| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 11, Number 4, December 2022, pages 117-120
Preeclampsia While on Venovenous Extracorporeal Membrane Oxygenation Support
Muhammad Abu-Rmaileha, Amy E. Hackmannb, Emily H. Adhikaric, Bethany L. Lussiera, d, e, f
aDepartment of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
bDivision of Cardiothoracic Surgery, Department of Surgery, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
cDivision of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
dDivision of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
eDivision of Neurological Critical Care, Department of Neurology and Neurosurgery, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
fCorresponding Author: Bethany L. Lussier, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75370, USA
Manuscript submitted September 17, 2022, accepted October 15, 2022, published online December 30, 2022
Short title: Preeclampsia While on VV-ECMO
doi: https://doi.org/10.14740/jcgo829
| Abstract | ▴Top |
The coronavirus disease 2019 (COVID-19) pandemic presented many challenges during pregnancy. Complications of COVID-19 in pregnancy were abundant, with higher rates of respiratory failure and preeclampsia. We present here a case of a woman at 24 weeks’ gestation requiring extracorporeal membrane oxygenation (ECMO) for refractory hypoxic respiratory failure and her management for presumed preeclampsia. Current methods to diagnose preeclampsia can be severely impacted by comorbid COVID-19 infection as well as the treatment with ECMO support. This is the first report detailing the challenges of using standard diagnostic criteria for diagnosis of preeclampsia while on ECMO and highlights the potential for and need for further research to identify and guide management of possible preeclampsia/eclampsia.
Keywords: Preeclampsia; Extracorporeal membrane oxygenation; Proteinuria; COVID-19
| Introduction | ▴Top |
Venovenous extracorporeal membrane oxygenation (VV-ECMO) is being used more often in refractory acute respiratory distress syndrome (ARDS), especially in younger patients [1]. It can often be difficult to sort the underlying disease process from the complications of VV-ECMO [1]. Additionally, the physiologic effects of VV-ECMO related to microvasculature and impaired autoregulation may be more complicated during pregnancy [2]. Today we describe a case of preeclampsia and possible eclampsia while on VV-ECMO support for coronavirus disease 2019 (COVID-19)-related ARDS and subsequent development of posterior reversible encephalopathy syndrome. We herein discuss the difficulties in differentiating disease processes while on VV-ECMO. All medical information has been deidentified and the submission was reviewed, and consent waived in compliance with medical ethics upon review by the Institutional Review Board.
| Case Report | ▴Top |
A 30-year-old pregnant woman, gravida 2, para 1, presented at 24 weeks’ gestation with dyspnea for 5 days secondary to COVID pneumonia. She was admitted to the medical intensive care unit (ICU) for progression to ARDS requiring intubation, therapeutic deep sedation, and paralysis, and inhaled nitric oxide for refractory hypoxic respiratory failure and subsequently required cannulation for initiation of VV-ECMO. While on VV-ECMO support, the paralysis was stopped and sedation was successfully weaned, and the patient became progressively hypertensive with systolic blood pressures over 160. Given concerns for comorbid preeclampsia, a 24-h urine protein was measured and found to be markedly elevated. The patient was also followed closely for coagulopathy and hemolysis felt to be related to ECMO circuit function with associated mild elevations in lactate dehydrogenase, mild thrombocytopenia, mild transaminase elevation, decreased fibrinogen and anemia (Table 1). The blood pressure was managed with intravenous analgesia with modest effect and nicardipine titration. At VV-ECMO day 14 the patient had a subacute rise in transaminases, thrombocytopenia, and persistent hypertension despite intravenous antihypertensives and analgosedation, and underwent cesarean section and successful delivery at 30 weeks 0 day of gestation. Her immediate post-partum course was complicated by hemoperitoneum requiring massive transfusion protocol. Clinically she improved rapidly and was undergoing slow wean of the VV-ECMO support and was successfully liberated on post-partum day 8 (VV-ECMO day 44). The patient’s hospital course was further complicated when she developed a generalized tonic-clonic seizure with gaze deviation that was responsive to intravenous lorazepam bolus on post-partum day 17. Computed tomography (CT) of the brain and subsequent CT angiogram showed focal frontal nonaneurysmal subarachnoid hemorrhage (Fig. 1). The patient had several additional witnessed and electrographic seizures over the subsequent 5 days and magnetic resonance imaging (MRI) brain showed white matter lesions consistent with posterior reversible encephalopathy syndrome (PRES) (Fig. 2). Transcranial Doppler ultrasound demonstrated mild diffusely elevated pulsatility indices consistent with increase distal cerebrovascular resistance with no evidence of vasospasm. Her antiepileptic and antihypertensives were increased with resolution of symptoms. The patient ultimately was liberated from VV-ECMO and ventilation, underwent extensive physical therapy and was discharged home.
 Click to view | Table 1. Review of Patient Laboratory Data |
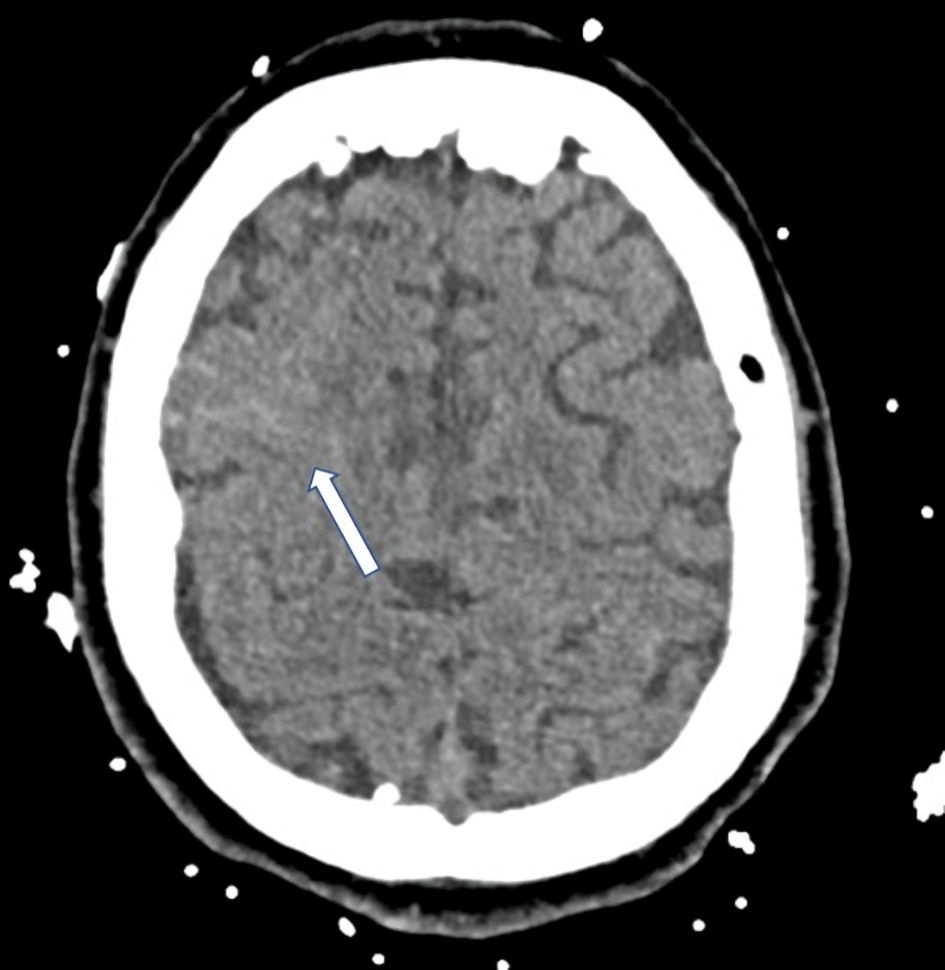 Click for large image | Figure 1. CT scan with small right frontal subarachnoid hemorrhage (arrow) with associated adjacent cerebral edema. CT: computed tomography. |
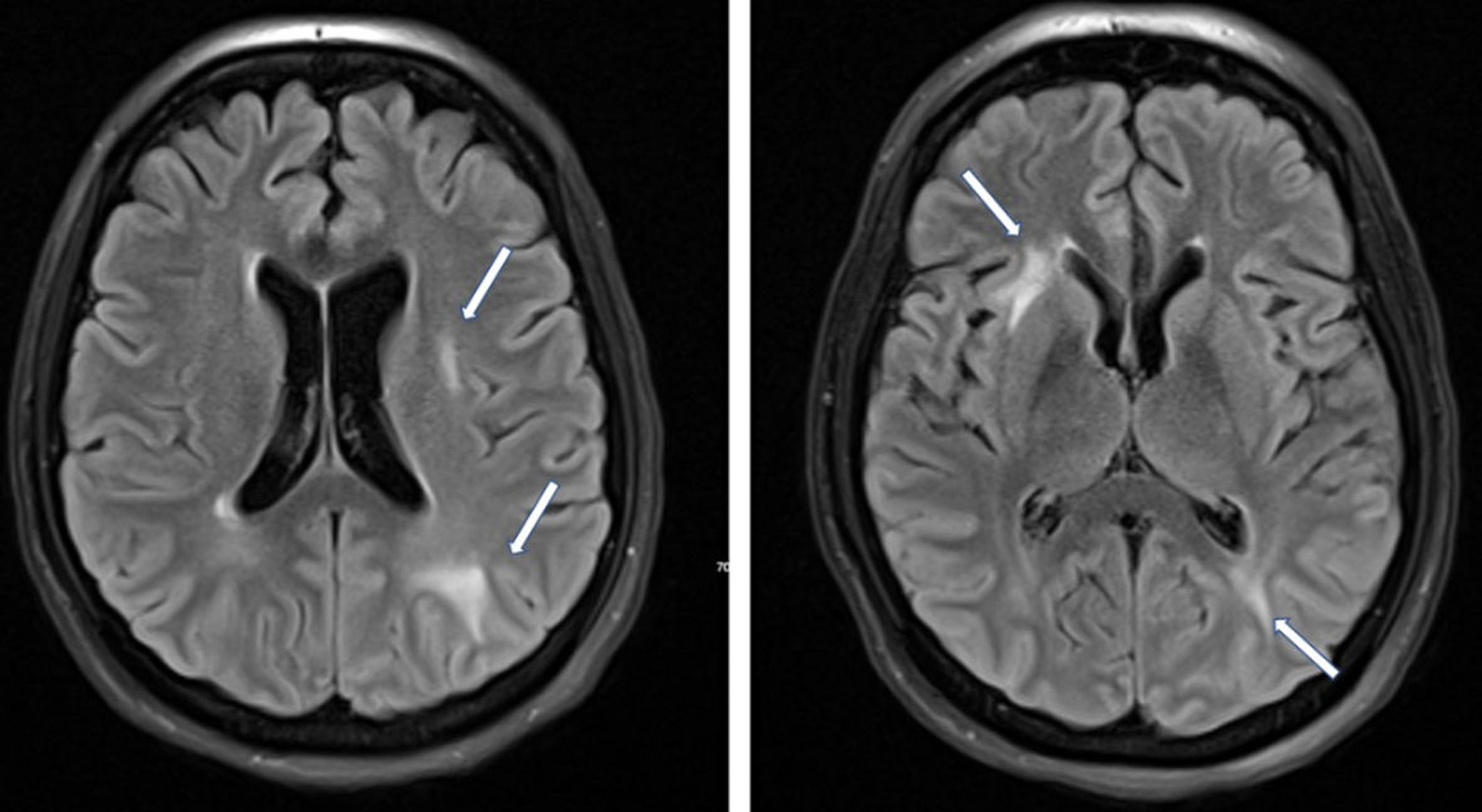 Click for large image | Figure 2. MRI axial FLAIR images demonstrating hyperintensities in the periventricular and parietal, occipital and frontal regions secondary to PRES (arrow). Diffusion-weighted imaging showed restricted diffusion along the right frontal convexity associated with subarachnoid blood (not shown). Diffusion-weighted images did not show evidence of acute infarct. MRI: magnetic resonance imaging; PRES: posterior reversible encephalopathy syndrome; FLAIR: fluid attenuated inversion recovery. |
| Discussion | ▴Top |
Differentiating between preeclampsia and VV-ECMO complications is challenging for physicians. The patient had preeclampsia with severe features based on the following: hypertension, proteinuria, and end organ dysfunction. However, ECMO can lead to liver and kidney dysfunction which can manifest as elevated creatinine, proteinuria, and transaminitis. Thus, it may be difficult to discern whether certain abnormal laboratory findings are due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, ECMO support, or preeclampsia, and this interplay may have treatment implications. For example, thrombocytopenia [3], liver function abnormalities, and renal dysfunction [4] are diagnostic criteria for preeclampsia with severe features and are also associated with worsening COVID-19 infection. There are currently no data to suggest the incidence of proteinuria in ECMO patients. Acute kidney injury (AKI) and volume overload are frequently observed in pediatric and adult ECMO populations. The need for combined ECMO and renal replacement therapy (RRT) is up to 50% [5, 6]. Patients with ARDS secondary to COVID-19 infection often develop AKI [6] making it hard to determine if end organ dysfunction is due to preeclampsia, ECMO, or the infectious process. There is also a strong association with COVID-19 and preeclampsia, especially among nulliparous women, with several prior cases of ARDS attributed directly to preeclampsia leading to need for ECMO [7].
In this case hypertension may have been the delineating feature; however, hypertension in the ICU can have multifactorial causes including pain, anxiety, trauma associated with the presence of ECMO cannula, etc. Despite delivery, the patient remained hypertensive and developed subsequent seizures. Her imaging was remarkable for PRES and nonaneurysmal subarachnoid hemorrhage as the likely the etiology of her new-set seizures. PRES is a known complication of eclampsia, and some studies demonstrate an incidence of up to 19% following delivery [8, 9]. Development of seizures and PRES within 6 weeks of delivery indicates a diagnosis of eclampsia in this patient. However, seizures and PRES are also known complications of ECMO support and associated coagulopathy, and there is no retrospective way to definitively differentiate etiology in a patient with multiple contributing conditions. PRES is documented in ECMO patients though the incidence is not known [10]. Cerebral blood flow and autoregulation can also be affected during VV-ECMO, including abrupt PaO2 and PaCO2 changes upon ECMO initiation [10]. Similarly, COVID-19 infection has been associated in small case series and case reports with neurologic sequelae including PRES. Although the patient also had a prolonged intubation period requiring VV-ECMO with fluctuating blood pressures, endothelial dysfunction secondary to COVID-19 could also have contributed to the PRES as we do not have information regarding timing of PRES in relation to active or chronic infection. The presence of SARS-CoV-2 in slow-flowing cerebral microcirculation may facilitate the interaction of the virus spike protein with endothelial angiotensin-converting enzyme 2 (ACE2) receptors causing impaired autoregulation and increasing the risk of capillary rupture [11]. This process could predispose to additional microvascular changes and hemorrhage in a hypertensive patient with multifactorial synergistic processes related to infection, hypoxia, post-partum hemorrhage, and impaired autoregulation in the setting of VV-ECMO, and preeclampsia all contributing to post-hypoxic leukoencephalopathy.
Proteinuria, thrombocytopenia, and neurologic sequelae including seizures and PRES can occur secondary to COVID-19-related infection, ECMO support, and as a complication of pregnancy. Differentiating the etiology of these findings is important and identifying additional criteria for diagnosis of preeclampsia in patients on ECMO support is paramount to maternal and fetal health. Bleeding and neurologic symptoms can occur as a complication of ECMO. While post-partum preeclampsia and eclampsia can occur 6 weeks after delivery, it would be unusual for her symptoms to occur so late. Untangling the exact etiology of this patient’s complications can make management challenging. New markers have been developed that help diagnose preeclampsia. Angiogenic markers that capture the pathophysiology of preeclampsia include vascular endothelial growth factor (VEGF) and placental growth factor (PlGF), as well as two anti-angiogenic proteins, soluble endoglin (sEng) and the truncated form of the full-length VEGF receptor type-1 (Flt-1), known as soluble fms-like tyrosine kinase 1 (sFlt-1). The sFlt-1/PlGF ratio has had the most success for predicting preeclampsia and the adverse outcomes in preeclampsia [12]. The issues with potentially using these biomarkers are limited availability for clinical application as they have not been validated outside of research, and it is unclear the effect ECMO has on these markers, suggesting opportunities for further investigation. Though we did not have access to biomarker testing in this patient, others have demonstrated self-limited preeclampsia-like syndrome associated with severe COVID-19 with normal sFlt-1/PlGF, which resolved after recovery from severe pneumonia [13].
Conclusions
In this case we present challenges in diagnosing preeclampsia and possibly eclampsia in a patient with hypertension, proteinuria, and COVID-19 infection who was maintained on ECMO support. Comorbidities and use of ECMO can confound common diagnostic criteria for preeclampsia and leave patients at high risk for misdiagnosis and development of eclampsia. This unique case details the challenges of applying common diagnostic criteria in uncommon circumstances. We report this case to support further exploration of alternative diagnostic methods and validation of biomarkers for clinical use to distinguish preeclampsia-like syndromes associated with severe COVID-19 and particularly those vulnerable patients on ECMO, for whom standard diagnostic criteria may not be reliable.
Acknowledgments
None to declare.
Financial Disclosure
We have no financial disclosures and there was no funding related to this report.
Conflict of Interest
There is no relevant conflict of interest to disclose.
Informed Consent
We obtained a direct waiver of informed patient consent from the institutional review board and ethics review. All data were deidentified.
Author Contributions
Muhammad Abu-Rmaileh performed the data collection and writing of the manuscript. Amy E. Hackmann was involved in manuscript planning and editing. Emily H. Adhikari was involved in manuscript editing. Bethany L. Lussier was involved in manuscript planning and writing of the initial manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Tramm R, Ilic D, Davies AR, Pellegrino VA, Romero L, Hodgson C. Extracorporeal membrane oxygenation for critically ill adults. Cochrane Database Syst Rev. 2015;1:CD010381.
doi pubmed - Pacheco LD, Saade GR, Hankins GDV. Extracorporeal membrane oxygenation (ECMO) during pregnancy and postpartum. Semin Perinatol. 2018;42(1):21-25.
doi pubmed - Kohs TCL, Liu P, Raghunathan V, Amirsoltani R, Oakes M, McCarty OJT, Olson SR, et al. Severe thrombocytopenia in adults undergoing extracorporeal membrane oxygenation is predictive of thrombosis. Platelets. 2022;33(4):570-576.
doi pubmed - Dado DN, Ainsworth CR, Thomas SB, Huang B, Piper LC, Sams VG, Batchinsky A, et al. Outcomes among patients treated with renal replacement therapy during extracorporeal membrane oxygenation: a single-center retrospective study. Blood Purif. 2020;49(3):341-347.
doi pubmed - Kielstein JT, Heiden AM, Beutel G, Gottlieb J, Wiesner O, Hafer C, Hadem J, et al. Renal function and survival in 200 patients undergoing ECMO therapy. Nephrol Dial Transplant. 2013;28(1):86-90.
doi pubmed - Alenezi FK, Almeshari MA, Mahida R, Bangash MN, Thickett DR, Patel JM. Incidence and risk factors of acute kidney injury in COVID-19 patients with and without acute respiratory distress syndrome (ARDS) during the first wave of COVID-19: a systematic review and Meta-Analysis. Ren Fail. 2021;43(1):1621-1633.
doi pubmed - Webster CM, Smith KA, Manuck TA. Extracorporeal membrane oxygenation in pregnant and postpartum women: a ten-year case series. Am J Obstet Gynecol MFM. 2020;2(2):100108.
doi pubmed - Martins JF, Cruz LR, Pereira DJ, Sousa JE, Martins P. Posterior reversible encephalopathy syndrome in a patient submitted to extracorporeal membrane oxygenation for COVID-19. Rev Bras Ter Intensiva. 2021;33(3):457-460.
doi pubmed - McDermott M, Miller EC, Rundek T, Hurn PD, Bushnell CD. Preeclampsia: association with posterior reversible encephalopathy syndrome and stroke. Stroke. 2018;49(3):524-530.
doi pubmed - Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C. Cerebral pathophysiology in extracorporeal membrane oxygenation: pitfalls in daily clinical management. Crit Care Res Pract. 2018;2018:3237810.
doi pubmed - Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18-22.
doi pubmed - Gu Y, Lewis DF, Wang Y. Placental productions and expressions of soluble endoglin, soluble fms-like tyrosine kinase receptor-1, and placental growth factor in normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2008;93(1):260-266.
doi pubmed - Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, Lopez-Martinez RM, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127(11):1374-1380.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
