| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 9, Number 4, December 2020, pages 102-107
Urethral Diameter at the Time of Birth and Its Implication on Urinary Incontinence
Ana S.B. Picolotoa, d, Joana Giosciab, Jose G.L. Ramosc
aDepartment of Obstetrics and Gynecology, UFRGS, Porto Alegre, RS, Brazil
bSchool of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS), Porto Alegre, RS, Brazil
cDepartment of Obstetrics and Gynecology, UFRGS, Porto Alegre, RS, Brazil
dCorresponding Author: Ana Selma Bertelli Picoloto, Hospital de Clinicas de Porto Alegre (HCPA), ObGyn Service, Rua Ramiro Barcelos, 2350 Porto Alegre/RS, 90035-007, Brazil
Manuscript submitted October 18, 2020, accepted December 8, 2020, published online December 15, 2020
Short title: Urethral Diameter and Urinary Incontinence
doi: https://doi.org/10.14740/jcgo705
| Abstract | ▴Top |
Background: Throughout life, many women develop urinary incontinence (UI), and vaginal birth has been implicated as one of the causes of this pathology, once it may cause damages to the pelvic floor and to the continence mechanism. The aim was to compare urethral diameter between primiparous women after vaginal birth and women after elective cesarean section, without previous vaginal birth, correlating these measurements with factors related to pregnancy and childbirth and with presence of UI within 6 months of childbirth.
Methods: Urethral diameter was measured in the immediate postpartum period (up to 2 days) by transperineal ultrasound at the level of the bladder neck and mid urethra. The data were collected on pregnancy and birth. Six months after childbirth, the participants were assessed for the presence of UI.
Results: Of 151 women, 73 delivered vaginally (group 1) and 78 had a cesarean section (group 2). Urethral diameter at the level of the bladder neck was significantly smaller in group 2 (P ≤ 0.0001). There was no significant difference between the two groups in urethral diameter at the mid urethra (P = 0.505). Only urethral diameter at the mid urethra was inversely correlated with UI at 6 months (P = 0.014). There was a positive correlation between presence of UI during pregnancy and at 6 months after childbirth (rs = 0.214, P = 0.016).
Conclusions: A significant difference was observed in the urethral diameter at the bladder neck according to the mode of birth. A positive correlation was also found between presence of UI during pregnancy and at 6 months postpartum.
Keywords: Labor; Pelvic floor; Transperineal ultrasound; Urethra; Urinary incontinence
| Introduction | ▴Top |
Urinary incontinence (UI) is a multifactorial condition that affects a significant number of women. Pregnancy per se can lead to changes in the lower urinary tract [1]. After childbirth, about 33% of women have UI and up to 10% develop some degree of fecal incontinence [2].
Significantly increased urethral mobility has been observed after vaginal birth compared with cesarean section in both nulliparous and multiparous women, being associated with a higher incidence of postpartum UI [3]. Reduced urethral closure pressure and functional urethral length have also been associated with vaginal birth, but not with cesarean section, indicating that mode of birth may play a role, more important than that of pregnancy itself, in the development of UI [4]. These results, however, remain controversial [5].
Transperineal (or translabial) ultrasound can objectively confirm the findings of the physical examination of the pelvic floor and, in some instances, may even improve diagnostic accuracy [6]. Increased use of ultrasound has allowed the effects of vaginal birth on the support of the anterior vaginal wall and bladder neck to be assessed more accurately [3]. In addition, the measurement of urethral diameter has been shown to be useful for the diagnosis of outcomes and complications after middle urethral sling surgery [7].
Urethral hypermobility is one of the most significant UI-related changes that can be diagnosed by ultrasound [8]. Studies have shown high reproducibility of ultrasound measurement of bladder neck descent [9, 10], which has been associated with an increased risk of developing stress UI (SUI) [11]. However, it is not clear whether the different modes of birth differently influence bladder neck mobility. A study of postmenopausal women has shown that urethral diameter is significantly increased in women with SUI due to intrinsic sphincter deficiency compared with women without UI or with UI without intrinsic sphincter deficiency [12].
The measurement of the urethral lumen may correspond to the anatomical integrity of the urethra and reflect the resting urethral tone. Therefore, altered measurements of this structure (especially increased lumen diameter) may reflect lesions caused by childbirth. Given the scarce literature on the measurement of urethral diameter postpartum and its relationship with UI or with pregnancy and birth characteristics, the present study was designed to compare the urethral diameter of primiparous women after vaginal birth with that of women after cesarean section, without previous vaginal birth. A secondary objective was to correlate urethral diameter with factors related to pregnancy and childbirth with presence of UI within 6 months of childbirth.
| Materials and Methods | ▴Top |
This was a matched cross-sectional study. The sample consisted of women with only one eutocic vaginal birth (group 1: “vaginal birth”) or only cesarean section(s) (group 2: “cesarean section”), aged 18 years or older, who had delivered at our institution and were in the immediate postpartum period (up to 2 days).
In our institution, the indication of elective cesarean section in primiparous patients is mainly due to non-cephalic presentation or failure to induce labor. Exclusion criteria were cesarean section after the active phase of labor (more than 6 cm of cervical dilatation), morbid obesity (body mass index (BMI) above 45), neurological disease or cognitive deficits, current symptoms of urinary tract infection, history of pelvic surgery, or history of major voiding dysfunction.
Sample size was calculated using WinPepi, version 11.32. To detect the presence of UI within 6 months of childbirth as the primary outcome, according to data available in the literature [13], with 80% power and 5% significance level, a sample size of 78 women per group was necessary. The study was approved by the institutional and national review board (Research Ethics Comittee, Hospital de Clinicas de Porto Alegre Porto Alegre/RS, Brazil (affiliate to the National Research Ethics Committee - CONEP) number 14-0321; registration number CAAE 34001014.1.0000.5327, approval date: June 8, 2018). This study was conducted in compliance with all the applicable institutional ethical guidelines for the care and welfare.
After selection, participants completed an unstructured questionnaire for information on previous medical history and pregnancy. Data on pregnancy and birth were also collected from medical records. Urethral diameter was measured by bedside transperineal (translabial) ultrasound at resting position, due to the immediate postpartum or cesarean section condition, which could compromise the proper execution of the Valsalva maneuver. All transperineal ultrasound examinations were performed by the same examiner using a 7.5-MHz linear-array transducer oriented along the sagittal plane, in the midline of the perineum, and covered with a vinyl sheath containing acoustic gel, with the patient in the lithotomy position, with the legs slightly flexed and abducted, and with a bladder volume of less than 100 mL. The standard sagittal image included an anterior view of the pubic symphysis, with the urethra and bladder neck immediately behind, a medial view of the vagina and cervix, and a posterior view of the rectum and anal canal [14]. The urethral diameter was measured at two sites (at the level of the bladder neck and at the mid urethra) and defined as the diameter of the lumen at these two sites, i.e., the distance between the two internal mucous membranes of the urethra (Fig. 1).
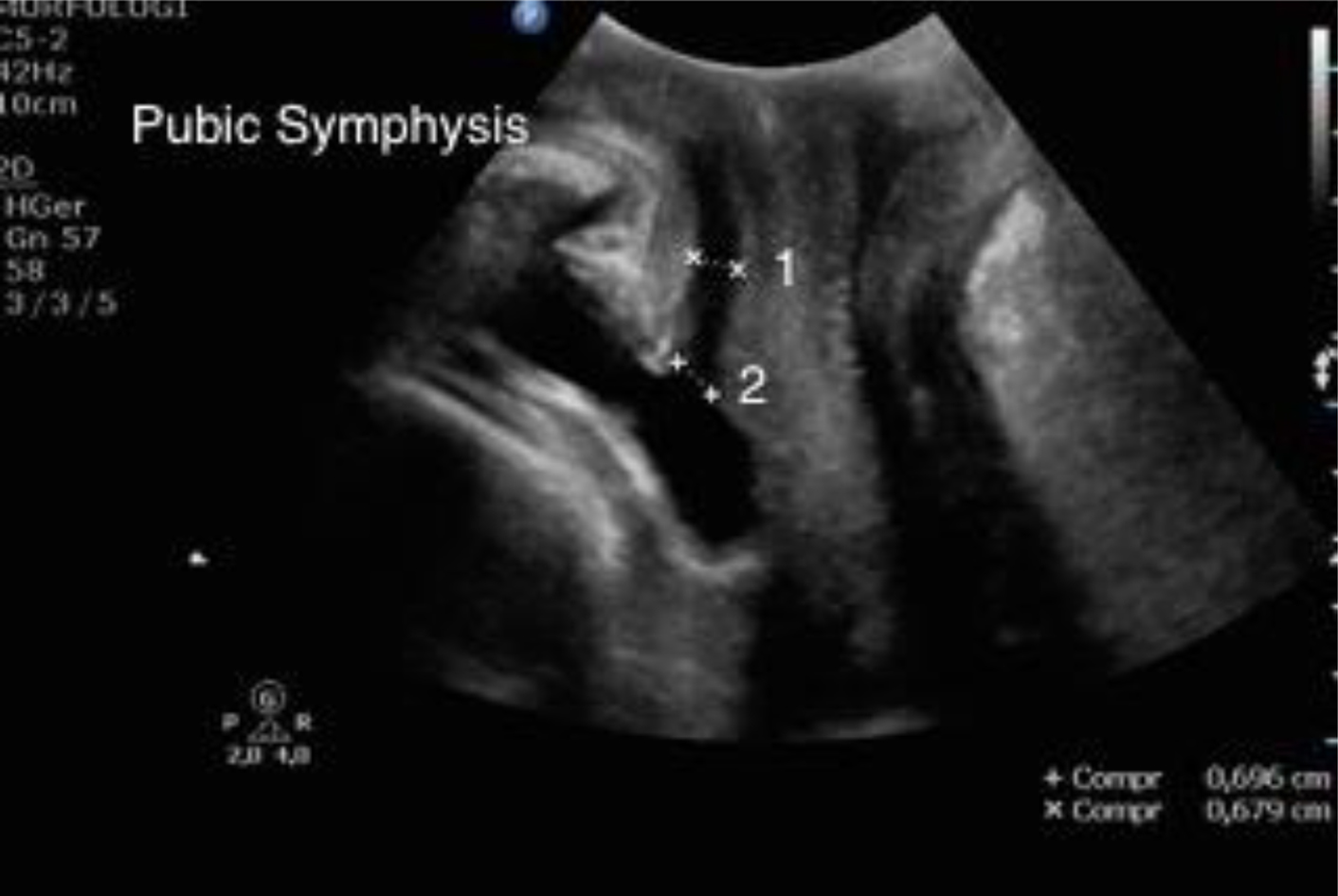 Click for large image | Figure 1. Pelvic floor ultrasound, midsagittal plane at rest. Arrow indicates the position of the symphysis pubis, i.e., point of reference for urethral measures. 1: urethral diameter at the mid urethra; 2: urethral diameter at the bladder neck. |
Secondary to ultrasound measurements, some gestational and intrapartum variables were analyzed (e.g., maternal age, height and parity; chronic or gestational comorbidities; presence of urinary tract infection during pregnancy; initial weight, final weight, and weight gain during pregnancy; BMI at the end of pregnancy; UI during pregnancy and time of symptom onset; mode of birth; duration of the active phase and newborn weight). Six months after childbirth, the participants were contacted by phone and asked about UI symptoms.
Regarding data processing, double data entry was performed and reviewed using SPSS, version 18.0 (SPSS Inc., released 2009; PASW Statistics for Windows, Chicago, IL, USA). Symmetric data were expressed as mean and standard error of the mean (SEM) or as median and 95% confidence interval (95% CI). Categorical variables were expressed as absolute (n) and relative (n%) frequencies. The Shapiro-Wilk test was used to determine data distribution. According to this, differences were analyzed for independent samples (Mann-Whitney or Student’s t-test, bivariate analysis) or with the Chi-square test (with adjusted residuals). Spearman ρ coefficients were estimated to determine correlations between the variables. All data were analyzed using SPSS, version 18.0. The level of significance was set at 5% for all analyses.
| Results | ▴Top |
A total of 205 women were assessed for eligibility, of whom 54 were excluded because they had one or more previous vaginal births. Therefore, the initial sample consisted of 151 women divided into two groups: those who had only one vaginal birth, cephalic presentation (group 1, n = 73) and those who had only cesarean section(s) (group 2, n = 78). After 6 months, 26 women were lost to follow-up, resulting in 65 women in group 1 and 55 in group 2 for analysis.
In Table 1, we can see differences between the two groups in age (P = 0.0001), maternal weight at the beginning of pregnancy (P = 0.008), BMI at the end of pregnancy (P = 0.010) and number of previous pregnancies (P ≤ 0.0001). Regarding maternal chronic or gestational comorbidities, there was a difference between the two groups only in the presence of chronic or pregnancy-induced hypertension (P = 0.013) (Table 2).
 Click to view | Table 1. Patients Characteristics |
 Click to view | Table 2. Maternal Clinical Characteristics |
Table 3 shows the results of the analysis of the two groups in the immediate postpartum period. Urethral diameter at the level of the bladder neck was significantly smaller in group 2 than in group 1 (P ≤ 0.0001). There was no significant difference between the two groups in urethral diameter at the mid urethra (P = 0.505). Presence of both SUI and urge UI during pregnancy (P = 0.324 and P = 0.817, respectively), and newborn weight (P = 0.626) were similar in both groups. Presence of UI at 6 months after childbirth did not differ between groups (P = 0.825).
 Click to view | Table 3. Perinatal and Maternal Characteristics |
Table 4 shows the correlations between urethral diameter measurements and presence of UI during pregnancy and at 6-month follow-up, as well as the correlations of these findings with maternal, newborn and gestational characteristics. There was a direct correlation between urethral diameter measured at the bladder neck and at the mid urethra (rs = 0.279, P = 0.001). Urethral diameter at the mid urethra was inversely correlated with presence of UI at 6-month follow-up (rs = 0.219, P = 0.014) but was not correlated with presence of UI during pregnancy (rs = 0.148, P = 0.07). Urethral diameter at the bladder neck did not correlate with urinary leakage at any assessment time point (rs = -0.073, P = 0.409 for UI during pregnancy and rs = -0.077, P = 0.425 for UI at 6-month follow-up).
 Click to view | Table 4. Correlations Among Studied Factors |
There was a direct correlation between presence of UI during pregnancy and at 6 months after childbirth in both groups (rs = 0.214, P = 0.016). Maternal age (rs = -0.3440, P ≤ 0.0001), maternal weight at the beginning of pregnancy (rs = -0.207, P = 0.017) and at the end of pregnancy (rs = -0.190, P = 0.029), and presence of previous pregnancies (rs = -0.223, P = 0.011) were inversely correlated with urethral diameter at the bladder neck but not with urinary leakage at any assessment time point (P > 0.05 for all factors). Weight gain during pregnancy, BMI at the end of pregnancy and presence of urinary tract infection did not influence any of the urethral diameter measurements, nor did they correlate with UI during pregnancy or at 6 months after childbirth (P > 0.05 for all). Presence of diabetes mellitus during pregnancy was positively correlated with urethral diameter at the mid urethra (rs = -0.165; P = 0.042) but was not correlated with urinary leakage at any assessment time point (P > 0.05). Presence of chronic hypertension/pregnancy-induced hypertension, however, was positively correlated with a larger urethral diameter at the bladder neck (rs = 0.269; P = 0.002). Newborn weight did not correlate with urethral diameter measurements, nor with UI during pregnancy or at 6-month follow-up (P > 0.05 for both). Urethral diameter at the mid urethra was positively correlated with the gestational trimester of onset of UI symptoms (rs = 0.166, P = 0.041) (Table 4).
| Discussion | ▴Top |
This study found that urethral diameter at the level of the bladder neck was larger in women who delivered vaginally than in women who had elective cesarean section. It has been shown that women with bladder neck descent have an increased risk of developing SUI [11], and the correlation between vaginal birth and urethral hypermobility is also evident in older women with pelvic floor dysfunction [15].
Peschers et al [3] found significantly increased urethral mobility in women after vaginal birth versus cesarean section, and this finding was associated with a higher incidence of postpartum UI. Cosimato et al [16] using transperineal ultrasound, found that the pubovesical angle was significantly increased in women after vaginal birth compared with women who had a cesarean section, and this alteration was directly related to UI symptoms. Measurements of the genital hiatus have also been compared between women after vaginal birth and after cesarean section, being higher in women who delivered vaginally [17]. A recent study investigating the relationship of ultrasound urethral descent measurements with postpartum SUI and mode of birth found that urethral descent during pregnancy was a risk factor for UI at 2 months after birth; however, no association was found between mode of birth and urethral descent [18].
Many studies have been conducted to investigate the association between postpartum UI and mode of birth with variable follow-up periods, but the results are conflicting [19-21]. In the present study, no statistically significant difference in the presence of UI at 6 months after childbirth was found between the vaginal birth and cesarean section groups. Likewise, Hutton et al [19] found no difference in the presence of UI at 3-month follow-up (5.5% in the cesarean section group versus 6.4% in the vaginal birth group; P = 0.31). In a similar study, the authors also concluded that cesarean section is not a protective factor against developing UI [20]. Conversely, a systematic review showed that women who delivered vaginally had a two-fold higher risk of developing UI within the first year postpartum than women who had a cesarean section [21].
The positive correlation between urethral diameter measured at the mid urethra and at the bladder neck may be explained anatomically, since both measurements were performed at the lumen of the urethra, or, perhaps, by the physiology of pregnancy and birth, which affects both of them equally. Similar to the findings of Pizzoferrato et al [18], who reported no association between UI during pregnancy and urethral descent, we found no correlation between urethral diameter at the bladder neck and UI during pregnancy or at 6-month follow-up. The inverse correlation between urethral diameter at the mid urethra and presence of UI at 6 months after childbirth could be clarified by increasing the sample size.
Similar parameters have been investigated in non-pregnant/non-postpartum women. Wlazlak et al [22] investigated the ability of 4D transperineal ultrasound to predict UI and found that urethral mobility had good positive predictive value for SUI in women seen for urodynamic testing due to symptoms of pelvic floor dysfunction. Kupec et al [23] also found a significant difference in the diameter of the urethral lumen at the mid urethra and in the incidence of urethral funneling between women with and without UI.
The prevalence of UI increased as pregnancy progressed in our sample, and urethral diameter at the mid urethra was positively correlated with the onset of UI symptoms. Rogers et al [24] also showed that UI symptoms increased as pregnancy progressed. This may be explained by the increasing pressure of the growing uterus and fetal weight on the pelvic floor and by hormonal changes that may reduce the strength of pelvic floor muscles, causing greater bladder neck and urethral mobility, leading to urethral sphincter incompetence [1, 18, 25].
The small difference observed in maternal weight at the beginning of pregnancy and BMI at the end of pregnancy probably did not influence our results, since weight at the end of pregnancy was similar in both groups. In contrast to our findings, previous studies have shown that weight gain during pregnancy is a risk factor for postpartum UI [1, 26]. Furthermore, we did not find a correlation of fetal weight with urethral diameter measurements, nor with UI during pregnancy or at 6 months after childbirth. This result is consistent with the findings of Gartland et al [25], who reported no correlation between fetal weight and urinary leakage.
The urethra is a key organ in the physiology of female UI, and it is of paramount importance to understand whether pregnancy and birth can affect its functioning. In the present study, the proposed use of transperineal ultrasound, which is a widely available, non-invasive technique, to investigate postpartum urethral changes was satisfactory. It is hoped that by following up our sample we may correlate the onset of UI symptoms in the perimenopausal period with the urethral diameter measurements obtained in the postpartum period, which would allow us to further clarify the associations between pregnancy, birth, urethral anatomy and UI, as suggested in a previous study in which increased urethral diameter was observed in postmenopausal women with UI due to intrinsic sphincter deficiency [12]. Further studies focusing on this line of research are required to clarify these and other still obscure issues concerning the anatomical changes caused by pregnancy and birth and their influence on the onset of UI symptoms in women.
Acknowledgments
We acknowledge all the staff of the Obstetric Center of the Hospital de Clinicas de Porto Alegre.
Financial Disclosure
There is no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Written informed consent was obtained from all participants prior to their inclusion in the study.
Author Contributions
AP: design, data collection, data analysis with statistics, manuscript preparation and writing; JGLR: design, supervision of the manuscript; JG: supported in sample collection.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Sangsawang B, Sangsawang N. Stress urinary incontinence in pregnant women: a review of prevalence, pathophysiology, and treatment. Int Urogynecol J. 2013;24(6):901-912.
doi pubmed - Hay-Smith J, Morkved S, Fairbrother KA, Herbison GP. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database Syst Rev. 2008;4:CD007471.
doi - Peschers U, Schaer G, Anthuber C, Delancey JO, Schuessler B. Changes in vesical neck mobility following vaginal delivery. Obstet Gynecol. 1996;88(6):1001-1006.
doi - van Geelen JM, Lemmens WA, Eskes TK, Martin CB, Jr. The urethral pressure profile in pregnancy and after delivery in healthy nulliparous women. Am J Obstet Gynecol. 1982;144(6):636-649.
doi - Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Munoz A. Pelvic floor disorders 5-10 years after vaginal or cesarean childbirth. Obstet Gynecol. 2011;118(4):777-784.
doi pubmed - Dietz HP, Shek C. Validity and reproducibility of the digital detection of levator trauma. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(8):1097-1101.
doi pubmed - Chan L, Tse V. Pelvic floor ultrasound in the diagnosis of sling complications. World J Urol. 2018;36(5):753-759.
doi pubmed - Schaer GN, Koechli OR, Schuessler B, Haller U. Improvement of perineal sonographic bladder neck imaging with ultrasound contrast medium. Obstet Gynecol. 1995;86(6):950-954.
doi - Schaer GN, Koechli OR, Schuessler B, Haller U. Perineal ultrasound for evaluating the bladder neck in urinary stress incontinence. Obstet Gynecol. 1995;85(2):220-224.
doi - Derpapas A, Digesu GA, Fernando R, Khullar V. Imaging in urogynaecology. Int Urogynecol J. 2011;22(11):1345-1356.
doi pubmed - Dietz HP, Wilson PD. Childbirth and pelvic floor trauma. Best Pract Res Clin Obstet Gynaecol. 2005;19(6):913-924.
doi pubmed - Oliveira FR, Ramos JG, Martins-Costa S. Translabial ultrasonography in the assessment of urethral diameter and intrinsic urethral sphincter deficiency. J Ultrasound Med. 2006;25(9):1153-1158; quiz 1159-1160.
doi pubmed - Boyles SH, Li H, Mori T, Osterweil P, Guise JM. Effect of mode of delivery on the incidence of urinary incontinence in primiparous women. Obstet Gynecol. 2009;113(1):134-141.
doi pubmed - Digesu GA, Robinson D, Cardozo L, Khullar V. Three-dimensional ultrasound of the urethral sphincter predicts continence surgery outcome. Neurourol Urodyn. 2009;28(1):90-94.
doi pubmed - Dietz HP, Clarke B, Vancaillie TG. Vaginal childbirth and bladder neck mobility. Aust N Z J Obstet Gynaecol. 2002;42(5):522-525.
doi pubmed - Cosimato C, Cipullo LM, Troisi J, Di Spiezio Sardo A, Tommaselli GA, Oro RR, Zullo F, et al. Ultrasonographic evaluation of urethrovesical junction mobility: correlation with type of delivery and stress urinary incontinence. Int Urogynecol J. 2015;26(10):1495-1502.
doi pubmed - Aydin S, Tuncel MA, Aydin CA, Ark C. Do we protect the pelvic floor with non-elective cesarean? A study of 3-D/4-D pelvic floor ultrasound immediately after delivery. J Obstet Gynaecol Res. 2014;40(4):1037-1045.
doi pubmed - Pizzoferrato AC, Fauconnier A, Bader G, de Tayrac R, Fort J, Fritel X. Is prenatal urethral descent a risk factor for urinary incontinence during pregnancy and the postpartum period? Int Urogynecol J. 2016;27(7):1003-1011.
doi pubmed - Hutton EK, Hannah ME, Ross S, Joseph KS, Ohlsson A, Asztalos EV, Willan AR, et al. Maternal outcomes at 3 months after planned caesarean section versus planned vaginal birth for twin pregnancies in the Twin Birth Study: a randomised controlled trial. BJOG. 2015;122(12):1653-1662.
doi pubmed - McKinnie V, Swift SE, Wang W, Woodman P, O'Boyle A, Kahn M, Valley M, et al. The effect of pregnancy and mode of delivery on the prevalence of urinary and fecal incontinence. Am J Obstet Gynecol. 2005;193(2):512-517; discussion 517-518.
doi pubmed - Thom DH, Rortveit G. Prevalence of postpartum urinary incontinence: a systematic review. Acta Obstet Gynecol Scand. 2010;89(12):1511-1522.
doi pubmed - Wlazlak E, Surkont G, Shek KL, Dietz HP. Can we predict urinary stress incontinence by using demographic, clinical, imaging and urodynamic data? Eur J Obstet Gynecol Reprod Biol. 2015;193:114-117.
doi pubmed - Kupec T, Pecks U, Graf CM, Stickeler E, Meinhold-Heerlein I, Najjari L. Size does not make the difference: 3D/4D transperineal sonographic measurements of the female urethra in the assessment of urinary incontinence subtypes. Biomed Res Int. 2016;2016:1810352.
doi pubmed - Rogers RG, Ninivaggio C, Gallagher K, Borders AN, Qualls C, Leeman LM. Pelvic floor symptoms and quality of life changes during first pregnancy: a prospective cohort study. Int Urogynecol J. 2017;28(11):1701-1707.
doi pubmed - Gartland D, Donath S, MacArthur C, Brown SJ. The onset, recurrence and associated obstetric risk factors for urinary incontinence in the first 18 months after a first birth: an Australian nulliparous cohort study. BJOG. 2012;119(11):1361-1369.
doi pubmed - Ng K, Cheung RYK, Lee LL, Chung TKH, Chan SSC. An observational follow-up study on pelvic floor disorders to 3-5 years after delivery. Int Urogynecol J. 2017;28(9):1393-1399.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
