| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 8, Number 3, September 2019, pages 97-102
Postpartum Cervical Manipulation, Vertebral Artery Dissection and Reversible Cerebral Vasoconstriction: A Case Report
Jodi L. Keanea, b, c, Lily Puiyi Liewa
aMonash Health, 246 Clayton Road, Clayton, Vic. 3168, Australia
bMonash University, Scenic Boulevard & Wellington Road, Clayton, Vic. 3168, Australia
cCorresponding Author: Jodi L. Keane, Monash Health, 246 Clayton Road, Clayton, Vic. 3168, Australia
Manuscript submitted August 17, 2019, accepted September 2, 2019
Short title: Vertebral Artery Dissection and RCVS
doi: https://doi.org/10.14740/jcgo576
| Abstract | ▴Top |
Stroke in the postpartum population is rare, but more common than non-pregnant states. Pathophysiology is more evenly split between hemorrhagic and ischemic etiologies and antecedent severe hypertensive disorders such as pre-eclampsia predominate. We describe a case of both potential minimally traumatic significant vertebral arterial dissection, posterior circulation ischemic stroke with lateral medullary syndrome and concomitant incidental reversible cerebral vasoconstriction on neuroimaging initially referred to the obstetric team as late postpartum pre-eclampsia and discuss diagnostic and management challenges.
Keywords: Pre-eclampsia; Stroke; Neck manipulation; Vertebral dissection; RCVS
| Introduction | ▴Top |
Although stroke risk is increased in the pregnant population compared to non-pregnant women, absolute incidence is low and stroke remains an uncommon event complicating around 13 in 100,000 pregnancies [1]
In older adults, etiologic frequencies are firstly ischemic events secondary to established atherosclerotic cerebrovascular disease or cardioembolic causes at 80-87%, followed by hemorrhagic stroke (which incorporates primary hemorrhage and subarachnoid bleeding) at 13-20% [2, 3]. In otherwise young healthy women of childbearing ages, the distribution is more evenly split between hemorrhagic and non-hemorrhagic causes [4].
Typical etiologies in peripartum women include ischemic events secondary to complications of pre-eclampsia and eclampsia, venous thrombosis and maternal thrombophilia [4], whereas hemorrhage occurs most frequently in the setting of eclampsia (44%) and rupture of prior often undiagnosed arteriovenous malformation (AVM) (37%) [4]. Of note, the majority of peripartum stroke are related to consequences of severe pre-eclampsia, and indeed this is a major direct cause of maternal death [5]. Similarly, postpartum stroke in a retrospective cohort has been found to be associated with cesarean section and, unsurprisingly, hypertensive disorders of pregnancy which encompasses pregnancy-induced hypertension and pre-eclampsia [6].
Postpartum cerebral angiopathy/reversible cerebral vasoconstriction (RCVS) is also a rare but potentially under-recognized cause of headache that can in extreme forms lead to ischemic stroke [7]. It is described in the postpartum context in women who receive adrenergic or serotonergic drugs but also occurs without these exposures and there is an association with migraine headache [7]. The exact etiology is incompletely elucidated [8] but typical radiologic findings include a “string of beads” appearance of the cerebral arteries corresponding to sites of vasospasm [9]. The course is typically relapsing-remitting over 1 - 3 months with complete resolution after this time. Complications vary in their timing with hemorrhagic sequelae and posterior reversible encephalopathy syndrome (PRES) typically occurring in the first week and ischemic events in the second with long-term deficits uncommon [8].
| Case Report | ▴Top |
A 30-year-old gravida 2 para 2 Mauritian woman was brought in by ambulance to the emergency department (ED) with a hypertensive crisis and severe headache at 17 days postpartum, later confirmed to be a posterior circulation stroke due to vertebral artery dissection in the setting of recent neck manipulation with incidental neuroimaging finding of RCVS.
Background
The patient’s past medical history was significant for chronic neck and shoulder pain which resulted in cessation of work as a hairdresser, for which she had sought manual therapies over the years, and chronic non-migraine headache.
There was no other past medical history of note. Body mass index was normal and there was no history of connective tissue disease, hypertension, cerebrovascular disease or risk factors for cerebrovascular disease such as smoking or hyperlipidemia. The patient consumed no regular medications and had no medication allergies.
Gynecologically there were diagnoses of mild endometriosis diagnosed with laparoscopy and polycystic ovarian syndrome, neither of which was currently symptomatic.
Obstetrically the patient had two uncomplicated pregnancies and term births, neither complicated by hypertension or pre-eclampsia. This pregnancy was managed by a senior consultant obstetrician antenatally and was uncomplicated. Blood pressure at 8 weeks gestation was 120/65 mm Hg and subsequently recorded with the range of 105 - 130/56 - 80 mm Hg.
The first pregnancy ended in an emergency cesarean section for obstructed labor at term and the most recent was a planned repeat cesarean birth.
Routine elective repeat lower uterine segment cesarean section (LSCS) was performed at 39 weeks and 1 day gestation under spinal anesthesia. The operation was uneventful with a reported blood loss of 450 mL. Post birth course was unremarkable with normal blood pressures recorded throughout the admission with discharge home on day 3 as per hospital protocol.
Presentation
Seventeen days postpartum, the patient attended the ED complaining of severe headache unrelieved by multimodal oral analgesia (paracetamol, diclofenac and immediate-release oxycodone). Headache was 10/10 in severity, generalized throbbing in nature associated with photophobia, diplopia, nausea and severe exacerbation of pre-existing neck pain. It was also worsened with any head or body movements. Antecedent history revealed 1 - 2 weeks of acute on chronic neck and shoulder pain on the left, for which she sought physical therapies; however, no further details were available on initial assessment (downstream history confirmed neck manipulation prior to presentation). There were no other reported neurological symptoms, infective symptoms, rash, sick contacts or any other illness, and the patient was otherwise well leading up to the event. The patient denied any head trauma, recreational drug use or features consistent with post dural puncture headache since the birth.
Five milligrams of intravenous morphine and 50 µg intravenous fentanyl were given for analgesia with effect, with resultant mild drowsiness and hypertension (initial blood pressure 170/100 mm Hg with automated machine) at this time. Further assessment and initial management were conducted in a resuscitation cubicle.
The obstetric team were requested to attend on the emergency presumptive diagnosis of postpartum severe pre-eclampsia. Intravenous access was obtained bilaterally with wide bore cannulae and blood was sent for urgent full blood examination (FBE), complete urine examination (CUE), liver function test (LFT) and venous gas while awaiting review.
On examination by the obstetric team, manual blood pressure with a correctly sized cuff was recorded to be 235/135 mm Hg. Heart rate was borderline sinus bradycardia at 60 beats per minute, suggesting possible Cushing response. Respiratory rate was 18 per minute and an oxygen saturation was 100% on room air and the patient was apyrexial.
Examination revealed a drowsy woman in pain with a Glasgow coma scale (GSC) of 14 (E3, V5, M6) but orientated to time, place and person.
Neurological examination, conducted in supine due to headache, severe hypertension and recent opiates, was unremarkable aside from brisk lower limb reflexes, without hyperreflexia, clonus or other evidence of neurological irritability. There were no obvious cerebellar signs at this time, although this examination was by necessity incomplete.
Abdominal examination revealed a fresh healing Pfannenstiel scar, a non-tender non-enlarged liver and absence of peritonism. Speculum and bimanual examinations were not indicated but minimal non-offensive lochia serosa was observed on perineal pad.
Electrocardiogram revealed a normal sinus rhythm and blood pressures were equal in bilateral upper limbs and no pulse deficit was felt to suggest an evolving type A aortic dissection.
As part of initial assessment and management, an in-dwelling urinary catheter was inserted. Bedside urinalysis (not seen by the specialty team) was reportedly 3+ protein without other findings; however, this was not substantiated on formal catheter protein quantification which was negative for proteinuria on a reliable specimen. Given the time frame since delivery and multiparous status with prior normal blood pressures, pre-eclampsia was felt clinically very unlikely by the obstetric team.
Severe headache and neck pain with history of treatment to the area raised concerns regarding a neurological event or dissection; however, given recent pregnancy, there was reluctance on part of other teams to lead care. Careful hypertensive control was initiated by the obstetric team as regardless of the diagnosis hypertensive control, stabilization, central nervous system imaging and intensive care unit disposition would be necessary.
Hypertension control was initiated with typical obstetric agents, within 30 min of presentation. The patient was given the following anti-hypertensive sequentially with her blood pressured controlled around 160 - 170/90 - 100 mm Hg: 1) 20 mg of nifedipine sublingually while obtaining intravenous access; 2) five 20 mg boluses of labetalol intravenously (total 100 mg); 3) two 5 mg and another 10 mg of hydralazine boluses (total 20 mg); 4) labetalol intravenous infusion at 20 mg/h, up to 60 mg/h within an hour and maintained for 12 h; 5) 4 g loading dose of magnesium sulphate intravenously after the return of a normal serum creatinine level, partly as synergy for hypertensive control and for membrane stabilization followed by an intravenous infusion of 1 g/h until firm control of blood pressure and confirmation of the non-obstetric diagnosis.
Investigations
Initial venous gas was within normal limits.
Presentation hematology including FBE, electrolytes and renal function, lactate dehydrogenase (LDH), reticulocytes and coagulation profile were all unremarkable and out of keeping with severe fulminating pre-eclampsia. Random glucose was normal at 6.6 mmol/L and there was a non-specific mild elevation of liver enzymes just above laboratory reference range consistent with expected postpartum LFT findings (Table 1).
 Click to view | Table 1. Initial Laboratory Investigations |
An urgent head computed tomography (CT) demonstrated left cerebellar ischemia/infarction without hemorrhage (Fig. 1). After blood pressure stabilization, the patient was transferred to intensive care for further management under the stroke unit.
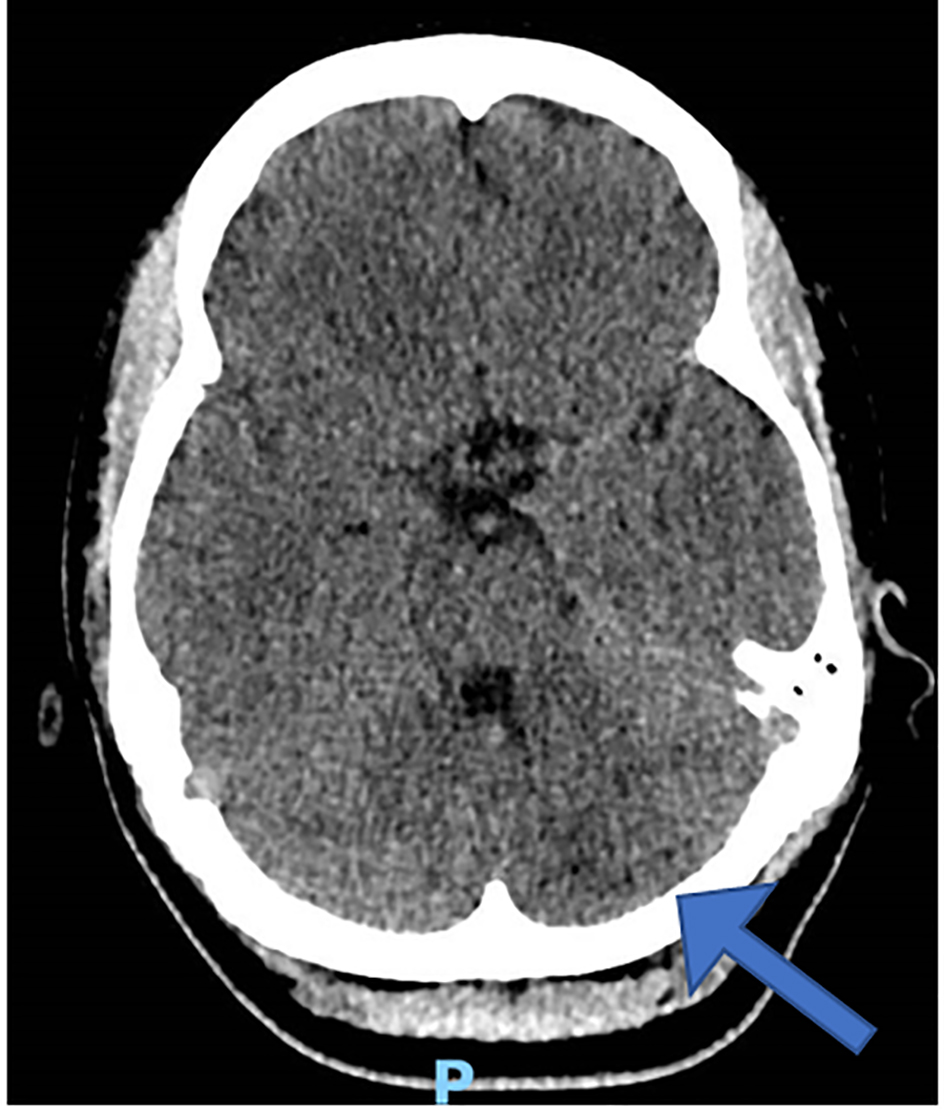 Click for large image | Figure 1. Initial head computed tomography. |
Formal urine protein/creatinine ratio was normal with urine protein excretion of 0.2 g/L.
Management
Subsequent brain magnetic resonance imaging (MRI) confirmed infarction involving the left medulla and cerebellar hemisphere, due to dissection of left vertebral and posterior inferior cerebellar arteries, which was later confirmed on a CT angiogram (Figs. 2 and 3). The later also showed changes consistent with postpartum RCVS syndrome.
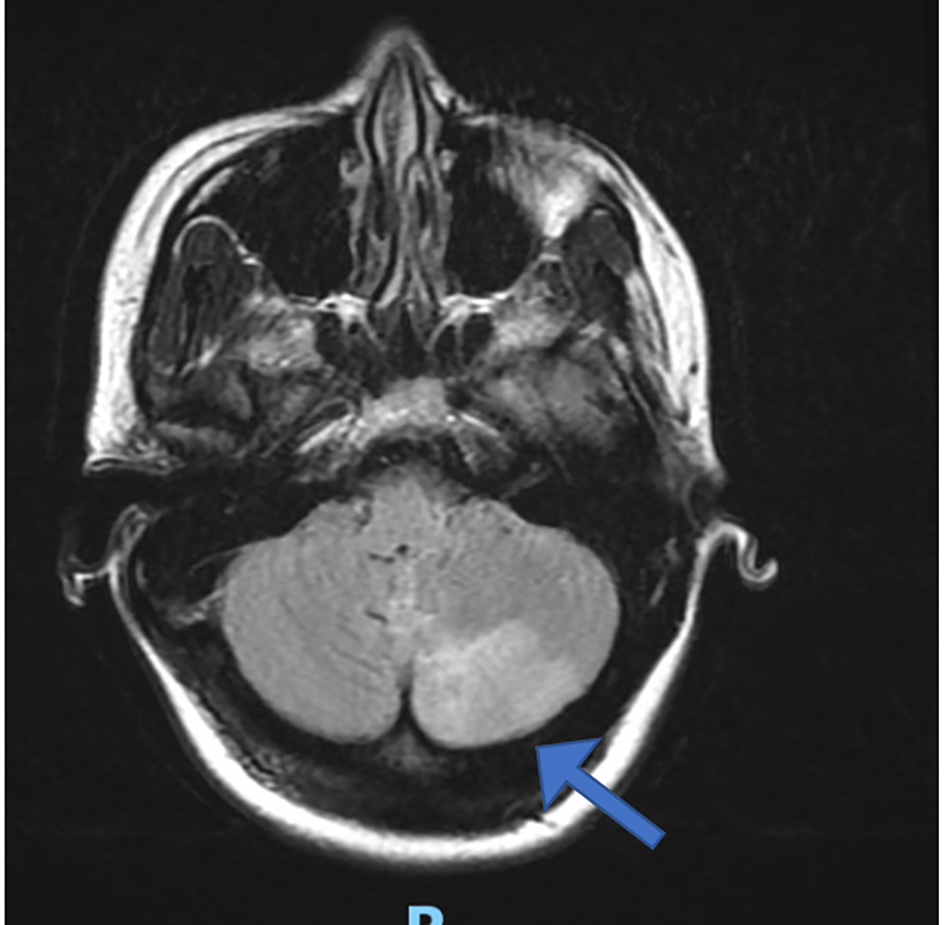 Click for large image | Figure 2. Brain magnetic resonance imaging with left cerebellar infarction (arrow). |
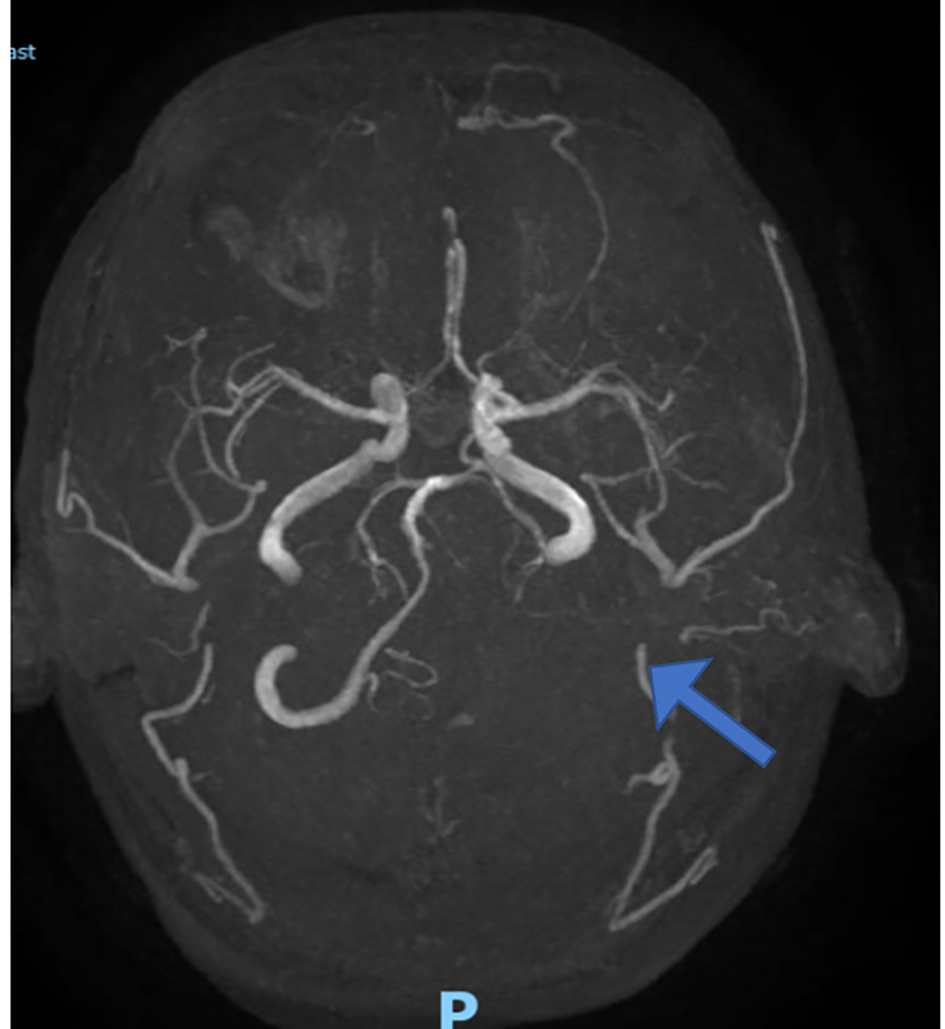 Click for large image | Figure 3. Computed tomography angiogram with left vertebral occlusion (arrow). |
Directed history revealed the patient frequented an osteopath where she had regular therapy for her chronic neck pain, which continued throughout her pregnancy and in particular postpartum including the day before presentation.
The patient developed ipsilateral Horner’s syndrome and signs consistent with left lateral medullary syndrome with mild lower limb weakness (4+ power on all muscle groups), mild oro-pharyngeal dysphagia, right facial droop and tongue deviation, which largely resolved with minimal residual deficit after a month of in-patient rehabilitation. She was commenced on aspirin 100 mg, atorvastatin 80 mg and amlodipine 5 mg daily as part of post stroke management.
Targeted MRI of neck and cervical spine did not demonstrate an organic cause for chronic pain.
Outcome
The patient was discharged for ongoing outpatient rehabilitation after this time with minor residual defects. Repeat imaging revealed recanalization of the distal left vertebral artery and flow demonstrated through the cervical vertebral artery with mild narrowing.
Final review with the obstetric team included a discussion regarding avoiding estrogen containing contraceptives with a history of ischemic stroke and recommendation for pre-pregnancy counselling before any subsequent pregnancy and tertiary obstetric care once pregnant.
| Discussion | ▴Top |
Stroke is rare in a previously well, normotensive postpartum woman. Pre-eclampsia at 17 days post birth in a woman without a history of hypertension is probably at least as uncommon, if not more so, than stroke in this setting. Despite this, the obstetric team are often the first team to lead the care as current or recent pregnancy not infrequently clouds clinical opinion. At best this is an opportunity for interdisciplinary cooperation but at worst, this can lead to inappropriate care or delayed management.
The differential diagnoses in this case were initially relatively broad ranging from ischemic or hemorrhagic stroke, severe migraine variant, meningitis, arterial dissection of the vertebrobasilar system (given the severe neck pain), aortic dissection, mechanical injury and very atypical pre-eclampsia, with iatrogenic meningitis and pre-eclampsia both being rendered very unlikely due to the healthy greater than 2-week interval since birth and neuraxial block. Radiological investigations and essentially normal pre-eclampsia investigations revealed the correct diagnosis relatively rapidly and permitted appropriate care. In establishing the correct diagnosis, it is important to particularly note the normal postpartum blood pressure measurements, normal biochemistry, negative formal protein quantification and absence of neurological irritability that one would expect in severe pre-eclampsia with marked hypertension. Furthermore, although LFTs were mildly abnormal, this is well described in the literature in a postpartum woman, particularly post cesarean section [10].
Neck manipulation is described of being capable of causing traumatic dissection of the vertebral arteries and posterior circulation stroke [11-13]. Not all authors agree as while the American Heart and American Stroke Association joint position statement reflects recognition of association between cervical spine manipulative therapies and stroke [13], there is controversy in the chiropractic literature with at least one relatively large case-control study questioning the link, suggesting patients seek manual therapies due to pain and symptoms from early dissection events, reversing causality [14]. Certainly, the vertebral circulation is vulnerable to tearing due to anatomical course through the vertebral column when rotatory forces such as those applied with rapid thrust and rotation manipulative therapies are used as treatment for neck pain performed by chiropractors but also performed by osteopaths, some physical therapists and other clinical craft groups. Pregnant and puerperal women are physiologically more vulnerable to arterial dissection due to elevated levels of relaxin and progesterone and decreased collagen synthetic activity affective vessel wall properties combined with the hemodynamic changes of increased cardiac output and endothelial sheer stress and thus multiple reports of arterial dissections exist in multiple circulations including coronary, carotid and other [15, 16].
Stroke is also well reported to cause a transient hypertensive response [17], in particular ischemic events [18] and for this reason, combined with absence of laboratory, urinary or clinical support of pre-eclampsia faced with a dissection in a vulnerable circulation temporally related to neck manipulation in the puerperium we repeat the key message of this case. Not all hypertension is pre-eclampsia; however, obstetricians are often the default early responders.
Dual pathology of dissection and ischemic stroke with RCVS as in the case is uncommon, and to the authors knowledge not previously reported in the literature.
It is difficult to ascertain with certainty the stroke event was related etiologically to neck dissection or the RCVS as both were present; however, radiological opinion by two senior radiologists favored the former scenario due to stroke territory concordance with vertebral dissection and absence of infarction in other territories also with RCVS including middle and anterior cerebral arteries.
RCVS typically presents with severe headache which may be described as thunderclap, posterior and then generalized with associated nausea and vomiting [7]. Unilateral neck pain as in this case is inconsistent with typical symptoms and although ischemic stroke may eventuate [8], a single territory corresponding to a dissection event renders the stroke more likely due to dissection than RCVS. Neuroimaging findings in RCVS include normal findings apart from cerebral vasoconstriction, convexity subarachnoid hemorrhage, intracerebral hemorrhage, cerebral infarction and reversible brain edema [7]. Which cerebellar ischemic infarcts are possible is not described as a common manifestation of RCVS [7, 9]. Interestingly both magnesium sulphate and nifedipine, familiar obstetric agents in severe hypertensive events are two treatment modalities used clinically to address vasospasm in RCVS [7].
Although stroke was suspected and caution was exercised with blood pressure control, obstetric teaching for careful sustained lowering of blood pressure to mild-moderate hypertension is also appropriate in RCVS and ischemic stroke where overaggressive lowering can worsen ischemic penumbra and exacerbate infarction.
Conclusion
It is important to maintain an appropriate index of suspicion for stroke and atypical causes of severe hypertension in the pregnant and puerperal woman, particularly when accompanied by atypical symptoms such as severe headache and neck pain. Obstetric staff are well trained in hypertension control and many agents used familiarly are appropriate in this setting, despite the non-obstetric diagnosis.
As first caregivers, retention of more general medical and diagnostic skills by obstetric staff is important as is interdisciplinary cooperation and good communication in the care of an unwell woman who is pregnant or puerperal.
This report echoes that other authors regarding caution with neck manipulation in pregnancy and the puerperium given absence of high level proven benefit, alternate therapeutic options and uncommon but severe nature of sequelae such as in this case.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written consent was obtained.
Author Contributions
JLK wrote, edited and revised manuscript. LPL wrote manuscript and collated clinical data herein. All authors reviewed final manuscript.
| References | ▴Top |
- Lanska DJ, Kryscio RJ. Risk factors for peripartum and postpartum stroke and intracranial venous thrombosis. Stroke. 2000;31(6):1274-1282.
doi pubmed - Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8(3):319-329.
doi pubmed - Feigin VL, Lawes CM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43-53.
doi - Sharshar T, Lamy C, Mas JL. Incidence and causes of strokes associated with pregnancy and puerperium. A study in public hospitals of Ile de France. Stroke in Pregnancy Study Group. Stroke. 1995;26(6):930-936.
doi pubmed - Humphrey MD, Bonello MR, Chughtai A, Macdaldowie A, Harris K, Chambers GM. Maternal dealths in Australia 2008-2012. MAternal deaths series no 5. Cat no. PER 70. Canberra, 2015.
- Witlin AG, Mattar F, Sibai BM. Postpartum stroke: a twenty-year experience. Am J Obstet Gynecol. 2000;183(1):83-88.
doi - Ducros A. Reversible cerebral vasoconstriction syndrome. Lancet Neurol. 2012;11(10):906-917.
doi - Singhal AB, Hajj-Ali RA, Topcuoglu MA, Fok J, Bena J, Yang D, Calabrese LH. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68(8):1005-1012.
doi pubmed - Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser MG. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130(Pt 12):3091-3101.
doi pubmed - David AL, Kotecha M, Girling JC. Factors influencing postnatal liver function tests. BJOG. 2000;107(11):1421-1426.
doi pubmed - Shanmugalingam R, Reza Pour N, Chuah SC, Vo TM, Beran R, Hennessy A, Makris A. Vertebral artery dissection in hypertensive disorders of pregnancy: a case series and literature review. BMC Pregnancy Childbirth. 2016;16(1):164.
doi pubmed - Stuber KJ, Wynd S, Weis CA. Adverse events from spinal manipulation in the pregnant and postpartum periods: a critical review of the literature. Chiropr Man Therap. 2012;20:8.
doi pubmed - Biller J, Sacco RL, Albuquerque FC, Demaerschalk BM, Fayad P, Long PH, Noorollah LD, et al. Cervical arterial dissections and association with cervical manipulative therapy: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(10):3155-3174.
doi pubmed - Kosloff TM, Elton D, Tao J, Bannister WM. Chiropractic care and the risk of vertebrobasilar stroke: results of a case-control study in U.S. commercial and Medicare Advantage populations. Chiropr Man Therap. 2015;23:19.
doi pubmed - Ulrich N, Johnson A, Jodry D, Dola C, Martin-Schild S, El Khoury R. Resolution of internal carotid dissection with middle cerebral artery occlusion in pregnancy. Case Rep Neurol Med. 2015;2015:398261.
doi pubmed - Wiebers DO, Mokri B. Internal carotid artery dissection after childbirth. Stroke. 1985;16(6):956-959.
doi pubmed - AlSibai A, Qureshi AI. Management of acute hypertensive response in patients with ischemic stroke. Neurohospitalist. 2016;6(3):122-129.
doi pubmed - Kwarciany M, Gasecki D, Kowalczyk K, Rojek A, Laurent S, Boutouyrie P, Skrzypek-Czerko M, et al. Acute hypertensive response in ischemic stroke is associated with increased aortic stiffness. Atherosclerosis. 2016;251:1-5.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
