacy of probiotics in reduction of preterm labor was found to be 50%). Lactobacilli induce apoptosis of pathogenic bacteria by competing against them to get nutrients (growth antagonism), vaginal niche colonization and maintaining vaginal pH by producing lactic acid and H2O2 [29]. This study was well designed and the result of this study has been supported by Simhan et al, which showed that a woman with pH ≥ 5 was significantly associated with spontaneous PTB. Therefore it is imperative that the intervention for BV with probiotics should be as early as less than 20 weeks [30]. If probiotics are started earlier, there is possibility to have a clinically meaningful reduction of risk of preterm delivery by 50%.
In another study to examine the effect of probiotic and pregnancy outcome, Myhre et al included 18,888 pregnancies from a nationwide cohort. It was conducted at the Norwegian Institute of Public Health on the basis of answers to a food-frequency questionnaire. Results of this study showed that among 18,888 pregnancies, there were 950 cases of sPTD which was mostly seen in women who did not consume probiotics (70.2%) compared to women who had consumed probiotics (20.8%). This study concluded that intake of probiotics might be associated with reduced risk of PTB. Results fit to the general hypothesis that probiotics function as a “rescue mechanism” by lowering overall inflammation in combination with providing a healthy vaginal microbiological environment. Successive reduction in sPTD may be achieved by targeting dietary health issues and evaluating intake of probiotics, with consideration of nutritional interventions early in pregnancy or pre-pregnancy. This prospective cohort study with large sample size has shown the dose-dependent effect of probiotics in reducing the risk of PTB. The results of this study implied that high intake of probiotics will have better effect than low intake [31].
Kaplas et al conducted a small double-blind, randomized controlled trial in pregnant women to study the effects of probiotic supplementation on placental phospholipid fatty acids. Fatty acids which are incorporated into phospholipids will be utilized in the metabolism of intrinsic placental fatty acids to synthesize either longer-chain fatty acid derivatives or inflammatory modulators. Importantly, they also serve as a reservoir to satisfy fetal demands for growth and development [32]. Dietary counselling with probiotics (group 1) resulted in higher concentrations of linoleic (18:2n-6) and dihomo-c linolenic acids (20:3n-6) compared with dietary counselling with placebo (group 2) or controls (group 3). The pregnancies were uncomplicated and the infants were delivered at term; there were no reports of malformations (major or minor), miscarriages, low birth weight, or preterm delivery [33]. Although studies in this regard are less, a study by Kankaanpaa et al has shown that probiotic supplementation has resulted in changes in the fatty acid composition of infants’ serum phospholipids. The results of these studies show that the intake of probiotics can modify the content and composition of placental phospholipid fatty acids [34]. Hence probiotic administration in pregnancy has dual health benefit for both the mother and the fetus. Selvaraj K and Selvaraj P evaluated the benefits of probiotics in cases of bad obstetric history (BOH) and for prevention of post-in vitro fertilization (IVF) pregnancy complications. In this study, 70 pregnant women in the study group were treated with probiotics (i.e. Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14) along with treatment for infertility, and were compared with similar number of 70 cases from control group who were not given probiotics but were treated for infertility earlier. In study group, 56 cases (80%) had successful delivery and 14 cases (20%) ended in fetal loss. Whereas in the control group 44 cases (63%) delivered, and 26 cases (37%) experienced fetal loss. This study proves that probiotics used in selected cases brings back the normal vaginal epithelium thereby increasing the resistance to pathogenic bacteria and preventing fetal loss especially in women with BOH and who is on treatment for infertility [35].
An open-labelled randomized trial conducted by Neri et al consisted of 84 pregnant women with BV in their first trimester. At fourth and eighth weeks post treatment, the probiotic group had a significant reduction of BV in comparison to both the acetic acid and control groups. The authors concluded that the continuous correction of both the vaginal pH and Lactobacillus spp flora was crucial for normal vaginal ecology, and was responsible for the high treatment response rate. During pregnancy, a local treatment that restores the vaginal flora without any systemic effects is more preferable to any other treatment [36]. Similar results were also seen in the study by Reid et al where Lactobacilli-dominant microbiota was restored in subjects with BV but not in controls, following 2 months of daily oral intake of Lactobacillus rhamnosus GR1 and Lactobacillus fermentum RC14 [37]. In vitro antagonistic effect of Lactobacillus on pathogens associated with BV and urogenital infections was seen in a study done by Strus et al [38].
Nishijima et al in their letter argued that the probiotics reduced the risk of genitourinary tract infections, more particularly BV by 81%. In a review by Parma et al, authors are of the opinion that probiotic supplementation with Lactobacillus proved to be crucial in hindering bacteria growth after antibiotic therapy; therefore this intervention can be considered as a new adjuvant treatment for preventing recurrence of BV, even in high-risk patients [39, 40]. Another study by Ya et al has shown that administration of vaginal probiotics after metronidazole therapy will increase the cure rate and reduce the number of patients suffering from BV recurrence [41]. Many other studies have also shown strong evidence between BV and PTB. Therefore it can be implied that reducing the risk of BV will also reduce the incidence of PTB.
An in vitro study done by Koscik et al focussed on the influence of Lactobacillus rhamnosus GR1 on proinflammatory cytokines and prostaglandins as they are activated during the cascade of infection or inflammation mediated preterm labor. Human amnion epithelial cells were treated with supernatant of cultured Lactobacillus rhamnosus GR1. Subsequent assay showed a decrease in proinflammatory cytokines and elevation of several chemokines and prostaglandin E2 by Lactobacillus rhamnosus GR1. This study concluded that administration of Lactobacillus GR1 is the most user friendly preventative therapy to prevent infection or inflammation mediated PTB. The major drawback of this in vitro study is that it may not be representative of in vivo study because the effects of Lactobacillus rhamnosus GR1 on cytokines and chemokines from intact membranes in vivo may not be accurately determined using the system of mixed amnion cell cultures used in this study. But this study will bring us a step closer in developing treatment for prevention of PTB and its complications [42].
A study was done by Yang et al in mice. The objective of this study was to assess the effect of Lactobacillus rhamnosus GR1 (GR1) and its supernatant (GR1 SN) on the prevention of LPS-induced PTB. Pregnant mice were pre-treated with intra-peritoneal injections of GR1 SN or oral GR1 live bacteria prior to intrauterine injection of LPS. Inflammatory markers like cytokines and chemokines in the maternal plasma, amniotic fluid and intrauterine tissues were measured. This doctoral thesis provided evidence that Lactobacillus rhamnosus GR1 SN is efficacious in reducing LPS-induced PTB and inflammation in pregnant mice [43].
A randomized double-blind, placebo-controlled trial was conducted by Rautava et al in pregnant women with allergic disease and atopic sensitization. They were randomly assigned to receive probiotics or placebo during last 2 months of pregnancy and initial 2 months of breast feeding. Infants were followed up to the age of 24 months. Eczema was seen in 71% of infants in placebo group and only 29% in probiotic group. Chronically persistent eczema was seen in 26% in placebo group and only 10% in probiotic group. This study has shown that supplementation of probiotics before and after pregnancy will safely reduce the risk of atopic disease in infants born to women with atopic disease [44]. Probiotics are safe to be administered in pregnancy. Similar results were also seen in a meta-analysis done by Doege et al, which showed a significant risk reduction for atopic eczema in children aged 2 - 7 years born to the mothers who had taken probiotics during pregnancy. Results were more significant for Lactobacilli [45]. Many studies have shown safety of probiotics in both mother and newborn when administered during pregnancy. A study conducted by Kopp et al assessed maternal and fetal safety of probiotics in pregnant women; Lactobacillus GG was given 4 - 6 weeks before expected delivery, followed by a post-natal period of 6 months. No significant difference in gestational age, birth weight, or method of delivery was found between the study group and the control group [17]. In another randomized trial by Huurre et al in 2008, pregnant women were exposed to Lactobacillus commencing from first trimester until the end of exclusive breast feeding. There were no malformations in the fetus, no significant differences in duration of gestation or incidences of cesarean sections. There were no adverse effects in breast-fed infants as probiotics are not systemically absorbed [46]. Nordqvist et al investigated the influence of timing of probiotics on the incidence of preeclampsia and preterm delivery. In the Norwegian Mother and Child Cohort Study 70,149 singleton pregnant mothers were included. Among the participants, 23.3% women consumed probiotic milk containing Lactobacilli before pregnancy, 37.6% of women during early pregnancy and 32.2% of women during late pregnancy. Probiotic milk intake in late pregnancy was significantly associated with lower preeclampsia risk (adjusted OR: 0.80 (95% CI 0.68 - 0.94), P value: 0.007). Probiotic intake during early pregnancy was significantly associated with lower risk of preterm delivery (adjusted OR: 0.79 (0.64 - 0.97), P value: 0.03). In this large cohort, the study group has observed that Lactobacilli consumption in early and late pregnancy reduced the incidence of PTB and preeclampsia respectively. Hence, in clinical practice it is prudent to administer a course of Lactobacilli before 20 weeks of pregnancy to prevent PTB and then administer once again in the third trimester to reduce the incidence of preeclampsia [47].
Lactobacilli can be administered both orally and intravaginally. In a study by Bodean et al 2013, it was reported that oral administration of Lactobacilli was found to be more effective in treating BV than vaginal route. In this study, patients who received oral probiotics had a very low recurrence rate of BV compared to patients who received vaginal probiotics and also a low follow-up rate, due to route of administration, which these women have found to be uncomfortable [19]. Advantages of oral route of administration of Lactobacilli are that it prevents the ascending pathogens to vagina from perineum and rectum, while the concern of the intravaginal approach may be the more invasive instillation of microbes. Also compliance is better with oral route when compared to vaginal route. No adverse effects are reported in any of the above clinical trials.
| Conclusions | ▴Top |
Prevention of PTB is better than administering tocolytic agents to prolong gestation. There is growing evidence for the usage of probiotic to prevent PTB. Lactobacilli play a potential protective role in maintaining vaginal health by acting against pathogens and also by maintaining vaginal pH. It is well known that vaginal infection is an important mechanism responsible for PTB. Probiotics containing Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 have the potential to reduce the recurrence of vaginal infections and therefore the incidence of PTB by 50%. The available data in medical literature shows that Lactobacillus rhamnosus GR1 and Lactobacillus reuteri RC14 are beneficial and safe for use in pregnancy to prevent PTB, if administered at or before 20 weeks of gestational age. Reduction of preeclampsia is an additional benefit, if the probiotic dose is repeated in the third trimester of pregnancy.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
DKR and SB contributed to the study concepts, design, definition of intellectual content, literature search, clinical studies, data acquisition and analysis, and manuscript preparation; GN contributed to definition of intellectual content, clinical studies, data analysis, manuscript editing and manuscript review; KS contributed to definition of intellectual content, literature search, manuscript editing and manuscript review.
| References | ▴Top |
- Report of a WHO Expert Committee. The prevention of perinatal mortality and morbidity. World Health Organ Tech Rep Ser. 1970;457:1-60.
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, et al. Global, regional, and national causes of under-5 mortality in 2000-15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027-3035.
doi - Goldenberg RL, Iams JD, Mercer BM, Meis P, Moawad A, Das A, Copper R, et al. What we have learned about the predictors of preterm birth. Semin Perinatol. 2003;27(3):185-193.
doi - Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189(1):139-147.
doi pubmed - Katz VL, Farmer RM. Controversies in tocolytic therapy. Clin Obstet Gynecol. 1999;42(4):802-819.
doi pubmed - Worldwide Atosiban versus Beta-agonists Study Group. Effectiveness and safety of the oxytocin antagonist atosiban versus beta-adrenergic agonists in the treatment of preterm labour. The Worldwide Atosiban versus Beta-agonists Study Group. BJOG. 2001;108(2):133-142.
doi - Gyetvai K, Hannah ME, Hodnett ED, Ohlsson A. Tocolytics for preterm labor: a systematic review. Obstet Gynecol. 1999;94(5 Pt 2):869-877.
doi pubmed - Othman M, Neilson JP, Alfirevic Z. Probiotics for preventing preterm labour. Cochrane Database Syst Rev. 2007;1:CD005941.
doi pubmed - Ugwumadu AH, Hay P. Bacterial vaginosis: sequelae and management. Curr Opin Infect Dis. 1999;12(1):53-59.
doi - Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Nikita L, Galuppi M, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4.
doi pubmed - Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, Poston L. A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study. BJOG. 2006;113(1):65-74.
doi pubmed - Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med. 1995;333(26):1737-1742.
doi pubmed - Lamont RF, Anthony F, Myatt L, Booth L, Furr PM, Taylor-Robinson D. Production of prostaglandin E2 by human amnion in vitro in response to addition of media conditioned by microorganisms associated with chorioamnionitis and preterm labor. Am J Obstet Gynecol. 1990;162(3):819-825.
doi - Shoji T, Yoshida S, Mitsunari M, Miyake N, Tsukihara S, Iwabe T, Harada T, et al. Involvement of p38 MAP kinase in lipopolysaccharide-induced production of pro- and anti-inflammatory cytokines and prostaglandin E(2) in human choriodecidua. J Reprod Immunol. 2007;75(2):82-90.
doi pubmed - Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16(2):206-215.
doi pubmed - Argiles JM, Carbo N, Lopez-Soriano FJ. TNF and pregnancy: the paradigm of a complex interaction. Cytokine Growth Factor Rev. 1997;8(3):181-188.
doi - Kopp MV, Goldstein M, Dietschek A, Sofke J, Heinzmann A, Urbanek R. Lactobacillus GG has in vitro effects on enhanced interleukin-10 and interferon-gamma release of mononuclear cells but no in vivo effects in supplemented mothers and their neonates. Clin Exp Allergy. 2008;38(4):602-610.
doi pubmed - Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, Bruce AW. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol Med Microbiol. 2003;35(2):131-134.
doi - Bodean O, Munteanu O, Cirstoiu C, Secara D, Cirstoiu M. Probiotics—a helpful additional therapy for bacterial vaginosis. J Med Life. 2013;6(4):434-436.
- Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16(4):658-672.
doi pubmed - Gardiner GE, Heinemann C, Bruce AW, Beuerman D, Reid G. Persistence of Lactobacillus fermentum RC-14 and Lactobacillus rhamnosus GR-1 but not L. rhamnosus GG in the human vagina as demonstrated by randomly amplified polymorphic DNA. Clin Diagn Lab Immunol. 2002;9(1):92-96.
doi pubmed - Petrova MI, Lievens E, Malik S, Imholz N, Lebeer S. Lactobacillus species as biomarkers and agents that can promote various aspects of vaginal health. Front Physiol. 2015;6:81.
doi pubmed - Reid G, Bocking A. The potential for probiotics to prevent bacterial vaginosis and preterm labor. Am J Obstet Gynecol. 2003;189(4):1202-1208.
doi - O'Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013;8(11):e80074.
doi pubmed - Kim SO, Sheikh HI, Ha SD, Martins A, Reid G. G-CSF-mediated inhibition of JNK is a key mechanism for Lactobacillus rhamnosus-induced suppression of TNF production in macrophages. Cell Microbiol. 2006;8(12):1958-1971.
doi pubmed - Donders GG, Van Calsteren K, Bellen G, Reybrouck R, Van den Bosch T, Riphagen I, Van Lierde S. Predictive value for preterm birth of abnormal vaginal flora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. BJOG. 2009;116(10):1315-1324.
doi pubmed - Riduan JM, Hillier SL, Utomo B, Wiknjosastro G, Linnan M, Kandun N. Bacterial vaginosis and prematurity in Indonesia: association in early and late pregnancy. Am J Obstet Gynecol. 1993;169(1):175-178.
doi - McDonald HM, O'Loughlin JA, Jolley P, Vigneswaran R, McDonald PJ. Vaginal infection and preterm labour. Br J Obstet Gynaecol. 1991;98(5):427-435.
doi pubmed - Krauss-Silva L, Moreira ME, Alves MB, Braga A, Camacho KG, Batista MR, Almada-Horta A, et al. A randomised controlled trial of probiotics for the prevention of spontaneous preterm delivery associated with bacterial vaginosis: preliminary results. Trials. 2011;12:239.
doi pubmed - Simhan HN, Caritis SN, Krohn MA, Hillier SL. Elevated vaginal pH and neutrophils are associated strongly with early spontaneous preterm birth. Am J Obstet Gynecol. 2003;189(4):1150-1154.
doi - Myhre R, Brantsaeter AL, Myking S, Gjessing HK, Sengpiel V, Meltzer HM, Haugen M, et al. Intake of probiotic food and risk of spontaneous preterm delivery. Am J Clin Nutr. 2011;93(1):151-157.
doi pubmed - Kaplas N, Isolauri E, Lampi AM, Ojala T, Laitinen K. Dietary counseling and probiotic supplementation during pregnancy modify placental phospholipid fatty acids. Lipids. 2007;42(9):865-870.
doi pubmed - Coleman RA, Haynes EB. Synthesis and release of fatty acids by human trophoblast cells in culture. J Lipid Res. 1987;28(11):1335-1341.
- Kankaanpaa PE, Yang B, Kallio HP, Isolauri E, Salminen SJ. Influence of probiotic supplemented infant formula on composition of plasma lipids in atopic infants. J Nutr Biochem. 2002;13(6):364-369.
doi - Selvaraj Kamala, Selvaraj Priya. Benefits of probiotic treatment in cases of bad obstetric history (BOH) and for prevention of post IVF pregnancy complications. J Obstet Gynecol India. 2009;59(4):336-339.
- Neri A, Sabah G, Samra Z. Bacterial vaginosis in pregnancy treated with yoghurt. Acta Obstet Gynecol Scand. 1993;72(1):17-19.
doi pubmed - Reid G, Burton J, Hammond JA, Bruce AW. Nucleic acid-based diagnosis of bacterial vaginosis and improved management using probiotic lactobacilli. J Med Food. 2004;7(2):223-228.
doi pubmed - Strus M, Malinowska M, Heczko PB. In vitro antagonistic effect of Lactobacillus on organisms associated with bacterial vaginosis. J Reprod Med. 2002;47(1):41-46.
- Nishijima K, Shukunami K, Kotsuji F. Probiotics affects vaginal flora in pregnant women, suggesting the possibility of preventing preterm labor. J Clin Gastroenterol. 2005;39(5):447-448.
doi pubmed - Parma M, Stella Vanni V, Bertini M, Candiani M. Probiotics in the prevention of recurrences of bacterial vaginosis. Altern Ther Health Med. 2014;20(Suppl 1):52-57.
- Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. Am J Obstet Gynecol. 2010;203(2):120 e121-126.
doi pubmed - Koscik RJE, Reid G, Kim SO, Li W, Challis JRG, Bocking AD. Effect of Lactobacillus rhamnosus GR-1 Supernatant on Cytokine and Chemokine Output From Human Amnion Cells Treated With Lipoteichoic Acid and Lipopolysaccharide. Reprod Sci. 2018;25(2):239-245.
doi pubmed - Yang S, Li W, Challis JR, Reid G, Kim SO, Bocking AD. Probiotic Lactobacillus rhamnosus GR-1 supernatant prevents lipopolysaccharide-induced preterm birth and reduces inflammation in pregnant CD-1 mice. Am J Obstet Gynecol. 2014;211(1):44 e41-44 e12.
doi pubmed - Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130(6):1355-1360.
doi pubmed - Doege K, Grajecki D, Zyriax BC, Detinkina E, Zu Eulenburg C, Buhling KJ. Impact of maternal supplementation with probiotics during pregnancy on atopic eczema in childhood—a meta-analysis. Br J Nutr. 2012;107(1):1-6.
doi pubmed - Huurre A, Laitinen K, Rautava S, Korkeamaki M, Isolauri E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: a double-blind placebo-controlled study. Clin Exp Allergy. 2008;38(8):1342-1348.
doi pubmed - Nordqvist M, Jacobsson B, Brantsaeter AL, Myhre R, Nilsson S, Sengpiel V. Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open. 2018;8(1):e018021.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
