| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Short Communication
Volume 12, Number 3, December 2023, pages 84-87
Association of Labor and Lactate Levels
Michelle Nguyena, e, Teri M. Kozikb, Mouchumi Bhattacharyyac, Anuja Ozad
aTouro University College of Medicine, Vallejo, CA, USA
bDepartment of Graduate Medical Education, St. Joseph’s Medical Center, Stockton, CA, USA
cDepartment of Mathematics, University of the Pacific, Stockton, CA, USA
dDepartment of Family Medicine, Commonspirit St. Joseph’s Medical Center, Stockton, CA, USA
eCorresponding Author: Michelle Nguyen, Touro University College of Medicine, Vallejo, CA, USA
Manuscript submitted September 25, 2023, accepted October 23, 2023, published online December 28, 2023
Short title: Lactate During Labor
doi: https://doi.org/10.14740/jcgo912
| Abstract | ▴Top |
Background: Lactate is widely used as a marker for sepsis in pregnant and non-pregnant individuals. During an infection, lactate rises both due to increased production and decreased clearance. During labor, hypoxia can be noted in the uterus due to contractions that increase in frequency and magnitude which may also contribute to rising lactate. Thus, this study aimed to determine whether labor affects lactate levels.
Methods: This retrospective observational study reviewed charts of pregnant women who had serum lactic acids drawn during active labor and prior to delivery regardless of whether they ultimately underwent a cesarean section or vaginal birth at Saint Joseph’s Medical Center (SJMC) in Stockton, CA. Patients were categorized into non-septic and septic groups based on the institution’s modified systemic inflammatory response syndrome (SIRS) criteria for pregnant women. Two-sample t-tests were used to compare the mean serum lactic acid, body mass index (BMI), hours of labor at time of lactic acid collection, age, gravida, and para between septic and non-septic groups. A regression analysis was also used to determine any relation between serum lactate levels and the hours of labor that had passed at the time of the serum lactate collection.
Results: Sixty-two patient charts were identified and reviewed. Thirty patients fit the septic group inclusion criteria. There was a statistically significant difference in serum lactate between septic versus non-septic patients (P = 0.0043, 95% confidence interval (CI): -2.32, -0.45), independent of labor length. There was no relation between serum lactic acid and the hours of labor that had passed at the time of the lab (P = 0.419). There was also no statistically significant difference in the mean BMI (P = 0.18, 95% CI: -0.85, 4.39), hours of labor (P = 0.57, 95% CI: -4.01, 2.24), age (P = 0.44, 95% CI: -1.56, 3.52), gravida (P = 0.91, 95% CI: -1.03, 0.92), or para (P = 0.98, 95% CI: -0.65, 0.63) between the non-septic and septic groups.
Conclusions: The primary results suggest that lactic acid remains an accurate indicator of sepsis status given that septic patients had a significantly elevated mean serum lactate. There was also no correlation between serum lactate and the hours of labor that had passed at the time of the lab collection.
Keywords: Lactic acid; Lactate; Maternal sepsis; Sepsis; Labor
| Introduction | ▴Top |
During an infection, greater amounts of lactate are produced as cells convert to anaerobic metabolism due to either lack of oxygen delivery or increased metabolic demand. Outside of sepsis, lactate is also produced due to local tissue hypoxia in the uterus and peaks at the time of delivery [1]. Serum lactate is a reputable predictor of mortality but is a nonspecific infection marker [2, 3]. Based on analysis of the Surviving Sepsis Campaign (SCC) database, lactate is a clear indicator of septic shock and need for resuscitation when over 4 mmol/L in all individuals [4, 5]. This value is used as part of the Sepsis Obstetrics Scoring System (SOS) for determining the need for intensive care unit (ICU) management [6]. However, little data provide guidance for lactate values between 2 and 4 mmol/L, especially in pregnant individuals.
Studies have long shown that the gravid uterus produces a net positive amount of lactic acid although the pathophysiology remains unclear [7]. The theory of uterine contractions contributing to elevated lactate as a predictor for various outcomes has led to research on multiple fronts. For example, lactate, albeit in amniotic fluid, can be a predictor of dysfunctional labor such as dystocia [8, 9]. While these studies refer to uterine lactate levels and not venous lactate levels, the results again suggest that the uterus is a significant producer of lactic acid. A meta-analysis of observational studies supports this theory in finding that while venous lactic acid may still be used as a screening tool for sepsis in pregnant women, it may be elevated during periods of labor, especially in later stages [10].
In non-pregnant individuals, normal lactate levels are defined as less than 2 mmol/L, between 2 and 4 mmol/L is hyperlactemia, and above 4 mmol/L is severe [11]. In pregnant women on the day of their delivery, the median serum lactate is 1.8 mmol/L with an interquartile range (IQR) of 1.3 - 2.5 [12]. The upper limit of this IQR overlaps with the definition of hyperlactemia. They also found that it was higher in women with vaginal deliveries versus cesarean sections, suggesting that labor does contribute to elevated lactate levels [12]. Another study finds that the mean serum lactate of women in labor is consistently above 2 mmol/L which falls within range of the definition for hyperlactemia [10]. While a limit of 4 mmol/L is defined as severe in all individuals, pregnant women who are septic and require treatment but do not require ICU management average a peak of 3.25 ± 1.92 mmol/L which also overlaps with the normal range for women in labor [13]. Due to the physiologic elevations in serum lactate during pregnancy and labor, there are many false positives. Thus, the purpose of this study was to provide more evidence in correlating a possible effect of labor on serum lactate. Perhaps then, a clearer mechanism for elevated lactate during pregnancy and labor may be established.
| Materials and Methods | ▴Top |
Design
This study was a retrospective chart analysis from a community hospital in the Central Valley of California which has approximately 3,300 live births per year. Two studies were referenced for the average serum lactic acid values in the septic and non-septic groups in the power analysis. According to Goyal et al, the average serum lactic acid value for pregnant women who are septic but do not require admission into the ICU at hour 0 is 3.25 ± 1.92 mmol/L [13]. According to Dockree et al, the average serum lactic acid in a pregnant woman who is in labor but is not septic is 1.8 mmol/L (IQR 1.3 - 2.5) [12]. Given these anticipated means for the septic and non-septic groups, we used a sample size calculator with an enrollment ratio of 1 and alpha of 0.05. To achieve a power of 80%, a minimum of 28 patients in each group was required [14].
Participants and procedure
Charts from mothers who gave birth between July 2020 and 2022 at St. Joseph’s Medical Center and had a venous lactate drawn were reviewed for age, gravida, para, and body mass index (BMI) in addition to rupture of membrane time for determining the hours of labor that had passed at the time of lactate collection. Their vitals, complete blood count, and comprehensive metabolic panels were also noted in order to classify patients into the non-septic or septic groups.
Laboring patients were identified as those who were admitted to labor and delivery for regular contractions and had rupture of membrane after regular contractions. Patients who ultimately underwent cesarean sections were still considered if they had been in labor and had serum lactic acid drawn prior to the surgery. Patients were also not excluded or separated into groups based on whether they had a prior C-section. The start time of labor was defined as the rupture of membrane time as this is routinely documented. Patients who experienced prelabor rupture of membranes were excluded as the start of labor would not be identifiable or consistent across all patients.
As charts were retrospectively reviewed, only routinely collected data could be analyzed. Serum lactate had been drawn on these patients per the clinician’s judgment for ruling out sepsis. While this may present some bias, the majority of patients ultimately did not fit the sepsis criteria. Patients were classified as septic if they met the institution’s modified systemic inflammatory response syndrome (SIRS) criteria for pregnant women which in addition to concern for infection with a valid source, include two or more elements from temperature ≥ 38 °C or < 36 °C, heart rate > 110, respiration > 24/min, and white blood cell (WBC) count > 15,000 or 10% bands or have signs of organ dysfunction as indicated by either systolic blood pressure (SBP) < 85 mm Hg/mean arterial pressure (MAP) < 65 mm Hg, SBP decrease of more than 40 mm Hg, acute respiratory failure as evidenced by a new need for invasive or non-invasive mechanical ventilation, tachypnea > 24/min, tachycardia > 110 bpm, leukopenia with WBC < 4,000, leukocytosis with WBC > 15,000, thrombocytopenia with platelet count < 100,000, hypotension with SBP < 85 mm Hg, creatinine > 1.2 mg/dL, urine output < 0.5 mL/kg/h for 2 consecutive hours, total bilirubin > 2 mg/dL (34.2 mmol/L), international normalized ratio (INR) 1.5 or partial thromboplastin time (PTT) > 60 s, or lactic acid level of over 2 mmol/L when outside of labor. Valid sources of infection were chorioamnionitis if documented as a diagnosis by a physician, positive blood or urine culture, positive coronavirus disease 2019 (COVID-19) test, or urinary tract infection on urinalysis. All chart data were de-identified prior to analysis. This study was approved by the Commonspirit Institutional Review Board and conducted in compliance with the ethical standards of the Health Insurance Portability and Accountability Act.
Charts were excluded for having venous lactates drawn outside of the window of labor or if the mother did not labor prior to a C-section. We identified the window of labor as after rupture of membranes and prior to delivery and included lactates drawn within 5 h of delivery based on lactate’s half-life of 1 h in a healthy individual [15].
Plan of analysis
Two-sample t-tests were used to compare the serum lactic acid, BMI, hours of labor, age, gravida, and para between the non-septic and septic groups. The 95% confidence intervals (CIs) were generated in addition to P-values. A multiple regression model was used with serum lactic acid as the response variable and both septic status and hours of labor at the time of lab collection as the explanatory variables. This was plotted to demonstrate how serum lactic acid correlated with the hours of labor that had passed at the time of the lab collection within the non-septic and septic groups.
| Results | ▴Top |
Of the 171 charts reviewed, 62 were included in this study. Thirty of those charts met the inclusion criteria for the sepsis group. A total of six patients in the non-septic group and eight patients in the septic group had comorbidities such as hypertension or diabetes. Four patients (12.5%) in the non-septic group and five patients (16.7%) in the septic group had pre-eclampsia. One patient (3.1%) in the non-septic group and no patients in the septic group (0%) had type 2 diabetes. One patient in the non-septic group (3.1%) and three patients (10%) in the septic group had gestational diabetes.
The results and demographics of the patient population are demonstrated in Table 1. There was a significant difference in the mean serum lactate between the septic (n = 30) and non-septic (n = 32) patients (P = 0.0043, 95% CI: -2.32, -0.45). There was no significant difference in the means of BMI (0.1811, 95% CI: -0.85, 4.39), hours of labor (0.5717), 95% CI, age, gravida, or para between the non-septic and septic groups. Per the regression analysis, there was no relation between serum lactate and the hours of labor at the time of lab collection (P = 0.419) as shown in Figure 1.
 Click to view | Table 1. Comparison of Means Between the Non-Septic and Septic Groups |
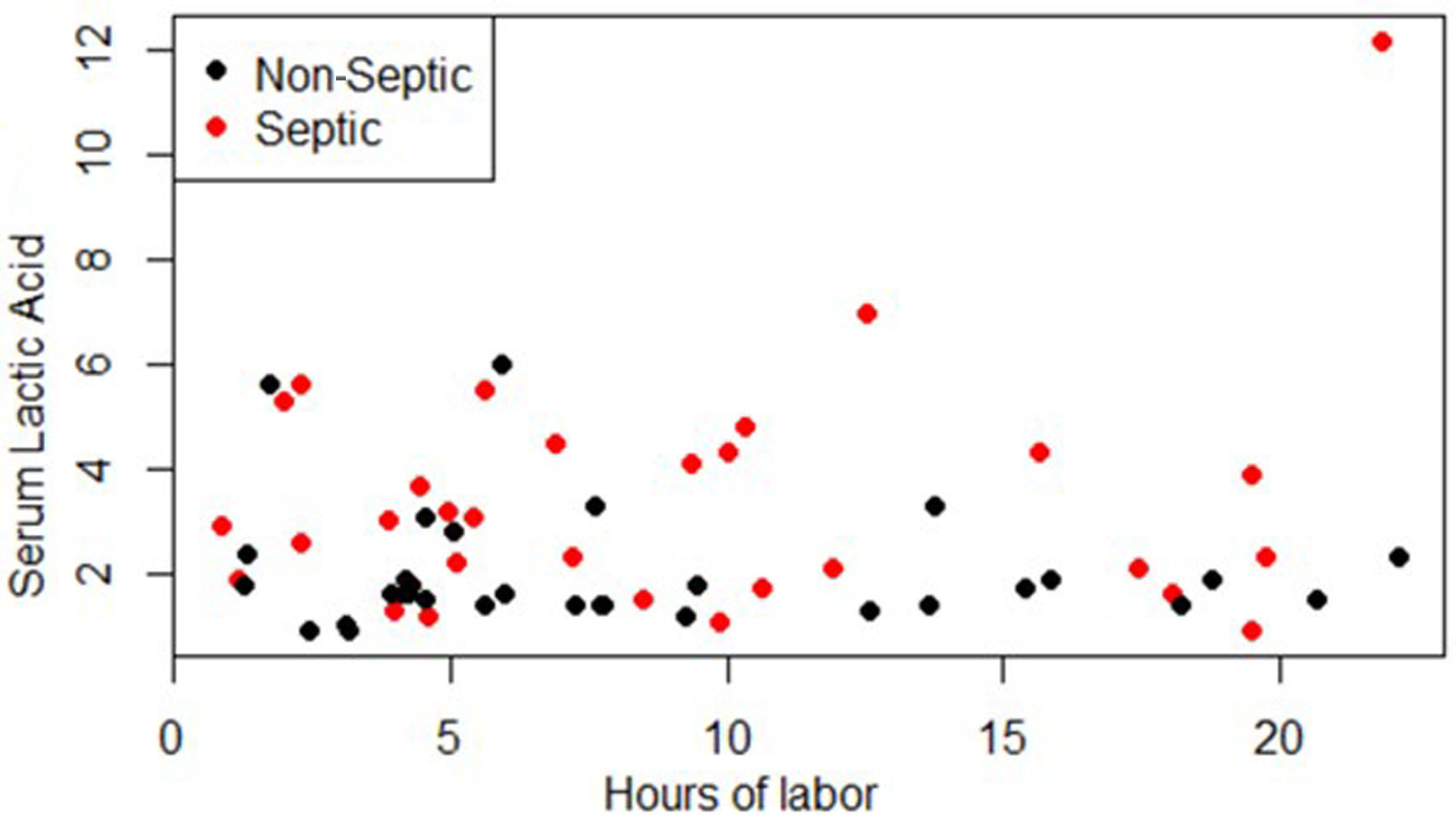 Click for large image | Figure 1. Serum lactic acid vs. hours of labor at time of lab collection. |
| Discussion | ▴Top |
The results confirm an overall association between sepsis and elevated serum lactic acid levels. However, the data cannot correlate a relationship between hours of labor and serum lactate levels regardless of septic status. The mean serum lactate levels for the non-septic and septic groups align with reports from other studies. The upper limit of serum lactate for sepsis varies between 2 and 4 mmol/L.
The lack of significant difference in the mean age, gravida, para, BMI, or hours of labor at the time of lactate drawn between the non-septic and septic groups demonstrate that the two groups were comparable from a demographic standpoint. This also suggests that there is no significant correlation between any of these factors and an increased risk of being septic.
Future studies could analyze the lactate clearance rate in septic patients during labor to determine whether the rate of clearance is an indicator of clinical outcomes. Since this study was limited by few patients with comorbidities, future studies could also examine whether comorbidities such as diabetes and high blood pressure affect lactate levels.
Acknowledgments
The investigators acknowledge St. Joseph’s Medical Center in Stockton, California for usage of their medical database.
Financial Disclosure
The authors have no financial disclosure or funding to disclose.
Conflict of Interest
The authors have no conflict of interest to disclose.
Informed Consent
Informed consent was not obtained as charts were reviewed retrospectively. A waiver of consent was approved by the institutional review board and all data were de-identified prior to analysis.
Author Contributions
MN designed the study, oversaw data collection, reviewed the literature, interpreted the data, and drafted the manuscript. TK and AO contributed to the design of the study, drafts of the manuscript, and provided critical reviews of the manuscript. MB analyzed and interpreted the data and reviewed drafts of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Marx GF, Greene NM. Maternal lactate, pyruvate, and excess lactate production during labor and delivery. Am J Obstet Gynecol. 1964;90:786-793.
doi pubmed - Alshiakh SM. Role of serum lactate as prognostic marker of mortality among emergency department patients with multiple conditions: A systematic review. SAGE Open Med. 2023;11:20503121221136401.
doi pubmed pmc - Liu Z, Meng Z, Li Y, Zhao J, Wu S, Gou S, Wu H. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51.
doi pubmed pmc - Casserly B, Phillips GS, Schorr C, Dellinger RP, Townsend SR, Osborn TM, Reinhart K, et al. Lactate measurements in sepsis-induced tissue hypoperfusion: results from the Surviving Sepsis Campaign database. Crit Care Med. 2015;43(3):567-573.
doi pubmed - Lee SM, An WS. New clinical criteria for septic shock: serum lactate level as new emerging vital sign. J Thorac Dis. 2016;8(7):1388-1390.
doi pubmed pmc - Stephens A, Baker A, Bell C, Barton J, PIneles BL, Sibai BM. Sepsis in obstetrics score predicts maternal adverse outcomes. American Journal of Obstetrics & Gynecology. 2022;226(1):S334-S335.
- Institute of Medicine (US). Subcommittee on Nutrition and Diarrheal Diseases Control. Nutrition issues in developing countries: Part I, diarrheal diseases, Part II, diet and activity during pregnancy and lactation. Chapter 3. National Academy Press; 1992. p.115.
- Wiberg-Itzel E, Pettersson H, Cnattingius S, Nordstrom L. Association between lactate concentration in amniotic fluid and dysfunctional labor. Acta Obstet Gynecol Scand. 2008;87(9):924-928.
doi pubmed - Wiberg-Itzel E, Pettersson H, Andolf E, Hansson A, Winbladh B, Akerud H. Lactate concentration in amniotic fluid: a good predictor of labor outcome. Eur J Obstet Gynecol Reprod Biol. 2010;152(1):34-38.
doi pubmed - Bauer ME, Balistreri M, MacEachern M, Cassidy R, Schoenfeld R, Sankar K, Clauw DJ, et al. Normal range for maternal lactic acid during pregnancy and labor: a systematic review and meta-analysis of observational studies. Am J Perinatol. 2019;36(9):898-906.
doi pubmed - Foucher CD, Tubben RE. Lactic acidosis. [Updated Jul 17, 2023]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470202/.
- Dockree S, O'Sullivan J, Shine B, James T, Vatish M. How should we interpret lactate in labour? A reference study. BJOG. 2022;129(13):2150-2156.
doi pubmed pmc - Goyal P, Agarwal R, Srivastava H, Kar R, Sikka M, Mohta M. Serial serum lactic acid in pregnancy-associated sepsis for maternal outcome. J Obstet Gynaecol India. 2020;70(5):342-348.
doi pubmed pmc - Rosner B. Fundamentals of biostatistics. 7th ed. Boston, MA: Brooks/Cole; 2011.
- Vincent JL, Quintairos ESA, Couto L, Jr., Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20(1):257.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
