| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 11, Number 1, March 2022, pages 14-18
Eclampsia in the Previable Period of 22w5d
Miriam Aiouba, Lindsay M. Serwatkab, c, Laura Harta
aDepartment of Obstetrics and Gynecology, Temple University Hospital, Philadelphia, PA 19140, USA
bLewis Katz School of Medicine, Philadelphia, PA 19140, USA
cCorresponding Author: Lindsay Serwatka, Lewis Katz School of Medicine, Medical Education and Research Building (MERB), Office of Student Affairs, Philadelphia, PA 19140, USA
Manuscript submitted July 29, 2021, accepted December 7, 2021, published online March 12, 2022
Short title: Eclampsia in the Previable Period
doi: https://doi.org/10.14740/jcgo763
| Abstract | ▴Top |
We herein report a case of eclampsia presenting at 22w5d gestation. The patient was a 25-year-old African American gravida 1 para 0 (G1P0) with no history of chronic hypertension or prior seizures. Her only antenatal issues included a remote history of marijuana use and an echogenic intracardiac focus noted on anatomy ultrasound. At 22w1d, she was noted to have blood pressure (BP) of 135/91 mm Hg in office. Four days later, she presented with generalized tonic-clonic seizures. In the emergency department (ED), obstetric providers were notified and recommended immediate initiation of 6 g bolus of intravenous (IV) magnesium. Fetal heart tones were present. Patient subsequently had another seizure in the ED. BP was 200s/130s mm Hg. Magnesium sulfate infusion was started and, with obstetric Doctor of Medicine at bedside, labetalol 20 mg IV was administered with improvement in BP to 160s/100s mm Hg. On labor and delivery, eclampsia diagnosis and severity, and preservation of mother’s life as preferred course were explained. After tearful discussion, patient and family proceeded with induction of labor. The patient exhibited signs of fluid overload and chest X-ray showed pulmonary edema. Repeat lab work was consistent with hemolysis, elevated liver enzymes, low platelet count syndrome . Due to persistent headache, a computed tomography of head was performed that showed posterior reversible encephalopathy syndrome. About 10 h after initial Cytotec dose, the patient delivered a pulseless intact fetus. Cerebrovascular involvement leading to seizures is termed eclampsia and is caused by massive release of excitatory neurotransmitters. Preeclampsia-eclampsia syndrome can present anywhere from 20 weeks through the postpartum period. However, eclampsia with associated complications is exceedingly rare in the second trimester. No reports of eclampsia at 22 weeks gestation have been found in the literature.
Keywords: Eclampsia; Preeclampsia; Previable period; PRES; HELLP; Seizure
| Introduction | ▴Top |
Preeclampsia-eclampsia was not formally classified as a disorder of pregnancy during ancient times. The syndrome, however, was first described by Hippocrates in 400 BC. In aphorism XXXI 507, he states that headache with heaviness and convulsions in pregnancy is considered bad [1]. At present, The American College of Obstetricians and Gynecologists defines preeclampsia as “a disorder of pregnancy associated with new-onset hypertension, which occurs most often after 20 weeks of gestation and frequently near term… Eclampsia is defined by new-onset tonic-clonic, focal, or multifocal seizures in the absence of other causative conditions such as epilepsy, cerebral arterial ischemia and infarction, intracranial hemorrhage, or drug use.” [2].
Herein, we report a case of a patient with eclamptic seizures at an early presentation of 22w5d, with subsequent induction of labor and delivery of a previable fetus. During her course, she was diagnosed with hemolysis, elevated liver enzymes, low platelet count syndrome (HELLP syndrome), posterior reversible encephalopathy syndrome (PRES) and pulmonary edema.
| Case Report | ▴Top |
Investigations
The patient was a 25-year-old African American gravida 1 para 0 (G1P0) whose only antenatal issues in the pregnancy were a remote history of marijuana use and an echogenic intracardiac focus noted on her anatomy ultrasound at 20w3d. For this, she had noninvasive prenatal testing (NIPT) that was negative for Down syndrome and showed a female fetus.
Patient was without prior seizure history and without a history of chronic hypertension and booking blood pressure (BP) at 13w2d was 119/82 mm Hg. Patient was without any other significant medical history and the patient and her family denied any history of prior seizures or elevated BP. Patient had no prior surgeries. She did not have any allergies and had a remote history of marijuana use but denied any other drug use.
At 22w1d, patient was noted to have BPs of 135/91 and 133/87 mm Hg in the office. She began to experience vague right upper quadrant pain 2 days later. At 22w5d, patient was at her normal shift at work when she began to feel unwell. She began vomiting and was sent home. At home, her stepfather witnessed her shaking and emergency medical services (EMS) was called for assistance. En route to hospital, patient had a witnessed generalized tonic-clonic seizure. Upon arrival to the emergency department (ED), obstetric providers were notified and recommended immediate start of 6 g bolus of intravenous (IV) magnesium. Patient subsequently had another witnessed generalized tonic-clonic seizure in the ED at which time BP was 200s/130s mm Hg. Magnesium sulfate infusion was begun and, with obstetric Doctor of Medicine (MD) at bedside, labetalol 20 mg IV was administered; BP subsequently improved to 160s/100s mm Hg. Upon evaluation in the ED, patient was post-ictal and very sleepy but responded correctly to questions about person, place, and time. She denied symptoms of preterm labor but did report right upper quadrant pain for the 2 days prior to presentation and intermittent headache the morning of her presentation. She denied visual changes, chest pain or difficulty breathing.
On presentation, patient was afebrile, and vitals included pulse in the 90-100 bpm, BP of 200s/130s mm Hg and oxygen saturation of 98% on room air. Her body mass index (BMI) was 37.3 kg/m2. She appeared very sleepy and post-ictal but was arousable and answered questions appropriately with intact memory. Family members standing nearby corroborated her reported history as accurate. She had evidence of tongue biting at the left of her tongue. Head was otherwise atraumatic and normocephalic. Rhonchi were present bilaterally and improved with deep inspirations. Her abdomen was soft, gravid, and non-tender to palpation. Fetal heart tones were obtained in the ED and were noted to be 147 bpm. On pelvic examination, no anatomic abnormalities were noted. Her cervical exam was closed, long and high. There was no blood or amniotic fluid noted on the examining glove; however, on the patient’s underwear, there was evidence of urinary incontinence. Patient’s extremities were normal, atraumatic, and without cyanosis. Trace edema was noted in the bilateral lower extremities. There were no focal neurologic deficits; sensation was intact and patient was moving all extremities well without involuntary movements. She did not have rashes or other skin abnormalities.
Diagnosis and treatment
After transfer to labor and delivery for stabilization, patient’s lab work resulted as follows: glucose was 84 mg/dL, white blood cell count was 11,000/mm3, hemoglobin was 12.1 g/dL, and platelets were 150,000/mm3. Creatinine was 0.97 mg/dL, alanine aminotransferase (ALT) was elevated at 102 U/L and aspartate aminotransferase (AST) was greater than twice the upper limit of normal at 86 U/L. Her remaining electrolytes were normal. Coagulation studies were normal, and fibrinogen was within normal range at 511 mg/dL. Urine drug screen was pan-negative, and urine protein-to-creatinine ratio was significantly elevated at 9.5.
Patient was again noted to have severe range BPs of 160-170/90-110 mm Hg. She subsequently received 40 mg IV labetalol with improvement in BPs. At bedside with family members present, diagnosis of eclampsia, severity of condition, and preservation of mother’s life as preferred course of action were explained. Recommendation for delivery of previable fetus was discussed and after tearful discussion, patient and family were amenable to induction of labor. About 6 h after administration of the magnesium sulfate bolus, patient reported labored breathing which improved some with 2 L oxygen via nasal cannula.
Given this new oxygen requirement and signs of fluid overload on exam as evidenced by dyspnea and lower-extremity edema, a chest X-ray (CXR) was performed, and the following labs drawn: magnesium level, repeat complete blood count (CBC), repeat comprehensive metabolic panel (CMP), and lactate dehydrogenase (LDH). Labor induction was begun with Cytotec (misoprostol) 600 µg loading dose followed by 400 µg every 3 h vaginally. Repeat lab work resulted significant for platelets 131,000/mm3, ALT 96 U/L, and AST 85 U/L. LDH was elevated at 576 U/L and magnesium level was normal at 5.0 mg/dL. Findings were consistent with hemolysis, elevated liver enzymes, low platelets (HELLP) syndrome. Magnesium was continued at infusion rate of 2 g/h. CXR showed pulmonary edema (Fig. 1). Furosemide 20 mg IV was administered which improved patient’s labored respiration.
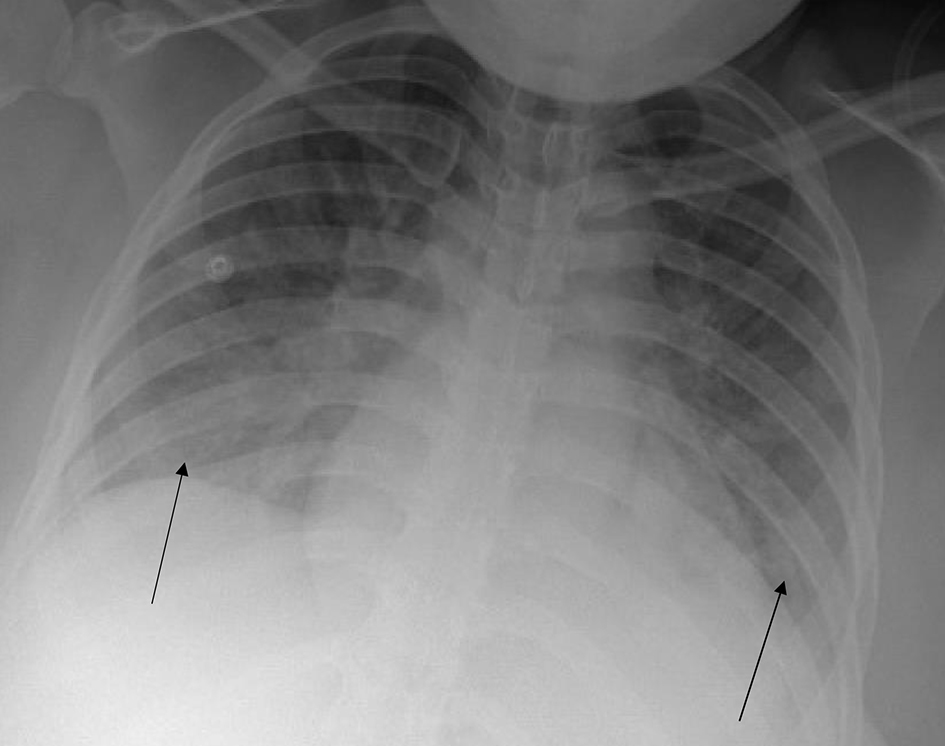 Click for large image | Figure 1. Chest X-ray prior to delivery. Arrows indicate diffuse pulmonary edema. |
Patient continued to complain of headache, as reported in her ROS, and CT of head was performed (Fig. 2). It showed confluent white matter hypodensity within the vertex and posterior parieto-occipital region, consistent with posterior reversible encephalopathy syndrome (PRES).
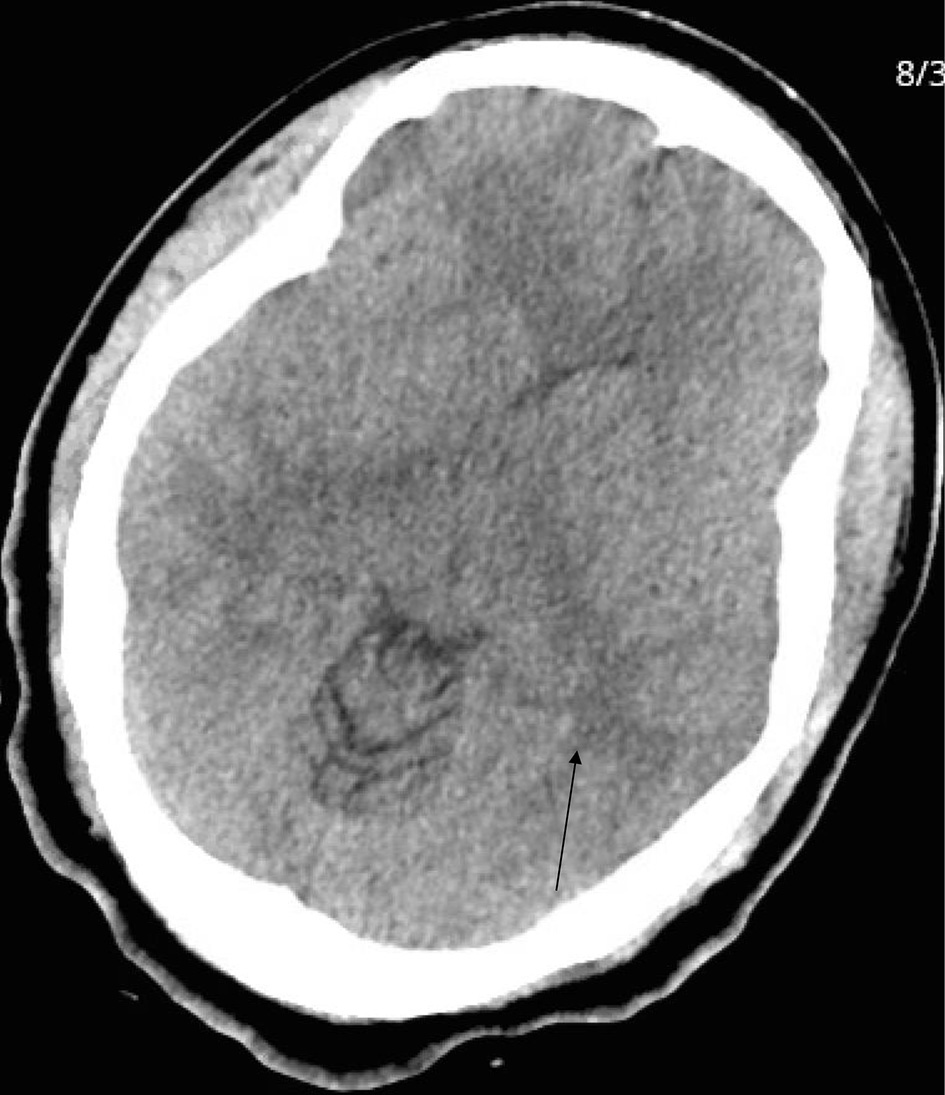 Click for large image | Figure 2. Head computed tomography. Arrow indicates confluent white matter hypodensity within the vertex and posterior parieto-occipital region, consistent with posterior reversible encephalopathy syndrome (PRES). |
As her labor progressed, patient received an additional 80 mg IV labetalol and total 25 mg IV hydralazine for severe range BPs > 160/110 mm Hg. She received a total 600 µg misoprostol loading dose and 300 - 400 µg vaginal misoprostol doses. Immediately prior to delivery, patient was noted to have a temperature of 102.7 °F and was treated with ampicillin, gentamicin and clindamycin for presumed chorioamnionitis/endometritis. About 10 h after initial misoprostol dose, patient delivered an intact fetus without heart tones or pulse. Placenta was delivered intact and estimated blood loss was 200 cc.
Following delivery, BPs initially improved to 130-140/40-60 mm Hg and the patient completed 24 h of postpartum magnesium sulfate for seizure prophylaxis. On postpartum day 1, patient was noted to have increased respiratory rate and repeat CXR as well as B-type natriuretic peptide (BNP) and echocardiogram were performed. BNP was elevated at 582 pg/mL and repeat CXR showed clear lungs (Fig. 3). Echocardiogram showed ejection fraction of 65% without regional wall motion abnormalities.
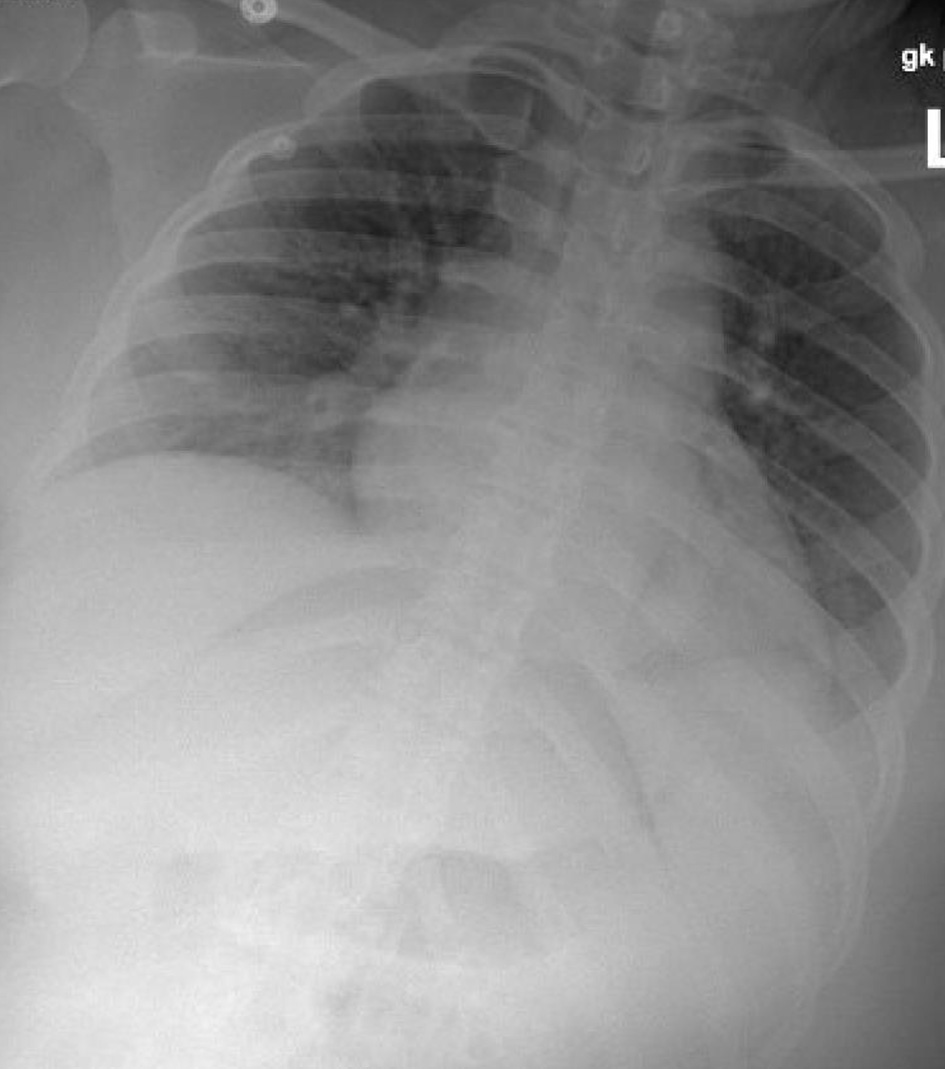 Click for large image | Figure 3. Chest X-ray post-delivery showing low lung volumes and clear lungs. |
Additionally, on postpartum day 1, patient’s BPs were noted to rise again. She was started on nifedipine and slowly titrated up for BP management.
On postpartum day 2, patient was noted to have new onset shortness of breath with ambulation. Electrocardiogram (EKG) was performed and showed normal sinus rhythm (Fig. 4). Computed tomography angiogram of chest was also performed and was negative for pulmonary embolism (Fig. 5). Bilateral sub-segmental atelectasis was visualized, and incentive spirometry was encouraged.
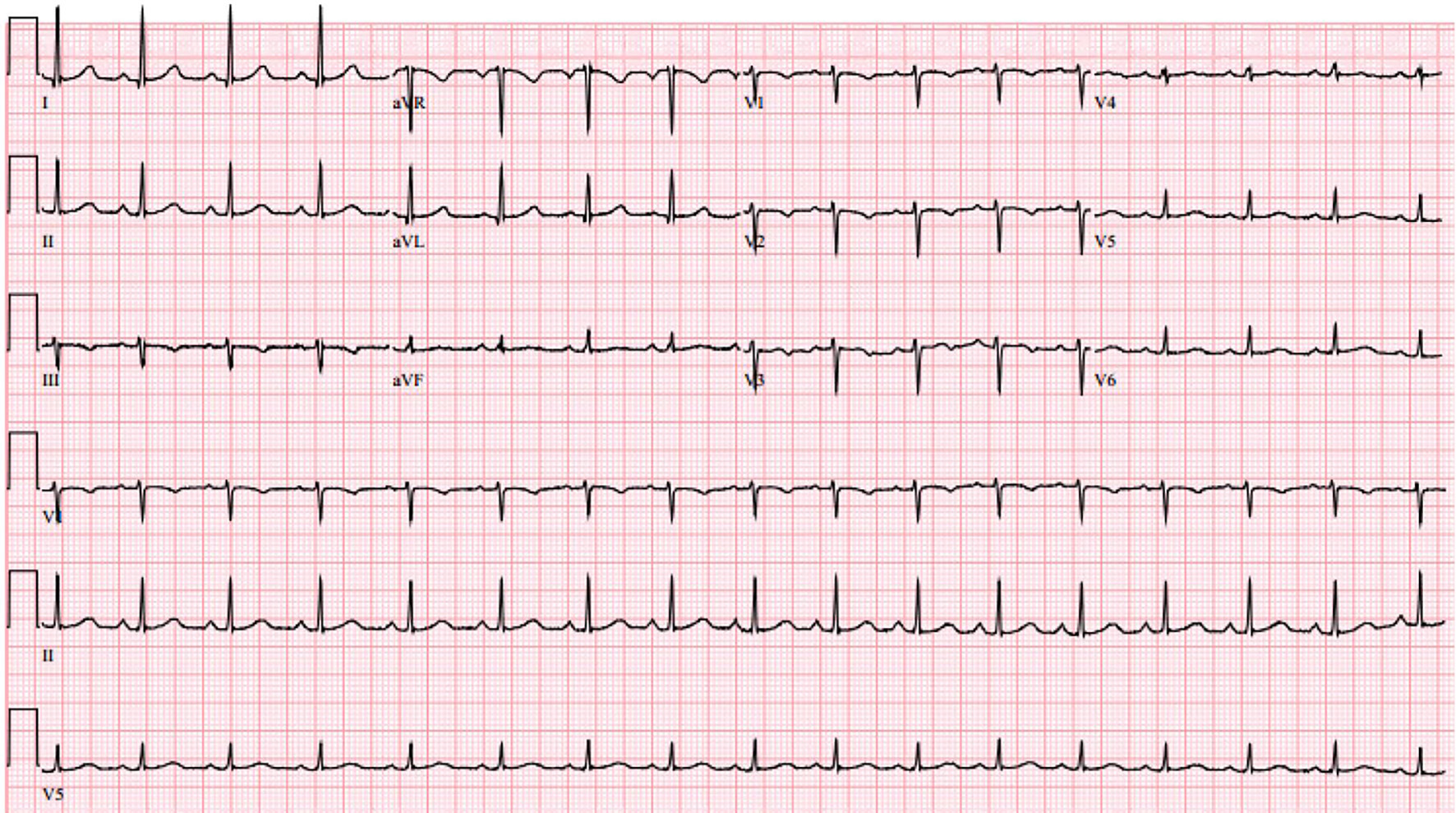 Click for large image | Figure 4. Electrocardiogram on post-partum day 2 showing normal sinus rhythm. |
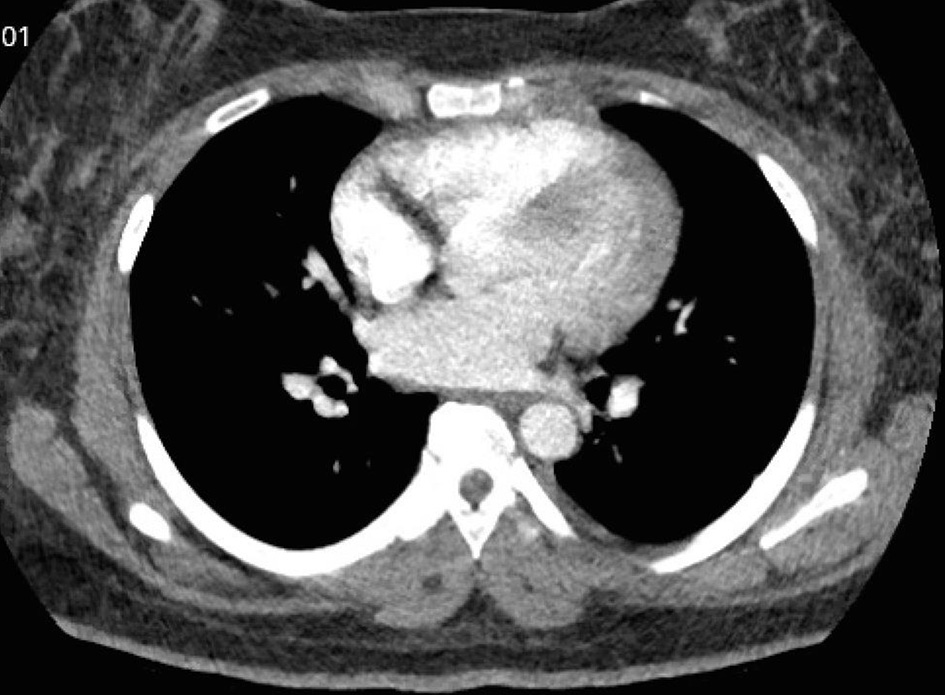 Click for large image | Figure 5. Computed tomography angiogram to rule out pulmonary embolism. No pulmonary embolism was visualized. |
Follow-up and outcomes
Patient was discharged to home on postpartum day 4 with a BP cuff and nifedipine 90 mg XL daily. She presented to the emergency room twice during the postpartum period with panic attacks and was diagnosed with post-traumatic stress disorder (PTSD) and underwent outpatient therapy and counseling. Patient followed up closely with obstetric team throughout the postpartum period.
Patient was subsequently diagnosed with chronic hypertension after she concluded the postpartum period and went on to have another pregnancy during which she began prophylactic aspirin in the first trimester. She carried that pregnancy to term and delivered a healthy neonate. No diagnosis of preeclampsia was given in her second pregnancy.
| Discussion | ▴Top |
This case report discusses a 25-year-old G1P0 at 22w5d who presented with eclamptic seizures and was subsequently diagnosed with HELLP syndrome, PRES syndrome, and pulmonary edema. She was actively stabilized and managed and was discharged to home on postpartum day 4. There is particular strength to this case report in that all details were well documented following the patient’s presentation with an eclamptic episode. There are no limitations or weaknesses to report.
In 2014, “10.8% of 3.8 million total deliveries”, as reported by the HCUP Statistical Brief, “had a diagnosis of hypertension complicating pregnancy, childbirth, and the puerperium” with preeclampsia-eclampsia as the most common etiology [3]. Preeclampsia-eclampsia syndrome can present anywhere from 20 weeks gestation through the postpartum period. Eclampsia is exceedingly rare in the second trimester and no previous case reports of eclampsia at 22 weeks gestation have been found in the literature.
To highlight the uniqueness of this case, Wojtowicz et al, in analyzing characteristics of early-onset preeclampsia (EOPE) versus late-onset preeclampsia (LOPE), reported < 0.1% of included pregnancies that developed preeclampsia (n = 214) developed it at a gestational age prior to 26.7 weeks [4].
Although the pathophysiology of the preeclampsia-eclampsia syndrome is not clearly understood, it is known that the syndrome is an abnormal response to placentation, given the only treatment is delivery and removal of the placenta. Additionally, internal placental factors are considered more strongly associated with EOP) than LOPE [4]. Current mechanisms that are proposed to explain the cause of preeclampsia include: 1) Placental implantation with abnormal trophoblastic invasion of uterine vessels; 2) Immunological maladaptive tolerance between maternal, paternal (placental), and fetal tissues; 3) Maternal maladaptation to cardiovascular or inflammatory changes of normal pregnancy; and 4) Genetic factors including inherited predisposing genes and epigenetic influences [5].
Guo et al discussed the differences in pathology between EOPE and LOPE to be related to distinct biomarkers identified in maternal blood, highlighting that “known pathogenic factors and diagnostic markers for preeclampsia” were identified at higher levels in EOPE placentae, but not in controls or LOPE placentae [6]. Their additional analysis suggests that placental dysfunction is the cause of EOPE, but not LOPE, and that the two presentations should be considered separate diseases entirely. A proposed distinction is that EOPE is caused by “abnormal function of (extravillous trophoblast cells) and fibroblasts in the maternal-fetal interface” whereas “LOPE shows gene expression alteration in peripheral blood.” [6]. While this explanation may be applicable to our patient, there is no way for knowing if this exact mechanism is why patient developed eclampsia at such an early gestational age, and further study is needed to better understand the distinct pathology of early-onset eclampsia.
Cerebrovascular involvement in preeclampsia that leads to seizures is termed eclampsia. These are caused by massive release of excitatory neurotransmitters leading to depolarization of network neurons, and subsequent bursts of action potentials [7]. Additionally, cerebrovascular involvement can manifest as PRES, as in the present case. The proposed mechanism of this is a combination of preeclampsia-associated interendothelial cell leak coupled with loss of normal cerebrovascular autoregulatory capacity due to sudden elevations in systemic BP [8]. This cerebral interendothelial cell leak is often a systemic phenomenon in women with preeclampsia and explains the pathophysiology underlying pulmonary edema as well.
Women with severe EOPE are at greatest risk of recurrence (ranging from 25% to 65%). Among women with a history of severe preeclampsia in the second trimester, 21% of subsequent pregnancies are complicated by recurrent severe preeclampsia in the second trimester. In those that develop recurrent preeclampsia, one-third occurs at < 27 weeks, one-third at 28 - 36 weeks, and one-third at > 37 weeks [9]. Current guidance of diagnosis and management of atypical preeclampsia-eclampsia can help minimize adverse pregnancy outcomes for active and future pregnancies, but such guidance may be limited for presentations of previable pregnancies [10]. The description of such a case will improve recognition and support prompt diagnosis, thereby decreasing overall morbidity and mortality of such a serious pregnancy complication as well as contribute to the evolving diagnostic criteria of hypertensive disorders of pregnancy [4].
Learning points
Eclamptic seizures are not a complication of only term or near-term status and can occur early in pregnancy, even in the previable period. The evaluation of management of eclampsia in the previable period will contribute to the evolving diagnostic criteria of preeclampsia, eclampsia, and other hypertensive disorders of pregnancy.
Acknowledgments
An abstract of this case report has previously been presented at ACOG District III Junior Fellow Day 2020.
Financial Disclosure
There are no disclosures of financial support or funding received for this study.
Conflict of Interest
There is no conflict of interest to declare.
Informed Consent
Patient’s informed consent for publication of this report was obtained.
Author Contributions
The manuscript was prepared by Miriam Aioub; Lindsay Serwatka aided with manuscript preparation; Laura Hart reviewed the manuscript and provided revisions.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
##w#d: # weeks, # days gestational age; G1P0: gravida 1 para 0; BP: blood pressure; ED: emergency department; OB: obstetric; MD: Doctor of Medicine; IV: intravenous; L&D: labor and delivery; HELLP syndrome: hemolysis, elevated liver enzymes, low platelet count syndrome; CT: computed tomography; PRES: posterior reversible encephalopathy syndrome; NIPT: noninvasive prenatal testing; EMS: emergency medical services; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CXR: chest X-ray; CBC: complete blood count; CMP: comprehensive metabolic panel; LDH: lactate dehydrogenase; PTSD: post-traumatic stress disorder; EOPE: early-onset preeclampsia; LOPE: late-onset preeclampsia
| References | ▴Top |
- The medical works of Hippocrates. Chadwick J, Mann WN, translators. England: Blackwell Scientific Publications; 1950.
- ACOG Practice Bulletin No. 202: Gestational hypertension and preeclampsia. Obstet Gynecol. 2019;133(1):1.
doi pubmed - Delivery Hospitalizations Involving Preeclampsia and Eclampsia, 2005-2014. HCUP Statistical Brief #222. April 2017. Agency for Healthcare Research and Quality, Rockville, MD. Accessed May 24, 2021. www.hcup-us.ahrq.gov/reports/statbriefs/sb222-Preeclampsia-Eclampsia-Delivery-Trends.pdf.
- Wojtowicz A, Zembala-Szczerba M, Babczyk D, Kolodziejczyk-Pietruszka M, Lewaczynska O, Huras H. Early- and late-onset preeclampsia: a comprehensive cohort study of laboratory and clinical findings according to the new ISHHP criteria. Int J Hypertens. 2019;2019:4108271.
doi pubmed - Cunningham F. Hypertensive Disorders. In: Williams Obstetrics. McGraw-Hill Education. 2018; p. 713-714.
- Guo F, Zhang B, Yang H, Fu Y, Wang Y, Huang J, Cheng M, et al. Systemic transcriptome comparison between early- And late-onset pre-eclampsia shows distinct pathology and novel biomarkers. Cell Prolif. 2021;54(2):e12968.
doi - Meldrum BS. Implications for neuroprotective treatments. Prog Brain Res. 2002;135:487-495.
doi - Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14(9):914-925.
doi - Sibai BM, Mercer B, Sarinoglu C. Severe preeclampsia in the second trimester: recurrence risk and long-term prognosis. Am J Obstet Gynecol. 1991;165(5 Pt 1):1408-1412.
doi - Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e481-487.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
