| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 9, Number 4, December 2020, pages 96-101
Gabapentin for Peri-Operative Pain Relief in Office Gynecological Surgery: A Double-Blind Randomized Controlled Trial
Oxana Zarudskayaa, Adam V. Levyb, d, Chad L. Crossc
aDepartment of Obstetrics and Gynecology, University of Toledo College of Medicine and Life Sciences, Toledo, OH, USA
bSchool of Medicine, University of Nevada Las Vegas, 1701 W. Charleston Bvld, Ste 290, Las Vegas, NV, USA
cMedicine Research, Biostatistics & Epidemiology, University of Nevada Las Vegas, 4505 S. Maryland Pkwy, Las Vegas, NV, USA
dCorresponding Author: Adam V. Levy, School of Medicine, University of Nevada Las Vegas, 1701 W. Charleston Bvld, Ste 290, Las Vegas, NV 89102, USA
Manuscript submitted August 11, 2020, accepted September 22, 2020, published online December 15, 2020
Short title: Gabapentin for Peri-Operative Pain Relief
doi: https://doi.org/10.14740/jcgo689
| Abstract | ▴Top |
Background: This study aims to validate the use of gabapentin in peri-operative pain management for outpatient gynecological (GYN) office procedures using surgical abortion at gestational ages less than 24 weeks.
Methods: We conducted a double-blind, randomized, placebo-controlled trial of gabapentin in an ambulatory surgical abortion center. Eligible participants were patients 18 years of age and older, English or Spanish speaking, seeking a surgical elective abortion with pain management consisting of local anesthesia and intravenous sedation at gestational ages from 5 to 23 6/7 weeks. The study site was Birth Control Care Center, an affiliated clinic with the University of Nevada Las Vegas School of Medicine. Patients received routine pain control plus either placebo or gabapentin 600 mg 1 - 2 h prior to surgery. The primary outcome measured was post-operative pain scores using a 100-point (100 mm) visual analog scale at 5 min after the procedure. Additionally, pain scores were obtained at 30 min and 24 h post-procedure. Other secondary outcomes measured were nausea and vomiting at 5 min and 24 h post-procedure.
Results: From May 2017 to March 2018, 219 patients were recruited to this study from 400 patients who were offered participation. A total of 109 were randomized to gabapentin and 110 were randomized to placebo. Demographic characteristics, pregnancy history and general health conditions were similar. Pain scores at 5 min, 30 min and 24 h after the procedure were not significantly different between arms (P = 0.28), though pain was reported to decrease between 30 min and 24 h post-procedure (P < 0.001) for both groups. Nausea and vomiting were also not significantly reduced in the gabapentin group (P = 0.32 and 0.47), respectively.
Conclusions: Patients who received gabapentin, in combination with the routine intravenous pain control and local anesthesia for office-based surgical abortion did not experience less pain at 5 min, 30 min, nor at 24 h after the procedure, compared to the placebo group. Gabapentin did not appear to reduce pain at 24 h after the procedure and is therefore unlikely to reduce narcotic use in this clinic sample treated with intravenous (IV) sedation. Further, gabapentin was not useful to reduce nausea and vomiting post-operatively in this clinic sample.
Keywords: Gabapentin; Peri-operative pain; Pain relief; Gynecology; Surgery
| Introduction | ▴Top |
Pain management during gynecological office surgical procedures relies on paracervical local anesthesia with or without intravenous sedation. General anesthesia used in some settings has both limited availability, higher risks and costs [1]. Despite these regimens, women undergoing in-office gynecological surgical procedures continue to report moderate pain during and/or after procedures [2]. Efforts to find more effective regimens have been unsuccessful [3, 4].
The use of gabapentin for the amelioration of peri-operative pain has been well demonstrated in numerous studies. A systematic review of 22 randomized controlled trials (RCTs) involving different surgical procedures demonstrated that a single dose of pre-operative gabapentin significantly reduced opioid consumption [5] and that given prior to hysterectomy or cesarean delivery resulted in significantly reduced pain scores [6, 7]. Gabapentin is a Food and Drug Administration (FDA) approved medication that is well tolerated and also appears to reduce nausea and vomiting, a common problem among patients undergoing different surgical procedures [6]. Overall, gabapentin is a safe drug. The most common side effects are noted to be dizziness, fatigue, somnolence, ataxia, peripheral edema, nystagmus, nausea and vomiting, headache, weight gain, asthenia, and amblyopia. Most of these side effects are associated with chronic usage. Other very rare side effects may include multi-organ sensitivity, withdrawal seizures (if given for epilepsy), drug reaction with eosinophilia and systemic symptoms (DRESSs), and suicidal behavior [5]. Drug interactions are both rare and mild if at all present. The peak plasma levels occur at 2 h and the half-life is 5 - 7 h.
The low cost and well-tolerated medication could vastly improve pain management in outpatient surgical settings impacting hundreds of thousands of women each year and may also be useful in other minor surgery settings as well. Gabapentin’s off-label uses have been well documented [8].
Our study sought to validate the use of gabapentin in the peri-operative pain management for outpatient office-based elective surgical abortion up to 23 6/7 weeks gestation by ultrasound. We speculated that adding a single dose of 600 mg gabapentin 1 - 2 h prior to the procedure to usual pain control regimens could reduce pain associated with the surgical procedure. Further, we believed that pre-operative gabapentin might reduce nausea, vomiting, and anxiety with few or no side effects.
| Patients and Methods | ▴Top |
Procedures and patient enrollment
This study (all procedures, forms, consents, treatments, data storage, and reporting) was approved by the University of Nevada, Reno Institutional Review Board (#734274) and we complied with all ethical standards for human studies research (all members CITI certified). We used a double-blind placebo controlled trial of gabapentin in the setting of an ambulatory surgical abortion center (Birth Control Care Center, Las Vegas, NV, USA) where patients received routine intravenous sedation (IVS) consisting of midazolam 4 mg, fentanyl 100 µg, and local para-cervical 1% lidocaine block anesthesia (para-cervical block anesthesia, PCB) plus either 600 mg of gabapentin or placebo 1 - 2 h prior to surgery. Patients scheduled to undergo surgical abortion between 5 and 23 6/7 weeks by ultrasound gestational age (UGA) were approached during the pre-operative counseling and offered participation. Patients included were 18 years of age or older, speaking English or Spanish, and eligible for office-based surgical pregnancy termination. Exclusion criteria included severe renal disease, current use of gabapentin or pregabalin, sensitivity or allergy to gabapentin, a contraindication to gabapentin, or missed abortion. Participants were not compensated and could withdraw from the study at any time.
The allocation and concealment method used was sequentially numbered, opaque pill containers, containing either gabapentin or placebo in identical appearing capsules. Randomization of these sequential containers was done by balanced fixed blocks of six using a random number generator to determine placebo from treatment. Both the patients and providers were blinded to the study interventions.
Recruitment was sequential and based on the patient qualifying and consenting to the study. A standardized script was used. All study staff were trained in the procedures and provided with a study protocol prior to beginning of the study. A standard operations manual was available to the study staff to refer to for operational details. The study investigators provided instructions concerning the recording of study data on clinical research forms. The study investigators had the responsibility to assure the quality of computerized data, the protocol development and the final study databases.
Screening was done using a standardized checklist and the informed consent was reviewed with all eligible participants. Once consent was obtained and signed, the participants received the randomized pre-medication. Treatment bottles had been sequentially numbered so that participants received the next available bottle in the sequence as they were recruited. Participants took the medication in front of the research staff. Baseline information was collected including age, marital and household status, race/ethnic group, gravidity, parity, last menstrual period (LMP), gestational age, body mass index (BMI), past medical and surgical history, medications, recreational drug use, prior abortions, and menstrual pain.
A master list for the randomized treatments was generated by the pharmacy staff and maintained by University staff. Unblinding was only available to the investigators after participants had completed treatment (more than 24 h).
Patients received misoprostol 400 µg buccal 90 min prior to surgery who were more than 12 weeks by ultrasound (UGA). Those patients more than 17 weeks by UGA had laminaria placed 1 day prior to surgery and patients at 20 to 23 6/7 weeks by UGA had laminaria placed over 2 days as well as digoxin 1 mg intrafetal injection 2 days prior to surgery. All procedures were performed by the attending physician and residents who were trained and credentialed for these procedures.
Patient questionnaires using 100 mm visual analog scales (VASs) were used by study staff prior to surgery and at 5 and 30 min after the procedure was completed as defined by speculum removal. We determined that our primary outcome measure was the pain score at 5 min as measured by the VAS scale. Secondary measures included pain at 30 min and 24 h following surgery, nausea and vomiting pre- and post-operatively, pre-procedure pain, and anxiety using the VAS [9]. Post-operatively patients received oxycodone 7.5 mg (quantity 10) for pain control at home. The 24-h follow-up contact was completed telephonically and included a study questionnaire.
Power calculations were performed to detect a 20-25-point VAS score difference between the treatment and placebo groups as we determined that this would be clinically relevant based on other pain studies [9-11]. This calculation yielded a need for approximately 100 patients in each arm based on 80% power and a type one error of 0.05.
Statistical procedures and data analysis
All data were transcribed by Dr. Oxana Zarudskaya from the clinical research forms into REDCap (institutionally managed by University of Nevada, Reno), a web-based, password protected relational database and forms were secured in locked files. Data were examined after transcription for accuracy, and descriptive statistics were utilized to determine if potential errors were made in data entry. Data were subsequently imported into SPSS (v.25) for analyses. Missing data were elements excluded from analyses. Potential differences in socio-demographic variables were examined using Chi-square analyses with Bonferroni-corrected post-hoc comparisons, except for “age”, which was tested using an independent samples t-test. Examination of VAS scores demonstrated skewness issues, and hence data were rank transformed for these variables. Kruskal-Wallis tests were used to examine potential differences in gestational age between study arms. Mann-Whitney tests were utilized for comparing the treatment arms on outcomes with VAS measurements, except for pain. The pain variable was time-based, and hence we examined potential temporal trends in pain at 5 min, 30 min, and 24 h post-procedure using a repeated-measures general linear model with baseline pain as a covariate; the repeated-measures analysis of variance (RM-ANOVA) provided the same statistical interpretation as the rank-based transformation, and hence the standard RM-ANOVA results are presented below.
| Results | ▴Top |
From May 2017 to March 2018 date, 400 patients were approached to participate in the study. A total of 219 patients consented to participate in the study, though three patients were ultimately excluded (one delivered the night before the scheduled procedure, and two were excluded owing to faulty pharmacy preparations). Hence, a total of 216 patients (107 gabapentin arm and 109 placebo arm) were included in the analyses (Fig. 1).
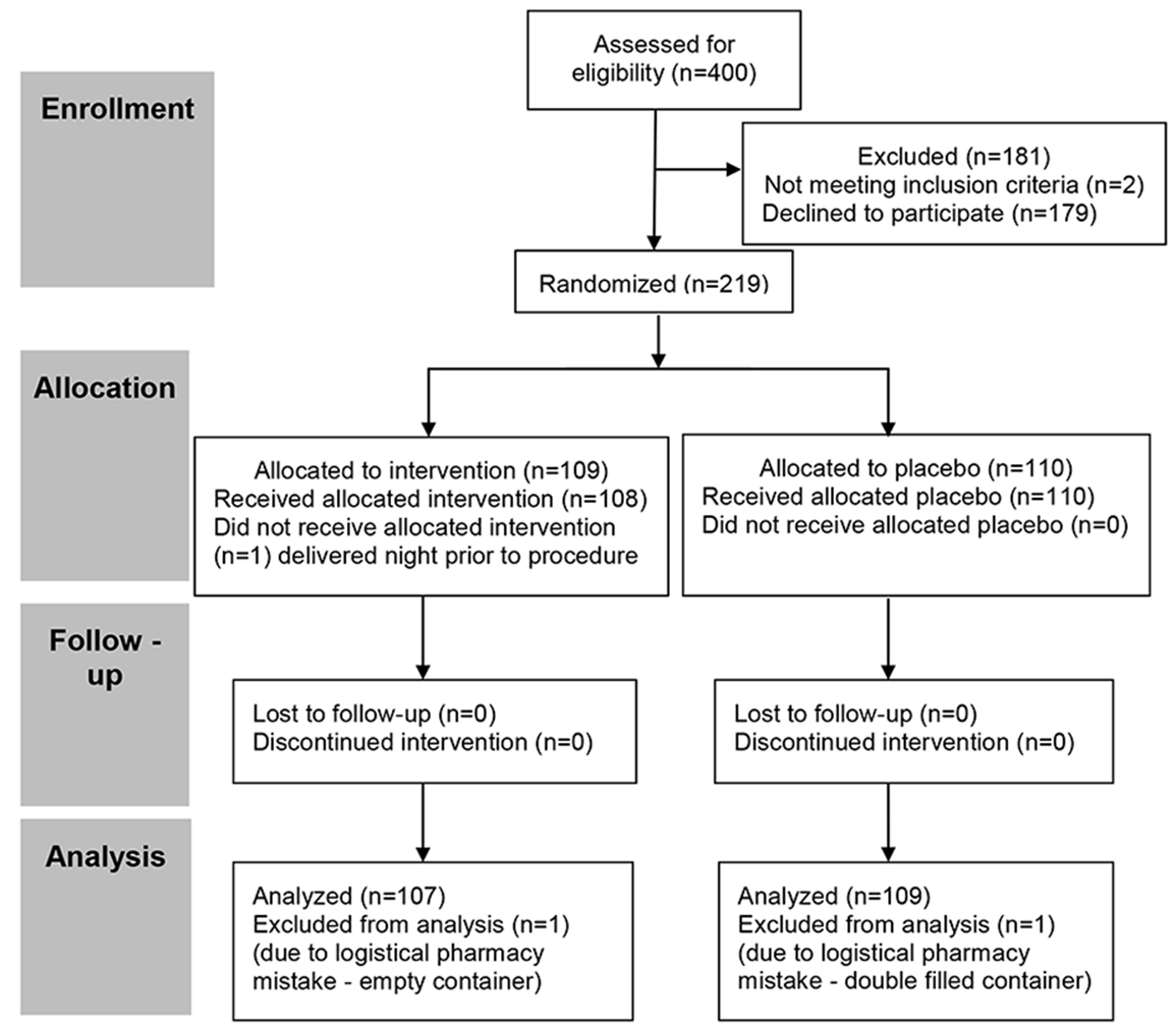 Click for large image | Figure 1. CONSORT flow diagram of participant recruitment, enrollment, randomization, follow-up, and analysis. CONSORT: Consolidated Standards of Reporting Trials. |
Patient socio-demographic and baseline health characteristics were relatively equitable between arms with some exceptions (Table 1). Overall race differed between arms (χ2 = 16.18, P = 0.013), and specifically between “White/Caucasian”, “other”, and “more than one race” categories (all P < 0.05). Ethnicity, however, did not differ between arms (χ2 = 1.67, P = 0.152). Living arrangements were distributed similarly apart from “Living with children”, which was significantly higher for the placebo arm (χ2 = 4.28, P = 0.039). In terms of baseline pregnancy and health history, those in the placebo group were more likely to report a history of vaginal deliveries (χ2 = 5.44, P = 0.020). There were no statistical differences in reported menstruation history (χ2 = 3.21, P = 0.524), BMI (χ2 = 4.30, P = 0.367), or age (t = 0.65, P = 0.517). Further, the distribution of gestational ages between study arms was nearly identical (χ2 = 0.05, P = 0.977).
 Click to view | Table 1. Patient Characteristics |
Patient symptoms, expectation, and satisfaction pre- and post-operatively were not different between placebo and gabapentin patients (all P > 0.15). Interestingly, VAS measures were high for both samples for nervousness associated with the procedure and for expected pain from the procedure. This did not translate into demonstrable differences in pain between arms (F = 1.19; P = 0.278), though there was a significant reduction of pain between 30 min post-procedure and 24 h post-procedure (F = 39.40, P < 0.001) for both groups. There were also no differences between groups in nausea (P = 0.321) or vomiting (P = 0.474) 24 h post-procedure. Further, and importantly, satisfaction, both overall and for pain, was nearly identical and near 100 for both arms (Table 2). Though it was anticipated that some differences may be found based on gestational age, this was not the case. Though those in the oldest gestational group expected more pain with the procedure (P = 0.022), actual reports of pain, nervousness, nausea, vomiting, and satisfaction were not statistically different among gestational age groups (all P > 0.10).
 Click to view | Table 2. Patient Symptoms, Expectations, and Satisfaction Pre- and Post-Operatively |
| Discussion | ▴Top |
In this double-blind randomized control trial, we demonstrated that patients who received the IVS, local PCB plus 600 mg of gabapentin 1 - 2 h prior to surgery did not experience less pain compared to the placebo arm at 5 min, 30 min, and 24 h post-procedure. These findings are in line with published data from a similar randomized control trial, evaluating the effect of oral gabapentin in conjunction with usual oral pain management regimens of lorazepam, ibuprofen, oxycodone, and acetaminophen for surgical abortion on pain 5 and 30 min post-procedure [12]. The choice of 5 and 30 min post-procedure as well as the 24 h evaluation was based on prior pain studies [12-14] and consistent with studies demonstrating that women experience most pain during suctioning in surgical abortion procedures [13, 14].
For medical terminations of pregnancies, the use of gabapentinoids, particularly pregabalin, as adjuncts to reduce the use of opiate pain medications has shown a modest effect while not reducing overall pain scores [15]. Similarly, opioid analgesia did not demonstrate a decrease in maximum pain scores for medical terminations in a 2018 study [16].
The successful use of gabapentin prophylaxis for prevention of post-operative nausea and vomiting has been well documented in abdominal surgeries [17]. However, in our population it did not demonstrate any benefit. Importantly, we also demonstrated that gabapentin did not significantly reduce pain at 24 h post-procedure. Therefore, it is very unlikely that patients who receive IVS for pregnancy termination would benefit from gabapentin pre-treatment in order to reduce narcotic use post-operatively. This is in stark contrast to the study by Gray et al, who demonstrated reduced narcotic use at 24 h in surgical abortion patients [12]. These patients’ surgical pain management was entirely oral medications and local anesthesia. This does not represent the majority of pain management methods used nationwide in abortion clinics as IVS is most common [18, 19]. Therefore, for the majority of patients having surgical abortion, gabapentin pretreatment does not appear to reduce pain, nausea, or narcotic use.
Strengths of this study include the double-blind randomized study design, which ensured that neither the patients nor the study staff were aware of which treatment the patient had received.
Limitations in the work presented here include the small number of patients at gestational age 19 - 24 UGA. Had this gestational age group been larger, it is possible that we may have demonstrated some differences for this group, as preliminary RM-ANOVAs with the limited number of patients provided some evidence that this group may differ from those of younger gestational age in combination with other model covariates, though this analysis was not powered for such a determination. We anticipate that future studies should include equitable samples across gestational age in order to fully address this potential result. Also, we did not directly measure narcotic use at 24 h. However, the measurement of perceived pain was not different between groups at 24 h thus supporting our view that narcotic use would also not be significantly different. We hope to collaborate and participate in a meta-analysis that could overcome our individual sample sizes to perhaps reveal increased statistical and clinically relevant results. However, it appears very convincing that our patients who received IVS and gabapentin pre-treatment did not see significant benefits.
Acknowledgments
We would like to express our thanks to State of Nevada Women’s health Grant support.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained prior to participation in study and approved by IRB of University of Nevada, Reno (#734274).
Author Contributions
AVL conceived the original idea, supervised the project, supervised data entry and analysis, and wrote the manuscript; OZ contributed to patient recruitment, data collecting/transferring, and manuscript writing; CLC contributed to statistical analysis and manuscript writing. All authors discussed the results and contributed to the final manuscript jointly.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Protocol Disclaimer
This research protocol is nearly identical to that performed by Gray et al (OB/GYN 2019; 134:611-619). This is by design as the protocol was developed in 2015 by collaboration among six investigators which included Gray, Haddad and Levy. The intention was to combine results for a prospective meta-analysis. This was not accomplished. However, this was a shared protocol to be freely used among the members of this collaboration.
| References | ▴Top |
- Renner RM, Jensen JT, Nichols MD, Edelman A. Pain control in first trimester surgical abortion. Cochrane Database Syst Rev. 2009;2:CD006712.
doi pubmed - Raymond EG, Weaver MA, Louie KS, Dean G, Porsch L, Lichtenberg ES, Ali R, et al. Prophylactic compared with therapeutic ibuprofen analgesia in first-trimester medical abortion: a randomized controlled trial. Obstet Gynecol. 2013;122(3):558-564.
doi pubmed - Jackson E, Kapp N. Pain control in first-trimester and second-trimester medical termination of pregnancy: a systematic review. Contraception. 2011;83(2):116-126.
doi pubmed - Chor J, Hill B, Martins S, Mistretta S, Patel A, Gilliam M. Doula support during first-trimester surgical abortion: a randomized controlled trial. Am J Obstet Gynecol. 2015;212(1):45 e41-46.
doi pubmed - Tiippana EM, Hamunen K, Kontinen VK, Kalso E. Do surgical patients benefit from perioperative gabapentin/pregabalin? A systematic review of efficacy and safety. Anesth Analg. 2007;104(6):1545-1556, table of contents.
doi pubmed - Alayed N, Alghanaim N, Tan X, Tulandi T. Preemptive use of gabapentin in abdominal hysterectomy: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1221-1229.
doi pubmed - Moore A, Costello J, Wieczorek P, Shah V, Taddio A, Carvalho JC. Gabapentin improves post cesarean delivery pain management: a randomized, placebo-controlled trial. Anesthesia and analgesia. 2011;112:167-173.
doi pubmed - Mack A. Examination of the evidence for off-label use of gabapentin. J Manag Care Pharm. 2003;9(6):559-568.
doi pubmed - Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4(7):407-414.
doi - Rowbotham MC. What is a "clinically meaningful" reduction in pain? Pain. 2001;94(2):131-132.
doi - Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485-489.
doi - Gray BA, Hagey JM, Crabtree D, Wynn C, Weber JM, Pieper CF, Haddad LB. Gabapentin for perioperative pain management for uterine aspiration: a randomized controlled trial. Obstet Gynecol. 2019;134(3):611-619.
doi pubmed - Wong CY, Ng EH, Ngai SW, Ho PC. A randomized, double blind, placebo-controlled study to investigate the use of conscious sedation in conjunction with paracervical block for reducing pain in termination of first trimester pregnancy by suction evacuation. Hum Reprod. 2002;17(5):1222-1225.
doi pubmed - Glantz JC, Shomento S. Comparison of paracervical block techniques during first trimester pregnancy termination. Int J Gynaecol Obstet. 2001;72(2):171-178.
doi - Friedlander EB, Soon R, Salcedo J, Davis J, Tschann M, Kaneshiro B. Prophylactic pregabalin to decrease pain during medication abortion: a randomized controlled trial. Obstet Gynecol. 2018;132(3):612-618.
doi pubmed - Colwill AC, Bayer LL, Bednarek P, Garg B, Jensen JT, Edelman AB. Opioid Analgesia for Medical Abortion: A Randomized Controlled Trial. Obstet Gynecol. 2019;134(6):1163-1170.
doi pubmed - Achuthan S, Singh I, Varthya SB, Srinivasan A, Chakrabarti A, Hota D. Gabapentin prophylaxis for postoperative nausea and vomiting in abdominal surgeries: a quantitative analysis of evidence from randomized controlled clinical trials. Br J Anaesth. 2015;114(4):588-597.
doi pubmed - O'Connell K, Jones HE, Simon M, Saporta V, Paul M, Lichtenberg ES, National Abortion Federation M. First-trimester surgical abortion practices: a survey of National Abortion Federation members. Contraception. 2009;79(5):385-392.
doi pubmed - O'Connell K, Jones HE, Lichtenberg ES, Paul M. Second-trimester surgical abortion practices: a survey of National Abortion Federation members. Contraception. 2008;78(6):492-499.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
