| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 9, Number 3, September 2020, pages 53-59
High-Grade Serous Carcinoma of Ovary With Choriocarcinomatous Differentiation: A Case Report and Review of Literature
Qandeel Sadiqa, Radhika Sekhria, Sonali Lanjewara, b
aDepartment of Pathology, University of Tennessee Health Science Center, Methodist University Hospital, 1265 Union Avenue, Memphis, TN, USA
bCorresponding Author: Sonali Lanjewar, Department of Pathology, University of Tennessee Health Science Center, Methodist University Hospital, 1265 Union Avenue, Memphis, TN 38119, USA
Manuscript submitted June 29, 2020, accepted August 1, 2020, published online September 9, 2020
Short title: High-Grade Serous Carcinoma of Ovary
doi: https://doi.org/10.14740/jcgo669
| Abstract | ▴Top |
Non-gestational choriocarcinoma (NGC) of the ovary is a rare, highly malignant neoplasm usually of germ cell origin. Rarely, NGC has been observed in association with ovarian epithelial neoplasms. So far 11 cases of NGC with choriocarcinomatous differentiation have been described in the relevant literature. Here we describe a case of NGC differentiation in a high-grade serous carcinoma of the ovary. A 53-year-old nulliparous, postmenopausal woman presented with abdominal pain and vaginal bleeding. Abdominal imaging revealed a complex pelvic mass and carcinomatosis. The patient underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy and peritoneal tumor debulking. Microscopic examination of the bilateral ovarian mass tissue revealed a high-grade serous carcinoma with a distinct component of choriocarcinomatous differentiation. The presence of choriocarcinomatous differentiation in epithelial malignancy is associated with a very poor prognosis. Our patient succumbed to disease within 2 months of diagnosis.
Keywords: Ovary; Non-gestational choriocarcinoma; High-grade serous carcinoma
| Introduction | ▴Top |
Choriocarcinoma, a tumor of most primitive trophoblast, is the most common gestational trophoblastic neoplasm seen in women of reproductive age. Non-gestational choriocarcinoma (NGC) in the ovary is either germ cell in origin or observed in association with epithelial neoplasms. NGC of germ cell origin is typically seen in children and young adults, with a prognosis less favorable than for gestational choriocarcinoma (GC) [1]. Choriocarcinomatous differentiation in epithelial neoplasms has a poor prognosis [2]. This rare differentiation has been reported in various organs, including the breast, esophagus, stomach, colon, and the female genital tract. The first two cases of ovarian epithelial neoplasms that differentiated into choriocarcinoma were described by Oliva et al in 1993 [3]. Nine more cases were reported subsequently. Here, we report a case of high-grade serous carcinoma of the ovary with choriocarcinomatous differentiation in the context of relevant information in the literature.
| Case Report | ▴Top |
A 53-year-old, nulliparous, post-menopausal Asian woman presented with abdominal pain, distension, and heavy vaginal bleeding. She was diagnosed with stage III C ovarian carcinoma at an outside clinic. Ultrasound examination revealed a complex pelvic mass measuring approximately 13.5 × 12.3 × 9.8 cm. The largest cystic portion of the mass was posterior midline with irregular internal borders and papillary projections and measured 5.9 × 5.4 × 4.6 cm. Computed tomography (CT) scan demonstrated bilateral ovarian masses, ascites and carcinomatosis consistent with ovarian cancer (Figs. 1, 2). Her carbohydrate antigen-125 (CA-125) levels were 25 U/mL. She underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy with peritoneal tumor debulking for the widespread intra-abdominal disease.
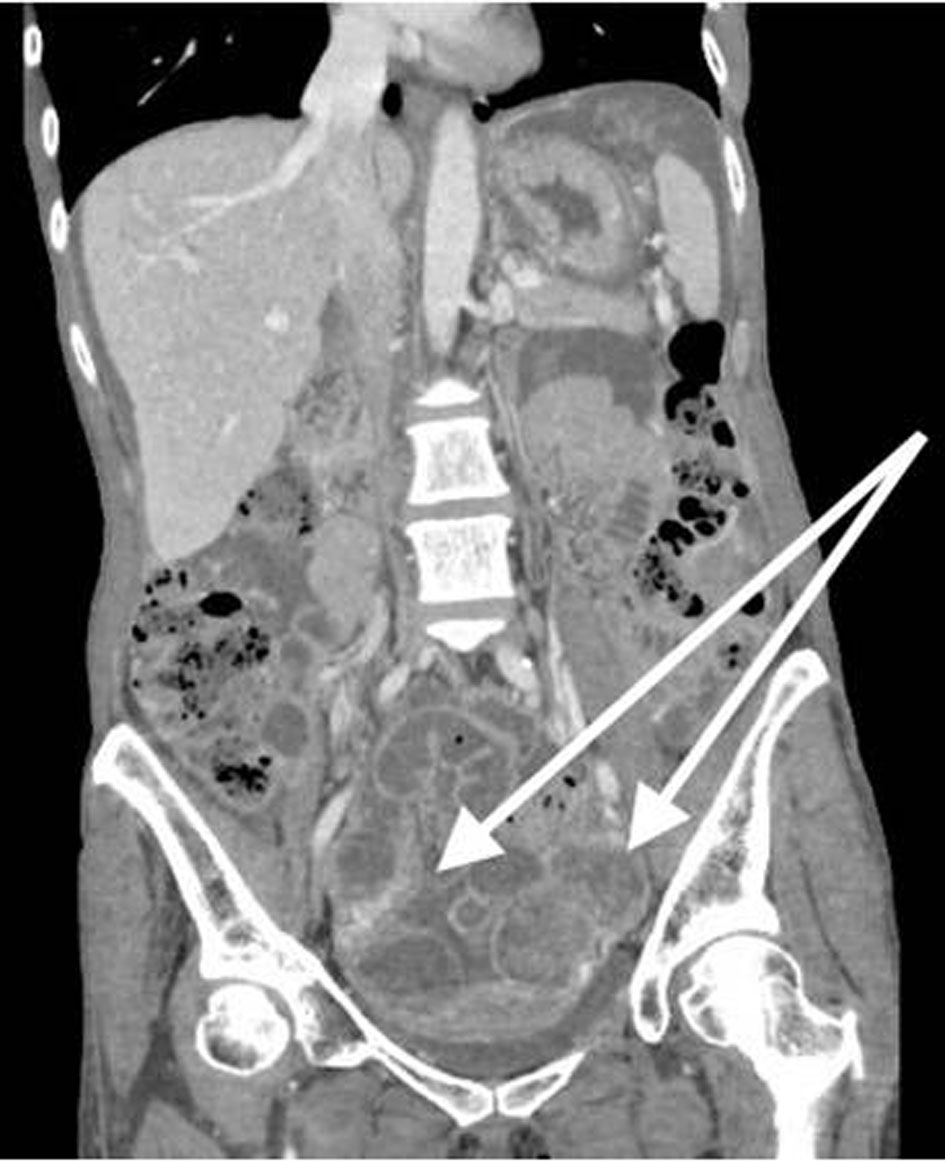 Click for large image | Figure 1. CT of abdomen and pelvis shows bilateral ovarian masses, and short arrow shows left ovarian mass. CT: computed tomography. |
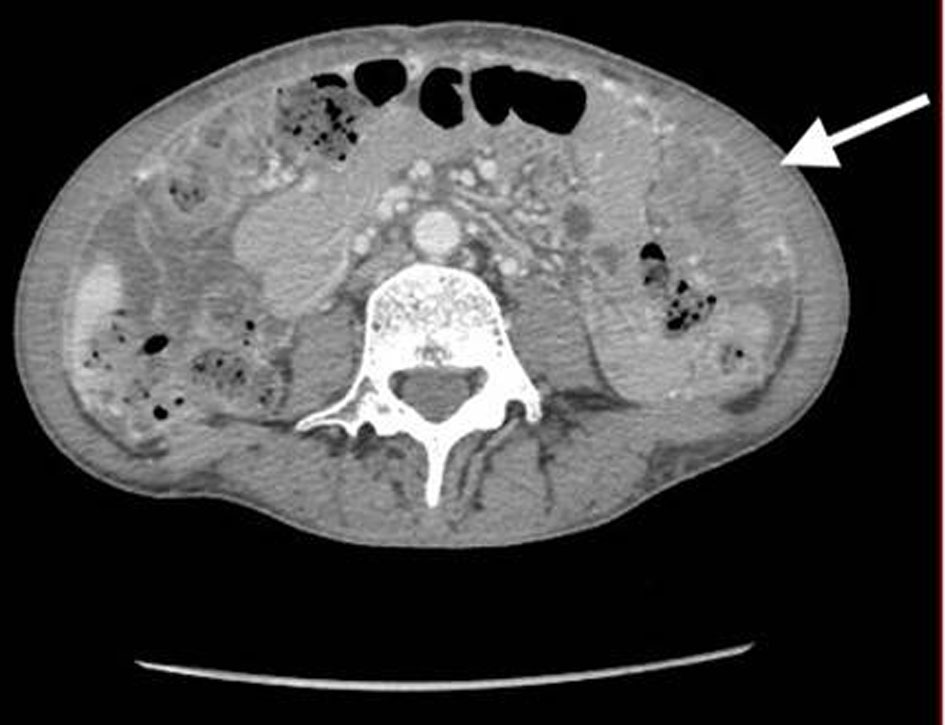 Click for large image | Figure 2. CT of abdomen and pelvis shows large bulky nodular mass in the omental carcinomatosis. CT: computed tomography. |
Pathology
Gross examination showed a tan-white, friable 9.4 × 6.2 × 4.7 cm left tubo-ovarian mass with papillary excrescences. The right ovary (8.2 × 3.8 × 3.0 cm) was solid and cystic and showed a friable papillary tumor involving approximately 55% of the ovary. Histopathological examination of the bilateral ovaries revealed a high-grade malignant neoplasm comprising two morphologically distinct tumors (Fig. 3). The epithelial component showed mixed papillary, glandular, and cribriform architecture with mitotically active, high-grade nuclei (Fig. 4). Adjacent to the tumor was a histologically distinct high-grade tumor growing in clusters and sheets and was found surrounding blood lakes. The tumor cells were polygonal, with centrally placed hyperchromatic, vesicular nuclei, prominent nucleoli, clear cytoplasm, and had a distinct cytoplasmic membrane (Figs. 5, 6). Admixed with these tumor cells was a second population of mononucleate pleomorphic cells with abundant eosinophilic cytoplasm. Rare multinucleated hyperchromatic syncytiotrophoblast-like, basophilic cells were identified. Extensive immunohistochemical studies were performed to verify trophoblastic differentiation while ruling out other ovarian neoplasms including clear cell carcinoma, and germ cell tumors (described below in the immunohistochemistry (IHC) section). Our case did not show the luteinization of ovarian stroma, which was attributed to steroidal hormones observed in a few case reports [3, 4].
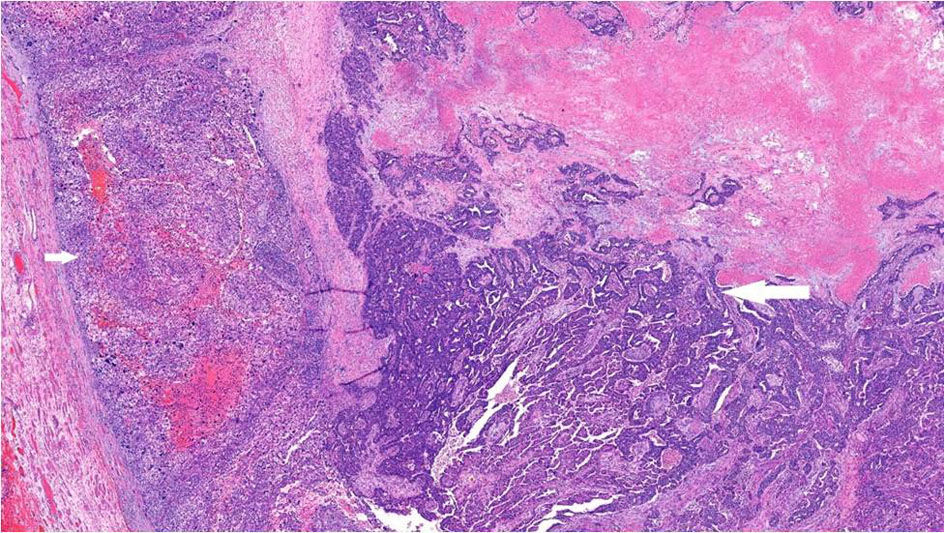 Click for large image | Figure 3. High-grade serous carcinoma with papillary and glandular architecture (long arrow) with adjacent choriocarcinomatous differentiation (short arrow), × 4. |
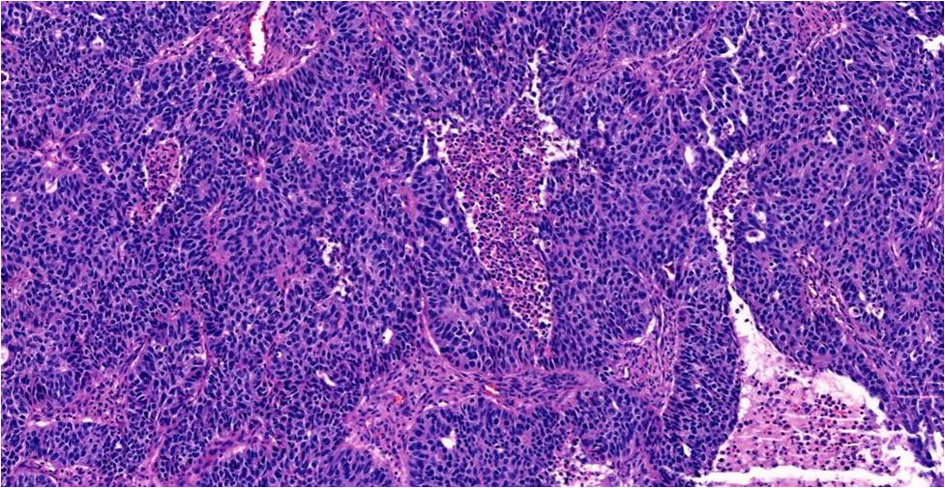 Click for large image | Figure 4. High-grade serous carcinoma with papillary and glandular architecture, × 20. |
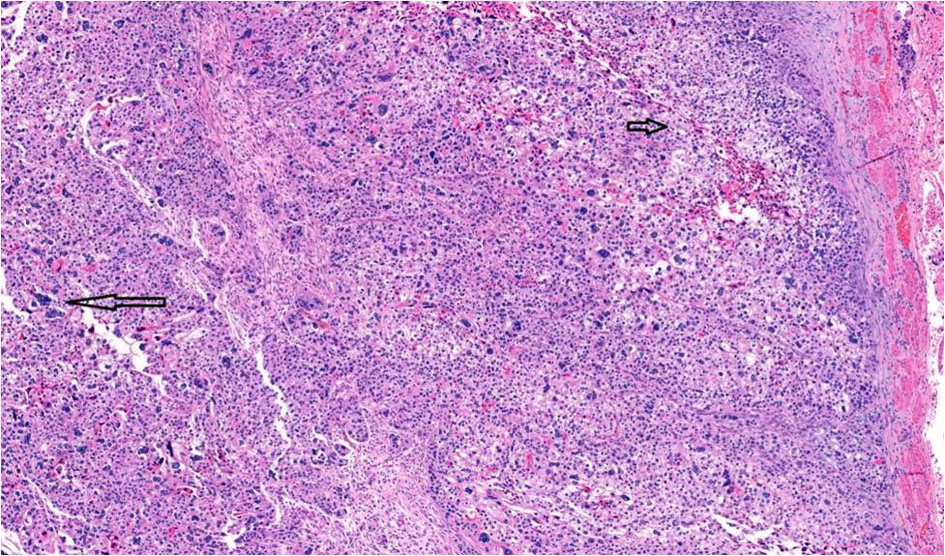 Click for large image | Figure 5. Choriocarcinomatous differentiation comprising an admixture of mononucleate cytotrophoblastic cells (short arrow) and multinucleate syncytiotrophoblast-like cells (long arrow), × 10. |
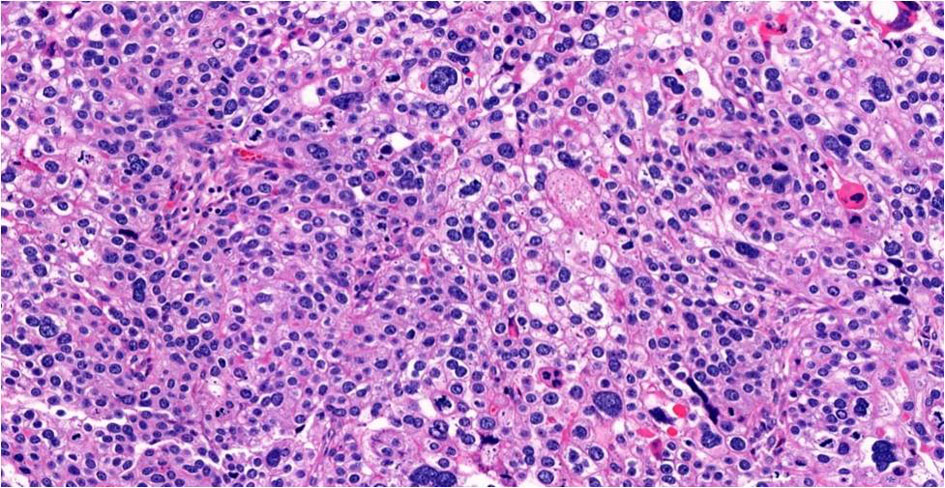 Click for large image | Figure 6. Choriocarcinomatous differentiation, × 20. |
Although serous and trophoblastic components were well-demarcated, the transition zone showed merging of the two components (Fig. 7). Intra-abdominal (bladder, ureters, rectosigmoid, transverse colon) carcinomatosis and lymph node metastasis were mainly comprised of choriocarcinomatous component.
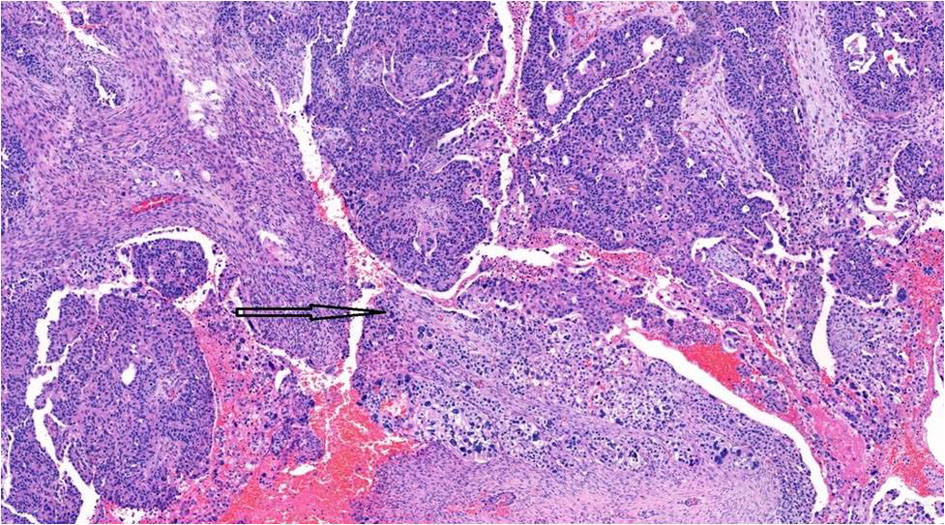 Click for large image | Figure 7. Transition zone (arrow) showing intermingling of epithelial and choriocarcinomatous components, × 10. |
IHC
The epithelial tumor showed diffuse and strong expression for p53 (Fig. 8) (DU-7, Leica; RTU), WT1 (wt49, Leica; RTU), and PAX8 (MRQ-SO Mab, Cell Marque; RTU) consistent with serous phenotype. GATA3 (LSO-823 Mab, Cell Marque, RTU), a highly sensitive marker for trophoblasts, was strongly and diffusely positive in the choriocarcinomatous component. This component was also strongly positive for P53, CK-OSCAR, placental alkaline phosphatase (PLAP) (NB-10, Roche; RTU), and negative for WT1, PAX8, ER (SP1, Roche; RTU), napsin A (IP64, Leica; RTY), alpha-fetoprotein (AFP) (Polyclonal, Roche; RTU), CD30 (JCM182, Leica; RTU), and programmed cell death ligand-1 (PD-L1) (NAT105, Cell marque). Trophoblasts showed patchy zonal staining for p63, supporting the presence of a dimorphic population of trophoblasts. Human chorionic gonadotrophin (hCG) immunostaining was negative. Summary of IHC study with review is enumerated in Table 1 [5-11].
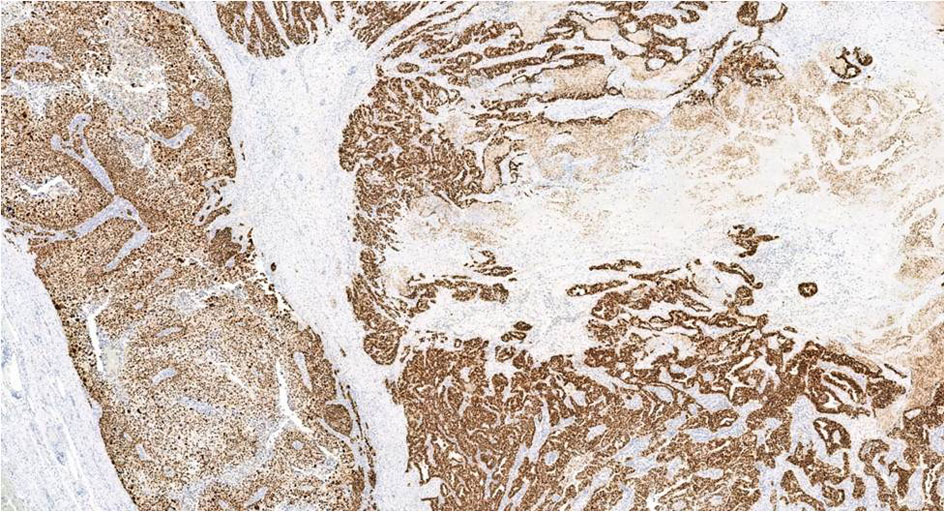 Click for large image | Figure 8. P53 IHC in both choriocarcinoma and serous carcinoma shows diffuse and strong nuclear positivity, × 4. IHC: immunohistochemistry. |
 Click to view | Table 1. Summary of IHC Findings in All Cases |
Follow-up
The patient presented with weakness, cachexia, and worsening ascites 6 weeks after surgery. Imaging revealed enhancement of liver capsule, liver distortion, diffuse bowel serosal enhancement, and circumferential thickening of the bowel wall with mesenteric enhancement, which was concerning for metastatic carcinoma. Her follow-up labs, including hCG (2.00 mIU/mL), carcinoembryonic antigen (CEA) (0.6 ng/mL) and AFP (4.0 ng/mL), were within reference ranges. She received the first cycle of chemotherapy comprised of paclitaxel and carboplatin. However, she succumbed to death within 2 months of surgical and medical management.
| Discussion | ▴Top |
Choriocarcinoma is a malignant gestational trophoblastic neoplasm comprised of an admixture of intermediate trophoblasts, cytotrophoblasts, and multinucleated syncytiotrophoblasts. It can be classified into: 1) GC or 2) NGC. The non-gestational tumor can be further sub-divided into germ cell origin or as a component of a somatic high-grade neoplasm. GC primarily arises in the uterus after full-term pregnancy or after a hydatidiform mole. Although rare, it can also occur at various ectopic sites (e.g. fallopian tubes [12], ovary [13], and greater omentum [14]). Non-gestational ovarian choriocarcinoma is mostly of germ cell origin. It is commonly found in children and young adults. Unlike GC, it spreads via both lymphatic and hematogenous pathways and also shows intra-abdominal spread. It has an intermediate prognosis between GC and NGC associated with somatic malignancies [15].
Choriocarcinomatous differentiation in somatic malignancy has been reported in various organ systems and has a worse prognosis than GC and NGC of germ cell origin. In the gastrointestinal (GI) tract, this differentiation is commonly found in the stomach and rectosigmoid carcinomas in elderly men [16]. This differentiation was noted in metastatic deposits of colonic adenocarcinomas. Other GI sites include the esophagus, sigmoid colon, and rectum [17-19]. The prognosis is poor with early metastasis and death within a year of diagnosis [19]. Lung adenocarcinoma and mammary carcinomas can also show this differentiation [20-23]. The occurrence of NGC with somatic malignancy in the urinary tract is a well-established phenomenon, with several cases reported in urinary bladder [24], kidney, and renal pelvis [25]. It is classified into three main morphologic subtypes: 1) Presence of only scattered syncytiotrophoblasts within urothelial carcinoma; 2) Presence of focal areas of complete trophoblastic differentiation; and alternatively, 3) Tumors manifesting only immunohistochemical evidence with no morphological identification of trophoblasts. Outcomes are worse in urothelial neoplasms containing syncytiotrophoblasts, necessitating the reporting of this morphological variant [26]. In the female reproductive tract, these tumors have been found in ovaries and the uterine corpus [27, 28], and, rarely, in cervical carcinoma. In the uterus, it is commonly seen in association with endometrioid endometrial adenocarcinoma and is predominantly found in postmenopausal women (median = 62.3 years). In the cervix, it is reported in association with squamous cell carcinoma, endocervical adenocarcinoma, and clear cell carcinoma [29-31]. NGC associated with somatic malignancy (NGC-SM) has a worse prognosis, with distant metastases and a short survival period [28, 32, 33]. The most common sites of metastasis are lung and liver.
A thorough search of the relevant literature in English was conducted to identify previously reported cases of ovarian epithelial tumors differentiating towards choriocarcinoma, with a total of 11 cases identified. We report the 12th case of ovarian neoplasm with choriocarcinomatous differentiation. NGC-SM is an extremely rare, possibly under-recognized, or under-reported entity. Clinicopathological features, histomorphology of co-existing epithelial tumors, and prognosis for reported cases are summarized in Table 2 [2-11]. NGC-SM is common in postmenopausal women but can be found in relatively younger [3, 11] and premenopausal women [2], with an age range of 29 - 65 years (mean: 51.7 years, median: 53 years). The common presenting symptoms include abdominal pain, distension, and vaginal bleeding. On imaging, tumors are typically solid and cystic, with size varying from 5 to 30 cm. Grossly, a separate nodule with hemorrhage and necrosis can be found adjacent to the epithelial tumor [3, 4, 6, 11]. The most common laboratory finding includes elevated serum levels of beta-hCG (β-hCG) [2-4, 6, 7, 9, 11, 27], CA-125, and normal levels of AFP, which is a yolk sac tumor marker. A normal β-hCG was reported by Shanmugasundaram et al [10], a finding similar to our case. Pre-procedure β-hCG was not performed in our patient due to low suspicion for choriocarcinomatous differentiation. Post-surgery, β-hCG was found to be within the reference range. CA-125 was elevated in six cases, including our case [3, 4, 7, 9, 10]. Variation in levels of neuron-specific enolase (NSE) has also been reported [6]. Xing et al performed PD-L1 on five somatic neoplasms with choriocarcinomatous differentiation. PD-L1 was positive in all tumors, with 90% of tumor cells positive in four tumors, and 50% positive in one tumor. In their study, PD-L1 was positive only in the choriocarcinomatous component, while the somatic component was completely negative [2].
 Click to view | Table 2. Summary of Clinico-Pathologic Features |
Choriocarcinomatous differentiation has been found in a wide range of benign and malignant ovarian neoplasms, including benign mucinous cystadenoma (n = 2), mucinous carcinoma (n = 2), endometrioid carcinoma admixed with other types (n = 2), undifferentiated carcinoma (n = 1), high-grade adenocarcinoma (n = 1), clear cell carcinoma (n = 1), and high-grade serous carcinoma, including our case (n = 3). A history of endometriosis was noted in two cases of endometrioid and clear cell carcinoma, respectively [2, 6]. Prognosis in these patients was typically poor. Seven out of 12 patients died of disease within 6 days to 15 months after diagnosis. Two patients had lung metastasis and brain metastasis respectively, while one patient had widespread peritoneal involvement (Table 2) [2-11]. One out of 12 patients showed a positive response to chemotherapy with no evidence of recurrence 7 months after following up [4]. It is important to note that choriocarcinomatous differentiation was not limited to high-grade epithelial neoplasms of the ovary but was also found in benign mucinous cystadenoma [3, 5] and low-grade endometrioid adenocarcinoma. One of the benign mucinous cystadenomas showed an abrupt transition to poorly differentiated carcinoma that differentiated into choriocarcinoma. Identification of this rare pattern of differentiation is crucial as it follows an aggressive clinical course, irrespective of the background ovarian disease.
The most accepted hypothesis for the presence of the choriocarcinomatous component is the theory of dedifferentiation or retro-differentiation. This idea has been supported by the identification of a transition zone between the tumor components [3, 4, 6]. Merging of two morphological components was also observed in our case, suggesting the possibility of retro-differentiation of the extra-embryonic component towards a more primitive tumor. Diffuse positive staining for p53 in both components also favors the same clonal origin for the two elements. Xing et al performed next-generation sequencing (NGS) on gynecological tumors with choriocarcinomatous differentiation and drew the inference that all tumor components shared somatic mutations and identical alleles upon single-nucleotide polymorphism (SNP) analysis, thus favoring the implication that all components have the same precursor origin [2].
It is imperative to differentiate between GC versus NGC, as this distinction has prognostic implications. This can be accomplished by short tandem repeat (STR) analysis using a set of highly polymorphic markers to determine the parental origin of tumors. GC usually can demonstrate either paternally derived DNA or both paternal and maternal contributions to the tumor, while NGC shows an identical homozygous allelic pattern in all tumor components, with no evidence of any paternal alleles [7]. The genetic origin of choriocarcinoma can also be established by fluorescent microsatellite genotyping of DNA.
As these tumors are typically chemotherapy resistant, the outcome is fatal. Routine PD-L1 testing may help in determining the role of checkpoint inhibitors as a therapeutic option [2].
Conclusions
Choriocarcinomatous differentiation in somatic high-grade malignancy can be found in various organ systems. Its recognition is important as it is associated with a worse prognosis. Choriocarcinomatous differentiation in the ovary is not limited to high-grade epithelial tumors but can also be observed in benign neoplasms of the ovary, well-differentiated endometrioid tumors, and other epithelial subtypes. Determination of the genetic origin of a tumor can differentiate NGC from GC. Knowledge of this differentiation and its identification is essential for prognostic information. There is a definite need for the development of standard management protocols, including routine PD-L1 testing.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
All authors have made substantial contribution to this case report. QS was involved in review of literature, creating tables and figures and made significant contribution to drafting result and discussion. RS contributed to drafting Introduction. SL helped in revising the paper in keeping with the intellectual contents including final approval of the draft before publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO classification of tumors of female reproductive organs. Lyon: IARC; 2014.
- Xing D, Zheng G, Pallavajjala A, Schoolmeester JK, Liu Y, Haley L, Hu Y, et al. Lineage-specific alterations in gynecologic neoplasms with choriocarcinomatous differentiation: implications for origin and therapeutics. Clin Cancer Res. 2019;25(14):4516-4529.
doi pubmed - Oliva E, Andrada E, Pezzica E, Prat J. Ovarian carcinomas with choriocarcinomatous differentiation. Cancer. 1993;72(8):2441-2446.
doi - Jimenez-Heffernan JA, Perna C, Martinez A, Casinello J, Cuevas J. Co-existent ovarian mucinous cystadenocarcinoma and ovarian choriocarcinoma. Arch Gynecol Obstet. 2002;266(4):235-237.
doi pubmed - Ozaki Y, Shindoh N, Sumi Y, Kubota T, Katayama H. Choriocarcinoma of the ovary associated with mucinous cystadenoma. Radiat Med. 2001;19(1):55-59.
- Hirabayashi K, Yasuda M, Osamura RY, Hirasawa T, Murakami M. Ovarian nongestational choriocarcinoma mixed with various epithelial malignancies in association with endometriosis. Gynecol Oncol. 2006;102(1):111-117.
doi pubmed - Oladipo A, Mathew J, Oriolowo A, Lindsay I, Fisher R, Seckl M, Yiannakis D. Nongestational choriocarcinoma arising from a primary ovarian tumour. BJOG. 2007;114(10):1298-1300.
doi pubmed - Hafezi-Bakhtiari S, Morava-Protzner I, Burnell MJ, Reardon E, Colgan TJ. Choriocarcinoma arising in a serous carcinoma of ovary: an example of histopathology driving treatment. J Obstet Gynaecol Can. 2010;32(7):698-702.
doi - Hu YJ, Ip PP, Chan KK, Tam KF, Ngan HY. Ovarian clear cell carcinoma with choriocarcinomatous differentiation: report of a rare and aggressive tumor. Int J Gynecol Pathol. 2010;29(6):539-545.
doi pubmed - Shanmugasundaram G, Sundaramoorthy E, Sudalaiandi S, Kondaveeti SS, Johnson T, Swaminathan R, Ramesh A. Double pathology: malignant epithelial ovarian tumor and germ cell tumor (choriocarcinoma), a rare coexistence. World J Oncol. 2015;6(4):421-425.
doi pubmed - Koyanagi T, Fujiwara H, Usui H, Ariga H, Machida S, Takei Y, Saga Y, et al. Ovarian nongestational choriocarcinoma and associated adenocarcinoma with the same germ cell origin determined by a molecular genetic approach: A case report. Pathol Int. 2016;66(9):529-534.
doi pubmed - Jwa SC, Kamiyama S, Takayama H, Tokunaga Y, Sakumoto T, Higashi M. Extrauterine choriocarcinoma in the fallopian tube following infertility treatment: implications for the management of early-detected ectopic pregnancies. J Minim Invasive Gynecol. 2017;24(5):855-858.
doi pubmed - Jia N, Chen Y, Tao X, Ou E, Lu X, Feng W. A gestational choriocarcinoma of the ovary diagnosed by DNA polymorphic analysis: a case report and systematic review of the literature. J Ovarian Res. 2017;10(1):46.
doi pubmed - Wan X, Li J, Xie X. Extrauterine choriocarcinoma of the greater omentum after tubal pregnancy: case report. Int J Gynecol Cancer. 2006;16(3):1476-1478.
doi pubmed - Blaustein's pathology of female genital tract. Sixth edition. p. 867-869.
- Fujiyoshi Y, Jiang S, Feng X. Intramucosal stomach adenocarcinoma metastasizing as a large intraabdominal mass with focal choriocarcinomatous differentiation. Int J Clin Exp Pathol. 2012;5(8):845-851.
- Motoyama T, Higuchi M, Taguchi J. Combined choriocarcinoma, hepatoid adenocarcinoma, small cell carcinoma and tubular adenocarcinoma in the oesophagus. Virchows Arch. 1995;427(4):451-454.
doi pubmed - Oh SK, Kim HW, Kang DH, Choi CW, Choi YY, Lim HK, Goo JJ, et al. Primary Adenocarcinoma with Focal Choriocarcinomatous Differentiation in the Sigmoid Colon. Korean J Gastroenterol. 2015;66(5):291-296.
doi pubmed - Mardi K, Gupta S, Gupta N, Mahajan V. Choriocarcinomatous differentiation in rectal adenocarcinoma: A rare occurrence. South Asian J Cancer. 2014;3(2):144-145.
doi pubmed - Buza N, Baine I, Hui P. Precision genotyping diagnosis of lung tumors with trophoblastic morphology in young women. Mod Pathol. 2019;32(9):1271-1280.
doi pubmed - Sung HJ, Maeng YI, Kim MK, Lee SJ, Kang SM, Bong JG, Oh HK. Breast carcinoma with choriocarcinomatous features: a case report. J Breast Cancer. 2013;16(3):349-353.
doi pubmed - Mohammadi A, Rosa M. Carcinoma of the breast with choriocarcinomatous features. Arch Pathol Lab Med. 2011;135(9):1097-1100.
doi pubmed - Resetkova E, Sahin A, Ayala AG, Sneige N. Breast carcinoma with choriocarcinomatous features. Ann Diagn Pathol. 2004;8(2):74-79.
doi pubmed - Rajabi B, Khoury J, Brewer C, Goodman OB, Jr. Urothelial bladder carcinoma with choriocarcinomatous differentiation presenting with a false-positive pregnancy test. Am J Med Sci. 2013;346(3):256-258.
doi pubmed - Grammatico D, Grignon DJ, Eberwein P, Shepherd RR, Hearn SA, Walton JC. Transitional cell carcinoma of the renal pelvis with choriocarcinomatous differentiation. Immunohistochemical and immunoelectron microscopic assessment of human chorionic gonadotropin production by transitional cell carcinoma of the urinary bladder. Cancer. 1993;71(5):1835-1841.
doi - Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22(Suppl 2):S96-S118.
doi pubmed - Carta G, Accurti V, Di Nicola M, Crisman G, Sollima L, Carta A, Patacchiola F. Uterine endometrioid carcinoma with focal area of choriocarcinomatous differentiation: case report. Eur J Gynaecol Oncol. 2014;35(6):731-733.
- Ishida M, Okabe H. Endometrioid adenocarcinoma with choriocarcinomatous differentiation: A case report and review of the literature. Oncol Lett. 2013;6(3):655-658.
doi pubmed - Chumworathayi B, Kleebkaow P. Primary non-gestational uterine cervical choriocarcinoma with metaplastic transformation from squamous cells. Asian Pac J Cancer Prev. 2007;8(4):642-644.
- Pavelka JC, Bryant DA, Vaccarello L. Adenocarcinoma of the uterine cervix with choriocarcinomatous metastasis. Gynecol Oncol. 2006;101(2):346-348.
doi pubmed - Mukonoweshuro P, McCluggage WG. Clear cell carcinoma of the cervix with choriocarcinomatous differentiation: report of an extremely rare phenomenon associated with mismatch repair protein abnormality. Int J Gynecol Pathol. 2017;36(4):323-327.
doi pubmed - Wakahashi S, Sudo T, Nakagawa E, Ueno S, Muraji M, Kanayama S, Itami H, et al. Endometrioid adenocarcinoma with high-grade transformation with serous and choriocarcinomatous differentiation - a case report. J Cancer. 2012;3:14-18.
doi pubmed - Yamada T, Mori H, Kanemura M, Ohmichi M, Shibayama Y. Endometrial carcinoma with choriocarcinomatous differentiation: a case report and review of the literature. Gynecol Oncol. 2009;113(2):291-294.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
