| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 10, Number 1, March 2021, pages 18-21
Myxoid Leiomyosarcoma of the Uterus: A Case Report With Magnetic Resonance Imaging Findings
Aya Tanabea, d, Tetsuo Maedab, Yasuo Nakatac, Shigeki Yoshidaa
aDepartment of Gynecology and Obstetrics, Chibune General Hospital, Osaka, Japan
bDepartment of Diagnostic Radiology, Chibune General Hospital, Osaka, Japan
cDepartment of Pathology, Chibune General Hospital, Osaka, Japan
dCorresponding Author: Aya Tanabe, Chibune Hospital, 3-2-29, Fukumachi, Nishiyodogawaku, Osaka City, Osaka, Japan
Manuscript submitted July 17, 2020, accepted December 14, 2020, published online December 30, 2020
Short title: M-LMS With MRI Findings
doi: https://doi.org/10.14740/jcgo675
| Abstract | ▴Top |
Myxoid leiomyosarcoma (M-LMS) of the uterus is extremely rare and its diagnosis is challenging. We report a case of the M-LMS in a 69-year-old female who referred to our hospital for abdominal discomfort and increased uterine mass lesion. Magnetic resonance imaging (MRI) demonstrated a well-defined intramural mass (approximately 8 cm in diameter) that exhibited isointensity to myometrium on T1-weighted images, and markedly high heterogeneous intensity and flow-void area suggestive of abundant blood flow on T2-weighted images. On diffusion weighted imaging, the major portion the mass showed high intensity, but it was considered to be T2 shine-through effect, because the mean apparent diffusion coefficient (ADC) value of the lesion was relatively high. Thus, we could not make the diagnosis of malignancy. However, considering the increase tendency of the mass at the postmenopausal status, the possibility of malignancy could not be ruled out, so a total abdominal hysterectomy with bilateral salpingo-oophorectomy was performed. The result of pathologic assessment is M-LMS. A diagnosis of M-LMS is difficult preoperatively. In the case that myxoid tumor is suspected, although malignancy is not definite, we might have to consider the possibility of malignancy from the age of the patient and the tendency of the lesion to increase.
Keywords: Myxoid leiomyosarcoma; MRI; Diffusion
| Introduction | ▴Top |
Only 3-7% of uterine malignancies are uterine sarcomas [1], and leiomyosarcoma (LMS) is the most common type of uterine sarcoma, consisting of up to 80% of uterine sarcomas when malignant mixed Mullerian tumors are excluded [2]. Among them, myxoid leiomyosarcoma (M-LMS) is extremely rare. It shows malignant process clinically, but convalescence is not bad as conventional LMS.
To the best of the authors’ knowledge, M-LMS of the uterus has been documented in 30 reports or so, and no imaging findings are available. Here the authors present the magnetic resonance imaging (MRI) findings of this rare tumor.
| Case Report | ▴Top |
A 69-year-old female, postmenopausal for 19 years, was referred to our hospital for abdominal discomfort and increased uterine mass lesion. She had no symptoms of hypermenorrhea or dysmenorrhea and no notable medical or family history. Physical examination revealed that she had an enlarged uterus and the transvaginal ultrasound revealed the presence of an 8-cm rounded uterine mass. The routine blood investigation results including serum lactate dehydrogenase (LDH) were normal. The levels of cancer antigen (CA)125 and CA19-9 were also within normal limits. We performed MRI of lower abdomen using a 3-T Magnetom Skyra (Siemens Healthcare, Erlangen, Germany). It demonstrated a well-defined intramural mass (approximately 8 cm in diameter) located in the center of the uterine fundus. The mass exhibited isointensity to myometrium on T1-weighted images, and markedly high heterogeneous intensity and flow-void area suggestive of abundant blood flow on T2-weighted images (Fig. 1a). Areas suggesting intra-tumoral hemorrhage or necrosis were not depicted. On gadopentetate dimeglumine (Gd-DTPA) contrast-enhanced images including fat suppressed T1-weighted images and dynamic study, the intra-tumoral vessels were rapidly enhanced in the early phase at 15 - 60 s after initiation of Gd-DTPA administration (Fig. 1b), and then the peripheral part of the mass was gradually enhanced (Fig.1c) on diffusion weighted imaging (DWI) with a b-value of 800 s/mm2. The major portion the mass showed high intensity, but it was considered to be T2 shine-through effect, because the mass showed also relatively high intensity on apparent diffusion coefficient (ADC) map and the mean ADC value of the lesion was relatively high (2.1 × 10-3 mm2/s) (Fig. 1d, e), which was atypical for malignancy. Thus, degenerated leiomyoma with edematous or myxoid change was suspected. However, considering the increase tendency of the mass at the postmenopausal status, the possibility of malignancy could not be ruled out, so a total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAHBSO) was performed.
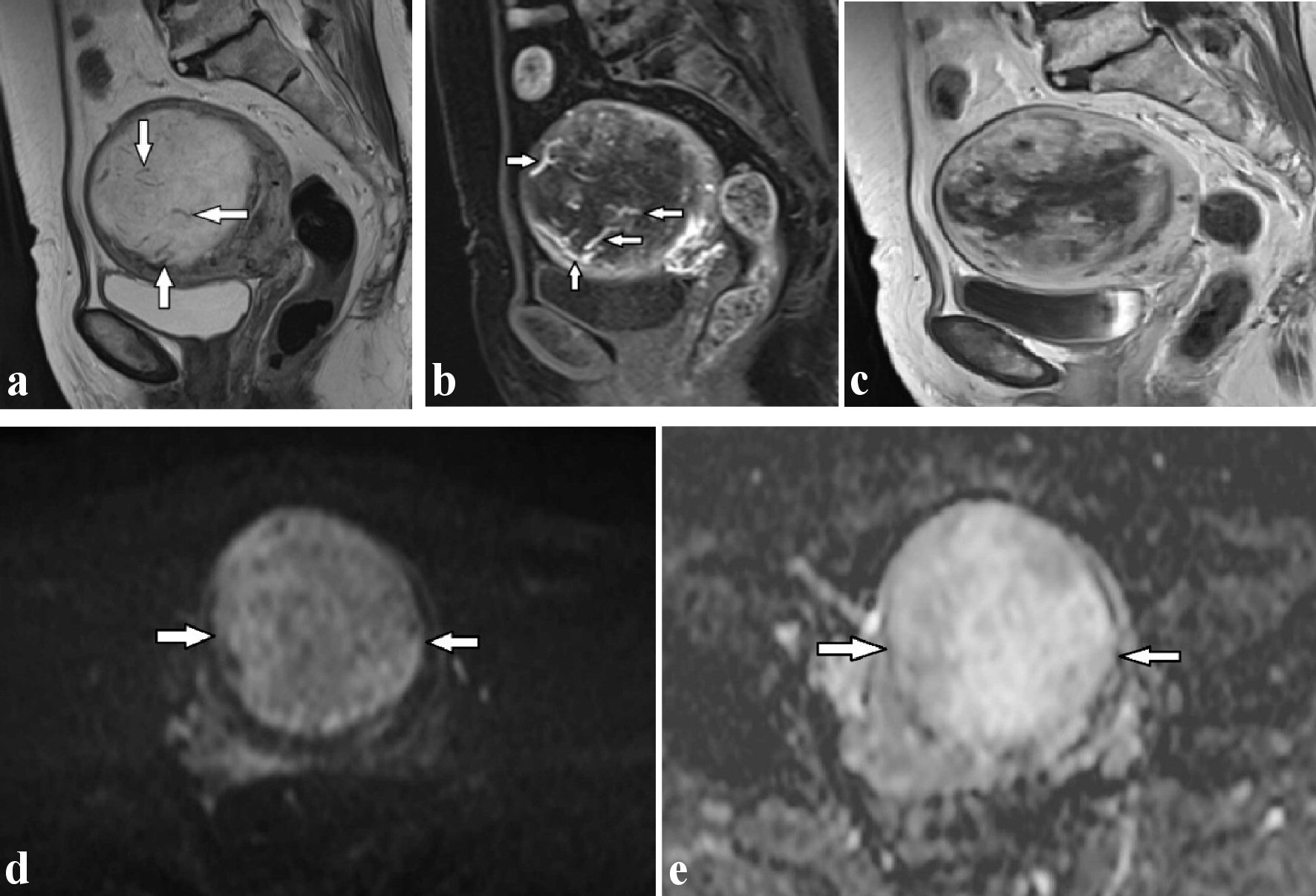 Click for large image | Figure 1. (a) T2-weighted sagittal image shows multiple flow-void signals (arrows) in the uterine mass revealing marked hyperintensity. (b) The flow-voids are found to correspond to dilated arteries located inside the mass in the early phase after administration of Gd-DTPA on dynamic T1-weighted images (arrows). (c) The peripheral part of the mass was gradually enhanced. Diffusion-weighted imaging (d) and apparent diffusion coefficient map (e) reveal the hyperintensity uterine mass (arrows), indicative of T2-shine through effect, which was suggestive of edematous change. Gd-DTPA: gadopentetate dimeglumine. |
A uterine mass measuring 76 × 67 × 79 mm was found. The tumor is grey-black tan in color with area of hemorrhage and foci of necrosis, and foci necrosis representing 40% of the tumor (Fig. 2a). There was no evidence of extrauterine disease. Both adnexa were unremarkable.
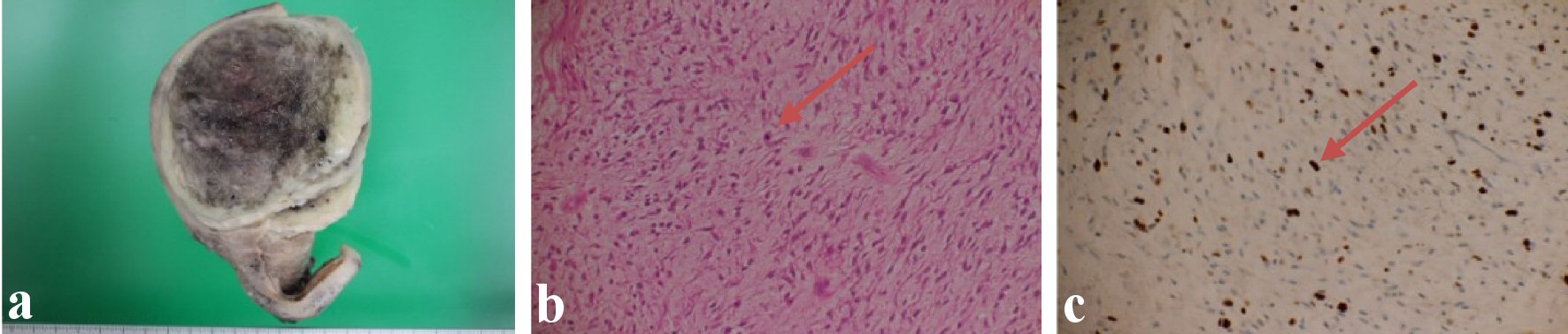 Click for large image | Figure 2. (a) Uterine tumoral mass with areas of hemorrhage and necrosis. (b) Atypical nuclear were seen in myxoid stroma (arrow). (c) Ki67 is 10-20% positive (arrow). |
On microscopic examination, the tumor was highly myxomatous, and large areas of the tumor were necrotic and hemorrhagic (Fig. 2b). This material showed positive for alcian blue.
The growth images of the tumor cells that have atypical nucleus were seen in the background of myxoid stroma.
The mitotic index accounts three to four mitotic figures per 10 high-power fields (HPFs). The presence of tumor cell necrosis is noted.
The following markers were used: smooth muscle actin (SMA), HHF35, caldesmon, CD34 and vimentin. The immunohistochemistry examination reveals the positivity for smooth muscle cell differentiation, the marker with diffuse positivity for myogenin is noted. The caldesmon is focal positive. Ki-67 is 10-20% positive which indicates the aggressiveness of the tumor. CD34 and HHF35 are negative (Fig. 2c), considering the diagnosis criteria (abundant myxoid matrix (positive for alcian blue), moderate atypia, ranging from low to high, tumor cell necrosis evidence and high mitotic index), the final diagnosis is the M-LMS of the uterus.
The surgical resection margins were free from tumor cells. The patient’s postoperative course and recovery were uneventful. According to the National Comprehensive Cancer Network (NCCN) guidelines, the additional therapy is to observe or chemotherapy. She refused it and has not exhibited signs of local recurrence or metastasis in 1.5 year after surgery.
| Discussion | ▴Top |
M-LMS of the uterus is a rare and aggressive neoplasm that characterized by infiltrative tumor borders and variability of other features (mitotic count, atypia, and necrosis), and knowledge of its clinical behavior and morphology remains limited [3]. This malignancy affects females with a mean age of 54 (a decade later than the mean age for leiomyomas), the mean tumor size was approximately 93 mm, and the prognosis was poor [4]. Burch et al identified infiltrative margins, intravascular extension, large size (> 80 mm), and p53 positivity as the most important features associated with aggressive behavior [5].
Diagnostic evaluation of LMS and making a distinction between benign leiomyoma and its malignant counterpart are challenging as those symptoms are often similar. Since an endometrial biopsy may not usually be helpful for the definitive diagnosis of uterine mesenchymal malignancies, MRI including methods such as DWI and dynamic contrast-enhanced study may play an important role in diagnosing these tumors and determining appropriate management. Until now, more than 30 cases have been reported in the English language literature. However, MRI findings are not described in those reports. Thus, the present case is considered to be the first case describing the MR features of this rare tumor. The current lesion showed tortuous flow-void areas on the noncontrast-enhanced MR images and enlarged intra-tumoral arteries on contrast-enhanced MR images, suggestive of hypervascularity in the mass.
These findings are often revealed in the cases of leiomyomas. However, in the postmenopausal status such as the present case, tumoral hypervascularity might suggest the possibility of malignancies because the intra-uterine vessels, giving blood supply to the uterine mass, usually decrease in size after menopause [6].
DWI-MRI, which measures the Brownian motion of molecules and highlights the increased cellularity of cancer tissue through quantitative evaluation using the ADC map, is an important method for differentiating between benign and malignant tumor including gynecological lesions. Sato et al suggest that MRI using a combination of signal intensity on DWI and ADC value is very effective, simple, and easy to apply clinically for differential diagnosis of LMS and leiomyoma [7]. In the present case, however, it was difficult to diagnosis malignancy on MRI, because it suggested T2 shine-through effect caused by a high T2 signal rather than by restricted diffusion. This phenomenon is most often observed with vasogenic edema. In addition, the ADC value of myxoid tumor was significantly higher than that of nonmyxoid tumors, because these high values directly reflect the high mucin and low collagen content in the lesion, representing a lesion composed of a large amount of water [8]. Accordingly, the MRI findings of the present case were compatible with the hypothesized pathophysiology. Thus, in the case that myxoid uterine tumor is suspected, DWI-MRI might not be effective for making a distinction between leiomyoma and malignant myometrial tumor such as M-LMS, and it may be better to decide on the treatment strategy from the patient’s age and growing trend of the lesion.
Differential diagnoses of M-LMS include myxoid leiomyoma, inflammatory myofibroblastic tumor and endometrial stromal tumor, the mass of which can display myxoid change [4].
In summary, M-LMS of the uterus is often visualized as a mass suggestive of T2 shine-through effect on MRI. In such cases, we might have to consider the possibility of the malignancy depending on the patient’s age and growing trend of the lesion.
Acknowledgments
None to declare.
Financial Disclosure
None of the authors have any financial support to disclose.
Conflict of Interest
The authors declare no conflicts of interest associated with this manuscript.
Informed Consent
Signed informed consent from the patient to present her care as a case report was obtained.
Author Contributions
AT, TM, YN, and SY contributed to diagnosis, treatment and follow-up of this case. AT wrote the first draft. TM contributed to revising the article. All authors read and approved the final manuscript.
Date Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- D'Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131-139.
doi pubmed - Wang WL, Soslow R, Hensley M, Asad H, Zannoni GF, de Nictolis M, Branton P, et al. Histopathologic prognostic factors in stage I leiomyosarcoma of the uterus: a detailed analysis of 27 cases. Am J Surg Pathol. 2011;35(4):522-529.
doi pubmed - Parra-Herran C, Schoolmeester JK, Yuan L, Dal Cin P, Fletcher CD, Quade BJ, Nucci MR. Myxoid leiomyosarcoma of the uterus: a clinicopathologic analysis of 30 cases and review of the literature with reappraisal of its distinction from other uterine myxoid mesenchymal neoplasms. Am J Surg Pathol. 2016;40(3):285-301.
doi pubmed - Barone A, Ambrosio MR, Rocca BJ, Mastrogiulio MG, Ambrosio A, Santopietro R. Myxoid leiomyosarcoma of the uterus: a case report. Eur J Gynaecol Oncol. 2014;35(3):322-324.
- Burch DM, Tavassolil FA. Myxoid leiomyosarcoma of the uterus. Histopathology. 2011;59(6):1144-1155.
doi pubmed - Wilde S, Scott-Barrett S. Radiological appearances of uterine fibroids. Indian J Radiol Imaging. 2009;19(3):222-231.
doi pubmed - Sato K, Yuasa N, Fujita M, Fukushima Y. Clinical application of diffusion-weighted imaging for preoperative differentiation between uterine leiomyoma and leiomyosarcoma. Am J Obstet Gynecol. 2014;210(4):368 e361-368 e368.
doi pubmed - Maeda M, Matsumine A, Kato H, Kusuzaki K, Maier SE, Uchida A, Takeda K. Soft-tissue tumors evaluated by line-scan diffusion-weighted imaging: influence of myxoid matrix on the apparent diffusion coefficient. J Magn Reson Imaging. 2007;25(6):1199-1204.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
