| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 2, Number 1, June 2013, pages 20-26
Comprasion of Ovarian Stromal and Uterin Artery Blood Flow Measured by Color Doppler Ultrasonography in Polycystic Ovary Syndrome Patients and Patients With Ultrasonographic Evidence of Polycystic
Mehmet Suhha Bostancia, d, Nevin Sagsozb, Volkan Noyanb, Aykan Yucelb, Kivilcim Gorenc
aDepartment of Gynecology and Obstetrics, Sakarya Education and Research Hospital, Sakarya, Turkey
bDepartment of Gynecology and Obstetrics, Faculty of Medicine, Kirikkale University, Kirikkale, Turkey
cDepartment of Gynecology and Obstetrics, Haci Hidayet Dogruer State Hospital, Kirikkale, Turkey
dCorresponding author: Mehmet Suhha Bostanci, Sakarya Egitim Arastirma Hastanesi Kadin Hastaliklari ve Dogum Klinigi, Sakarya, Turkey
Manuscript accepted for publication March 14, 2013
Short title: Comprasion of Ovarian Stromal and Uterin Artery Blood
doi: https://doi.org/10.4021/jcgo85w
| Abstract | ▴Top |
Background: In this study, the difference between ovarian stromal and uterine artery blood flow measured with color Doppler ultrasound between polycystic ovary syndrome (PCOS) patients and patients with ultrasonographic evidence of polycystic were evaluated.
Methods: Serum hormone levels and ultrasonographic and color Doppler analysis were evaluated for 20 patients with PCOS and 20 healthy women with ultrasonographic evidence of polycystic.
Results: There were significant difference between uterine artery pulsatility index (PI), uterine artery resistance index (RI), ovarian stromal artery PI and ovarian stromal artery RI measurements of PCOS and healthy women with ultrasonographic evidence of polycystic groups (P values respectively; P < 0.01, P < 0.05, P < 0.01, P < 0.01). In PCOS group ovarian stromal artery RI values were inversely correlated with androstenedione levels (r = -0.536; P < 0.05). Also in PCOS group uterine artery PI values were positively correlated with androstenedione levels (r = 0.536; P < 0.05).
Conclusion: In patients with PCOS uterine artery PI and RI values are higher than women with ultrasonographic evidence of polycystic while ovarian artery PI and RI values were lower. Ovarian stromal artery RI value is inversely proportional to the serum androstenedione level while exchange value of uterine artery PI varies in direct proportion to the serum androstenedione level for PCOS patients.
Keywords: Polycystic ovary syndrome; Uterine artery pulsatility index; Resistance index; Ovarian stromal artery; Androstenedione
| Introduction | ▴Top |
Polycystic ovarian syndrome (PCOS) was first described by Stein and Leventhal in 1935 [1]. PCOS is a heterogeneous pathological condition characterized by reproductive disorders, and frequently associated with hyperandrogenism, obesity, hyperinsulinemia and insulin resistance [2-4]. PCOS is the most common female endocrinopathy, and its frequency is about 6-8% in the reproductive period [2]. Although polycystic ovaries can be found in approximately 33% of the female population, they are not necessarily associated with the typical symptoms and PCOS, which may be expressed at some time during the fertile life span when provoked by, for example, weight gain or insulin resistance [3, 4]. In 2003, a joint ESHRE/ASRM consensus meeting produced a refined definition of PCOS: namely the presence of two out of the following three criteria: (1) oligo- and/or anovulation, (2) hyperandrogenism (clinical and/or biochemical), and (3) polycystic ovaries, with the exclusion of other etiologies [1]. The morphology of the PCO was redefined as an ovary with 12 or more follicles measuring 2 - 9 mm in diameter and/or increased ovarian volume (> 10 cm3) [5].
Ultrasound assessment of ovarian morphology is considered to be essential in the diagnosis of PCOS and the gold standard for defining polycyctic ovary (PCO) [6]. The polycystic ovary is the morphological ovarian phenotype in women with the polycystic ovary syndrome. However, not all women with polycystic ovaries demonstrate the clinical and biochemical features that define the PCOS [6].
The introduction of transvaginal Doppler sonography has contributed markedly to the refinement of ultrasound diagnosis. In addition, it has provided much new morphological and pathophysiological information on blood flow dynamics within the female pelvis [7, 8]. It has been shown that in patients with polycystic ovarian syndrome important changes in ovarian vascularization occur at the level of the intraovarian arteries. Battaglia and co-workers [9], Zaidi and co-workers [10] and Aleem and co-workers [11] successively confirmed that, in patients with PCOS, significant changes occur within the intraovarian vessels. Furthermore, uterine artery resistance was shown to be increased in PCOS [9-11]. Zaidi and co-workers [10] and Aleem and co-workers [11], confirming that Doppler analysis of ovarian stromal arteries in PCOS may be useful to improve the diagnosis, and to provide further information about the pathophysiology and evolution of the syndrome. These hypotheses have been recently confirmed either by using two- [12] or three-dimensional [13] color Doppler systems analysis.
Only little information exists in the literature regarding the details of ultrasound parameters in women with PCO only and PCOS, which may be important in the understanding of the pathophysiology of PCOS. An understanding of vascular changes in women with PCO may allow us to gain further insights into the underlying pathophysiology of the condition and differences between PCOS and PCO only patients.
This study aimed to show differences in their ovarian stromal and uterine blood flow and whether there is a correlation between these patterns and specific hormonal parameters.
| Materials and Methods | ▴Top |
The study protocol was approved by the local Ethics Review Committee. Women with PCOS (n = 20) and PCO only (n = 20) attending the Kirikkale University Medical Faculty Hospital Gynecology Clinic participated in the study after giving written informed consent. PCOS was previously diagnosed on the basis of the criteria of ESHRE/ASRM consensus. Those with ≥ 12 antral follicles at least in one ovary on transvaginal scanning and not had the criteria of ESHRE/ASRM consensus for diagnosis of PCOS were considered as PCO only. Hirsutism was assessed by the Ferriman-Gallwey score (FGS), as modified by Hatch [14] and body mass index (BMI) was computed as the ratio of weight divided by the height squared (kg/m2) [5]. Polycystic ovaries were diagnosed on the ultrasound scan if there were 12 or more cysts in the ovary, 2 - 8 mm in diameter and arranged around a dense stroma or scattered throughout an increased amount of stroma for the PCO only group [10].
Hormonal assays and ultrasonographic and color Doppler analysis were performed for all women in the early follicular phase, between the 2nd and the 5th days of their spontaneous menstrual cycle. None had hypertension (systolic blood pressure > 140 mmHg and/or diastolic blood pressure > 90 mmHg). Furthermore, they had not received hormonal therapy for at least 6 months before the study.
Ultrasound examinations were performed with a 6.5-MHz vaginal transducer (Siemens, Acuson EC7 Transvaginal Probe, USA) equipped with color Doppler. Ovarian follicle distribution, number were recorded. The spatial peak temporal average intensity of ultrasound for B mode and Doppler examinations was < 50 mW/cm2, which is well within the safety limits recommended by the Bioeffects Committee of the American Institute of Ultrasound in Medicine [15].
Doppler flow measurements of the uterine and ovarian stromal vessels were performed transvaginally with a 6.5-MHz color Doppler system (Siemens, ACUSON, Antares™, USA). All the patients were studied between 8.00 and 11.00 to exclude effects of circadian rhythmicity on the uterine blood flow [16]. Furthermore, participants rested in a waiting room for at least 15 min before being scanned and completely voided their bladder in order to minimize external effects on blood flow [17]. For ovarian stromal blood vessels measurements color signals were sought in the ovarian stroma at the maximum distance from the surface of the ovary. Blood vessels located near the wall of a follicle were not measured too. Whereas several blood vessels were detected inside the ovarian stroma, only the vessel with the lowest downstream impedance was selected for Doppler measurements [9]. No correction was made for the angle of insonation of the Doppler beam for stromal ovarian arteries, since the angle of insonation for small vessels cannot be determined. For uterine artery measurements color flow images identified the ascending branches of the uterine arteries which were sampled lateral to the cervix in a sagittal plane. The angle of insonation was always adjusted to obtain maximum color intensity and when good color signals were obtained, blood flow velocity waveforms were recorded with the sample volume placed across the vessel and the pulsed Doppler mode entered for uterine artery measurements. Resistance index (RI) and pulsatility index (PI) were electronically calculated according to the following formula: PI = (S - D)/mean, RI = (S - D)/S, where S is the peak shifted Doppler frequency, D is the minimum Doppler shifted frequency and ‘mean’ is the mean maximum Doppler shifted frequency over the cardiac cycle. Four waveforms were recorded from each artery examined on both sides, and the average measurements from these four waveforms were included in the calculations. After that according to the follicule number left or right side measurements was selected for accurate measurement uterine and stromal ovarian Doppler measurement. No significant differences between the PIs of the left and right uterine arteries were observed, and, therefore, the average value of both arteries was used. Similarly, the lowest PIs of the stromal arteries were not significantly different between the left and right ovaries and the mean value was used. Ultrasound and color Doppler analyses were performed by a single examiner who was unaware of the hormonal status of the scanned patients.
Blood samples were collected from each patient on the same day as Doppler flow analysis between 8.00 a.m. and 10.00 a.m., and the following hormones were assayed: luteinizing hormone (LH), follicle stimulating hormone (FSH), prolactin (PRL), estradiol (E2), total testosterone (total T), free testosterone (free T), androstenedione (A), 17α- hydroxyprogesterone (17-OHP), dehydroepiandrosterone sulfate (DHEAS) and serum sex hormone binding globulin (SHBG). Blood samples for progesterone (P) were collected from each patient on the 21st day of the menstruel cycle between 8.00 a.m. and 10.00 a.m. for detection of ovulation. Serum FSH, LH, PRL, E2, total T, free T, A, 17- OHP, DHEAS, SHBG, P concentrations were measured using commercially available kits (Roche kits, Germany).
Statistical analysis
The Statistical Package for the Social Sciences (release 11.5; SPSS, Inc., Chicago, IL) was used to store and analyze the data. Statistical analysis was performed for evaluating differences in mean Doppler and hormonal measurements between PCOS patients and the PCO only patients. The data were tested by means of Kolmogorov- Smirnov test. Student t-tests were used for parametric data and the χ2, Mann Whitney test for non parametric data. Correlation was estimated using Sperman correlation coefficient. Proportions were compared by χ2 test. P < 0.05 was considered statistically significant. All values given are the mean ± SD. Stepwise multiple regression analysis was performed in order to assess correlations.
| Results | ▴Top |
The overall clinical results of patients with PCOS, PCO only groups are reported in Table 1. Mean age was similar in all women (PCOS group 25.65 (± 3.133), PCO only group 26.11 (± 3.428). The Ferriman-Gallwey score was significantly higher in PCOS patients PCOS group 8.7 (± 1.80), PCO only group 3.06 (± 1.58), P < 0.001, 17 patients in PCOS group (85%) and 14 patients in PCO only group were not smoke. For PCOS group 3 (15%) and for PCO only group 4 (22.2%) patient were smoking 1 - 10 cigarettes per day. There was not any heavy smoker in both groups. All patients in PCOS group had oligomenorrhea for menstrual cycle. For PCO only group 16 patients (88.9%) had normal menstrual cycle, only 2 patients (11.1%) had oligomenorrhea.
 Click to view | Table 1. Clinical Data of the Polycystic Ovary Syndrome-Patients and PCO Only Patients |
The biochemical finding of patients with PCOS, PCO only groups are reported in Table 2. As for PCOS and PCOS only patients, the plasma levels of PRL, SHBG, DHEAS, E2 did not differ significantly (P values respectively; P = 0.06, P = 0.10, P = 0.25, P = 0.67). In PCOS patients, LH, total T, free T and A levels were significantly higher than in the PCO only patients (P values respectively; P < 0.01, P < 0.05, P < 0.01).
 Click to view | Table 2. Biochemical Data of the Polycystic Ovary Syndrome-Patients and PCO Only Patients |
The Doppler findings of patients with PCOS, PCO only groups are reported in Table 3. No significant differences between the PIs of the left and right uterine arteries were observed, and, therefore, the average value of both arteries was used. Similarly, the lowest PIs of the stromal arteries were not significantly different between the left and right ovaries and the mean value was used (PCOS group; right and left uterin artery PI P = 0.379, right and left uterin artery RI P = 0.965, right and left ovarian stromal artery PI P = 0.141, right and left ovarian stromal artery RI P = 0.486, PCO only group; right and uterin artery PI P = 0.895, right and left uterin artery RI P = 0.639, right and left ovarian stromal artery PI P = 0.935, right and left ovarian stromal artery RI P = 0.657). There were significant difference between uterine artery PI, uterine artery RI, ovarian stromal artery PI and ovarian stromal artery RI measurements of PCOS and PCO only groups (p values respectively; P < 0.01, P < 0.05, P < 0.01, P < 0.01) (Fig. 1).
 Click to view | Table 3. Doppler Findings of the Polycystic Ovary Syndrome-Patients and PCO Only Patients |
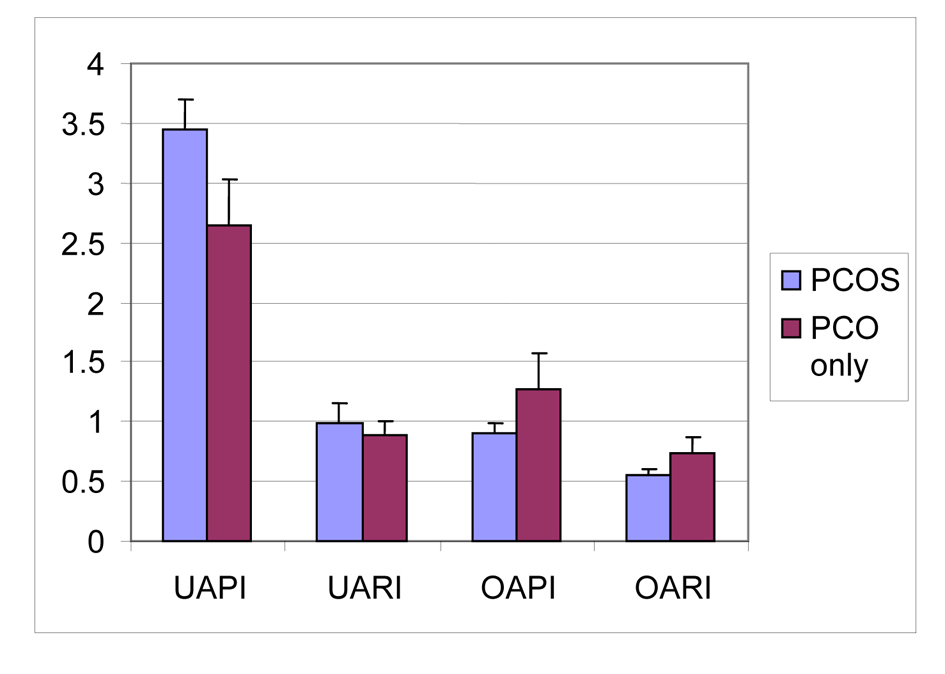 Click for large image | Figure 1. Mean values of uterin artery PI, uterin artery RI, ovarian stromal artery PI, ovarian stromal artery of PCOS and PCO only groups. UAPI: uterine artery pulsatility index; UARI: uterine artery resistance index; OAPI: ovarian stromal artery pulsatility index; OARI: ovarian stromal artery resistance index. |
In PCOS group ovarian stromal artery RI values were inversely correlated with androstenedione levels (r = -0.536; P < 0.05). Also in PCOS group uterine artery PI values were positively correlated with androstenedione levels (r = 0.536; P < 0.05). A significant positive correlation between higher serum LH levels and lower RI values of ovarian stromal artery was found for PCOS patients (P < 0.05).
| Discussion | ▴Top |
PCOS is the most common female endocrinopathy, affecting 6-8% of women in their reproductive years and etiology still unknown exactly [2]. The clinical significance of PCO only remains unclear although it is a common finding (14-23%) in normal women of reproductive age [18]. It has been suggested that development of a state of chronic anovulation may result in the classical picture of PCOS, displaying numerous follicles in the early stages of development and atresia, and dense stromal tissue [19]. It is still debatable whether the isolated finding of PCO in normal ovulatory women without the clinical or endocrine features of PCOS is a normal variation or a part of the spectrum of PCOS.
PCOS in patients being overweight or even obesity is a condition often encountered [20]. As our study, as cases PCOS cases compared with PCO Loverro et al found the BMI values PCOS group higher than PCO group [21]. In our study, increased BMI observed with a correlation between DHEAS levels and a similar relationship as a result of work done by Park and colleagues also found [22].
Values observed in patients PCOS, FGS increase depends, has been shown in many studies done on patients PCOS [23-26]. Values observed in patients PCOS FGS increase depends, of value only PCOS cases FGS compared to normal population but also increased according to the group PCO their study also show Loverro and colleagues [21].
Angiogenesis plays an important role in both the follicular and luteal phases of an ovarian cycle [27]. Hypersecretion of LH during the follicular phase of the menstrual cycle occurs in PCOS and is associated with hyperplasia of the ovarian theca and stromal cells. Elevated LH levels may be responsible for increased stromal vascularization by influencing neoangiogenesis, catecholaminergic stimulation and leukocyte and cytokine activation [28].
In the present study, Doppler parameters (RI and PI) of uterine and ovarian stromal blood flow in patients with PCOS and PCO only women were measured. Pulsatility and resistance indices of ovarian vessels were significantly lower in PCOS women when compared with PCO only women.
A significant positive correlation between higher serum LH levels lower RI values of ovarian stromal artery has been reported for PCOS patients [29, 30]. This was also confirmed by our results for PCOS group. Similar results were not found in PCO only group in our study.
In the present study for PCOS group uterine artery PI values were positively correlated with androstenedione levels but Resende et al was found no correlation was between PI and luteinizing hormone, testosterone or androstenedione levels [31].
The uterine artery PI was significantly greater in the PCOS group when compared for the PCO only group. In PCOS group as previously observed [21, 29, 32], uterine artery PI was shown to be positively correlated with DHEAS and AS. Similar results were not found in PCO group in our study. Androgens are known to have direct vasoconstrictive effects on vascular tissues [33], thereby increasing vascular resistance. Furthermore, PCOS patients have an increased predisposition to atherosclerosis with resultant hardening and thickening of vessel walls [34] which may also result in increased systemic vascular resistance. In this case, they carry long-term PCOS of patients with myocardial infarction and are considered coronary artery disease is important. Uterine artery Doppler values and serum of patients with PCO, as value similar correlations between these patients atherosclerotic risks it can not be displayed and did not move has not yet clarified.
Only little information exists in the literature regarding the details of Doppler ultrasound parameters in women with PCO only and PCOS, which may be important in the understanding of the pathophysiology of PCOS. PI and RI values of uterine artery and ovarian stromal arteries of patients with PCO in our study is different from PCOS cases and close to the value specified in the normal population and this results differs with some other studies [35, 36]. PCO cases this situation, as distinct from the normal value PCOS observed in patients with serum androgen levels and hormonal values occurred as a result suggest.
For patients in the diagnosis and long-term PCOS color Doppler ultrasound measurement of changes in clinical follow-up evaluation and clinical results of the etiology of the disease may be more useful for understanding also is thought by us. However, follow-up of patients in PCO color Doppler ultrasound may be useful for assessing the PCOS, atherosclerosis and other complication development. Longitudinal studies with careful follow-up are necessary to confirm and expand the above findings.
| References | ▴Top |
- Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol 1935; 29:181-188.
- Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89(6):2745-2749.
doi pubmed - Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle modification in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13(6):251-257.
doi - Homburg R. Polycystic ovary syndrome - from gynaecological curiosity to multisystem endocrinopathy. Hum Reprod. 1996;11(1):29-39.
doi pubmed - Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47.
doi - Balen AH, Laven JS, Tan SL, Dewailly D. Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update. 2003;9(6):505-514.
doi pubmed - Kurjak A, Zalud I, Jurkovic D, Alfirevic Z, Miljan M. Transvaginal color Doppler for the assessment of pelvic circulation. Acta Obstet Gynecol Scand. 1989;68(2):131-135.
doi pubmed - Kurjak A, Jurkovic D, Alfirevic Z, Zalud I. Transvaginal color Doppler imaging. J Clin Ultrasound. 1990;18(4):227-234.
doi pubmed - Battaglia C, Artini PG, D'Ambrogio G, Genazzani AD, Genazzani AR. The role of color Doppler imaging in the diagnosis of polycystic ovary syndrome. Am J Obstet Gynecol. 1995;172(1 Pt 1):108-113.
doi - Zaidi J, Campbell S, Pittrof R, Kyei-Mensah A, Shaker A, Jacobs HS, Tan SL. Ovarian stromal blood flow in women with polycystic ovaries—a possible new marker for diagnosis? Hum Reprod. 1995;10(8):1992-1996.
pubmed - Aleem FA, Predanic M. Transvaginal color Doppler determination of the ovarian and uterine blood flow characteristics in polycystic ovary disease. Fertil Steril. 1996;65(3):510-516.
pubmed - Ajossa S, Guerriero S, Paoletti AM, Orru M, Floris S, Mannias M, Melis GB. Uterine perfusion and hormonal pattern in patients with polycystic ovary syndrome. J Assist Reprod Genet. 2001;18(8):436-440.
doi pubmed - Pan HA, Wu MH, Cheng YC, Li CH, Chang FM. Quantification of Doppler signal in polycystic ovary syndromeusing three-dimensional power Doppler ultrasonography:a possible new marker for diagnosis. Hum Reprod 2002; 17:2011-2016.
- Hatch R, Rosenfield RL, Kim MH, Tredway D. Hirsutism: implications, etiology, and management. Am J Obstet Gynecol. 1981;140(7):815-830.
pubmed - Taylor KJ, Burns PN, Wells PN, Conway DI, Hull MG. Ultrasound Doppler flow studies of the ovarian and uterine arteries. Br J Obstet Gynaecol. 1985;92(3):240-246.
doi pubmed - Zaidi J, Jurkovic D, Campbell S, Okokon E, Tan SL. Circadian variation in uterine artery blood flow indices during the follicular phase of the menstrual cycle. Ultrasound Obstet Gynecol. 1995;5(6):406-410.
doi pubmed - Battaglia C, Artini PG, D'Ambrogio G, Galli PA, Genazzani AR. Uterine and ovarian blood flow measurement. Does the full bladder modify the flow resistance? Acta Obstet Gynecol Scand. 1994;73(9):716-718.
doi pubmed - Clayton RN, Ogden V, Hodgkinson J, Worswick L, Rodin DA, Dyer S, Meade TW. How common are polycystic ovaries in normal women and what is their significance for the fertility of the population? Clin Endocrinol (Oxf). 1992;37(2):127-134.
doi - Yen SSC. Chronic anovulation caused by peripheral endocrine disorders. In Reproductive Endocrinology 1986; 441-499 Eds SSC Yen and RB Jaffe. WB Saunders, Philadelphia.
- Rasgon NL, Rao RC, Hwang S, Altshuler LL, Elman S, Zuckerbrow-Miller J, Korenman SG. Depression in women with polycystic ovary syndrome: clinical and biochemical correlates. J Affect Disord. 2003;74(3):299-304.
doi - Loverro G, Vicino M, Lorusso F, Vimercati A, Greco P, Selvaggi L. Polycystic ovary syndrome: relationship between insulin sensitivity, sex hormone levels and ovarian stromal blood flow. Gynecol Endocrinol. 2001;15(2):142-149.
pubmed - Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421.
pubmed - Barth JH, Catalan J, Cherry CA, Day A. Psychological morbidity in women referred for treatment of hirsutism. J Psychosom Res. 1993;37(6):615-619.
doi - Moncada E. Familial study of hirsutism. J Clin Endocrinol Metab. 1970;31(5):556-564.
doi pubmed - Azziz R, Carmina E, Sawaya ME. Idiopathic hirsutism. Endocr Rev. 2000;21(4):347-362.
doi pubmed - Zaidi J, Jacobs H, Campbell S, Tan SL. Blood flow changes in the ovarian and uterine arteries in women with polycystic ovary syndrome who respond to clomiphene citrate: correlation with serum hormone concentrations. Ultrasound Obstet Gynecol. 1998;12(3):188-196.
doi pubmed - Abulafia O, Sherer DM. Angiogenesis of the ovary. Am J Obstet Gynecol. 2000;182(1 Pt 1):240-246.
doi - Findlay JK. Angiogenesis in reproductive tissues. J Endocrinol. 1986;111(3):357-366.
doi pubmed - Battaglia C, Artini PG, Salvatori M, Giulini S, Petraglia F, Maxia N, Volpe A. Ultrasonographic patterns of polycystic ovaries: color Doppler and hormonal correlations. Ultrasound Obstet Gynecol. 1998;11(5):332-336.
doi pubmed - Ng EH, Chan CC, Ho PC. Are there differences in ultrasound parameters between Chinese women with polycystic ovaries only and with polycystic ovary syndrome? Eur J Obstet Gynecol Reprod Biol. 2006;125(1):92-98.
doi pubmed - Resende AV, Mendes MC, Dias de Moura M, Mendonca HC, Gomes Premoli AC, Reis RM, Berezowski AT. Doppler study of the uterine arteries and ovarian stroma in patients with polycystic ovary syndrome. Gynecol Obstet Invest. 2001;52(3):153-157.
doi pubmed - Adali E, Kolusari A, Adali F, Yildizhan R, Kurdoglu M, Sahin HG. Doppler analysis of uterine perfusion and ovarian stromal blood flow in polycystic ovary syndrome. Int J Gynaecol Obstet. 2009;105(2):154-157.
doi pubmed - Christian RC, Dumesic DA, Behrenbeck T, Oberg AL, Sheedy PF, 2nd, Fitzpatrick LA. Prevalence and predictors of coronary artery calcification in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88(6):2562-2568.
doi pubmed - Dahlgren E, Janson PO, Johansson S, Lapidus L, Oden A. Polycystic ovary syndrome and risk for myocardial infarction. Evaluated from a risk factor model based on a prospective population study of women. Acta Obstet Gynecol Scand. 1992;71(8):599-604.
doi pubmed - Ehrmann DA, Rosenfield RL, Barnes RB, Brigell DF, Sheikh Z. Detection of functional ovarian hyperandrogenism in women with androgen excess. N Engl J Med. 1992;327(3):157-162.
doi pubmed - Graf MJ, Richards CJ, Brown V, Meissner L, Dunaif A. The independent effects of hyperandrogenaemia, hyperinsulinaemia, and obesity on lipid and lipoprotein profiles in women. Clin Endocrinol (Oxf). 1990;33(1):119-131.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
