| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 12, Number 3, December 2023, pages 117-122
Sal-Like Protein 4-Negative Gestational Trophoblastic Neoplasia
Chiho Koia, d, Reiko Yonedab, Yoshihiro Ohishic, Shun Akamineb, Miyako Maeharaa, Katsuko Egashiraa, Yosuke Ueokaa
aDepartment of Obstetrics and Gynecology, Hamanomachi Hospital, Fukuoka, Japan
bDepartment of Pathology, Hamanomachi Hospital, Fukuoka, Japan
cDepartment of Pathology, Aso Izuka Hospital, Fukuoka, Japan
dCorresponding Author: Chiho Koi, Department of Obstetrics and Gynecology, Hamanomachi Hospital, Chuo-ku, Fukuoka 810-8539, Japan
Manuscript submitted October 8, 2023, accepted November 11, 2023, published online December 28, 2023
Short title: SALL4-Negative GTN
doi: https://doi.org/10.14740/jcgo922
| Abstract | ▴Top |
Gestational trophoblastic neoplasia (GTN) is a group of pregnancy-related malignancies caused by trophoblast cells, including invasive mole, choriocarcinoma, placental site trophoblastic tumor (PSTT), and epithelioid trophoblastic tumor (ETT). The diagnosis of GTN is often made by monitoring the serum level of human chorionic gonadotropin (hCG) with histological confirmation. Distinguishing between these entities is important for determining the appropriate treatment, but the differential diagnosis is not always straightforward. We report a case of GTN in a 35-year-old woman who had massive vaginal bleeding and persistently elevated hCG after intrauterine curettage for hydatidiform mole. She underwent total abdominal hysterectomy, and the histopathological examination revealed a biphasic proliferation of prominently atypical syncytiotrophoblast cells. The tumor had a high mitotic count, and the Ki-67 index was 50-90%. Immunohistochemical staining for atypical trophoblast cells showed weak diffusely in most but strong positive in partial for hCG, positive for human placental lactogen (hPL), negative for p63, and negative for Sal-like protein 4 (SALL4). Immunohistochemical results rather likely suggested PSTT; however, a high Ki-67 index, and hematoxylin and eosin stain (H&E) findings tended to be indicative of choriocarcinoma. She has been on a good course without recurrence for more than 3 years after surgical treatment. SALL4 has been reported to be a reliable immunohistochemical marker which is expressed in 100% of choriocarcinoma but not in PSTT or ETT. Our search of the literature did not reveal any SALL4-negative choriocarcinoma. We report a case of SALL4-negative GTN that could not be categorized as either choriocarcinoma or PSTT with a review of the literature. This rare case is valuable and may be helpful in the diagnosis of GTN in the future.
Keywords: Gestational trophoblastic neoplasia; Choriocarcinoma; PSTT; SALL4
| Introduction | ▴Top |
Gestational trophoblastic disease (GTD) refers to a group of benign and malignant conditions related to pregnancy, and the latter is known as gestational trophoblastic neoplasia (GTN). GTN is a rare tumor group arising from the malignant transformation of placental tissue and includes invasive mole, choriocarcinoma, placental site trophoblastic tumor (PSTT) and epithelioid trophoblastic tumor (ETT) [1, 2]. The common trophoblastic stem cell develops along two lines of trophoblastic differentiation, namely villous and extravillous [3]. Invasive mole and choriocarcinoma are derived from villous trophoblasts, whereas PSTT and ETT are derived from extravillous trophoblasts [2, 4]. Choriocarcinoma can be thought of as a more undifferentiated trophoblastic tumor compared to PSTT and ETT because the latter two show phenotypic characteristics of extravillous intermediate trophoblast cells [5].
Choriocarcinoma is the most frequent GTN. Because of its rapid growth and strong tendency to metastasize, it is considered the most aggressive form of GTN. The incidence of choriocarcinoma is estimated to be 3 per 100,000 pregnancies in Europe and North America, compared to 23 per 100,000 in Southeast Asia [6]. Choriocarcinoma is preceded by any type of gestational event: previous benign molar pregnancies (50%), abortions or ectopic pregnancies (25%), and term or preterm deliveries (25%) [7]. The most common clinical manifestation is vaginal bleeding associated with high serum hCG levels, and other symptoms are directly associated with metastatic disease [8]. Choriocarcinoma responds well to chemotherapy [6]. Patients with choriocarcinoma often wish to preserve their fertility, so they are frequently treated with chemotherapy alone rather than hysterectomy.
Whereas, PSTT is the rarest form of GTN, with an estimated incidence of 1 in 100,000 pregnancies, representing only 0.23-3% of all GTNs, and has the highest mortality rate [6, 7]. In PSTT, long-term survival (about 90% at 10 years) is expected for stage I after hysterectomy. However, 10-15% of cases are advanced at onset, and mortality can be up to 25% if metastasis occurs [9]. PSTT mostly occurs following healthy pregnancies, as previous complete mole history and abortion are observed in only 16% and 13%, respectively [8, 10]. The most common symptom of PSTT is vaginal bleeding, similar to choriocarcinoma, whereas serum human chorionic gonadotropin (hCG) levels are lower although elevated compared to choriocarcinoma [1]. PSTT can be curative if the lesion is limited to the uterus. However, PSTT is often resistant to several chemotherapies because PSTT cells invade uterine muscle fibers and tend to spread through lymphatic pathways. Due to these characteristics, surgery is the most appropriate treatment; however, cases with extrauterine extension have poor prognosis [7, 9].
GTN may be cured in 98% of cases if a correct diagnosis is made initially [5]. It is clearly important to distinguish choriocarcinoma from PSTT and ETT because their clinical management and prognosis differ significantly as mentioned above. Recent reports indicate that grayscale ultrasonography, spectral and power/color Doppler techniques play an important role in the diagnosis, staging, and management of GTD [11]. It has also been reported that ultrasonography may predict delayed response to chemotherapy and drug resistance in stage I low-risk GTN [12]. A recent history of molar gestation, high levels of hCG, histopathologic characteristics, and diffuse hCG immunostaining are features of choriocarcinoma. Some specific tissue biomarkers are also becoming more prevalent [1, 8, 13]. However, despite the difference in characteristic clinicopathological features, it is not always easy to make an accurate diagnosis.
| Case Report | ▴Top |
A 35-year-old woman, gravida 7 para 5 visited her previous doctor following a positive result on a pregnancy test at 11 weeks of gestation. Transvaginal ultrasonography demonstrated a polycystic tumor in the uterus, and the blood level of hCG was 56,000 mIU/mL, which indicated a possibility of trophoblastic disease. She underwent intrauterine curettage, and the specimens were histopathologically diagnosed as total hydatidiform mole. After curettage, the level of hCG did not decline steadily. She was referred to our hospital because of abundant vaginal bleeding 12 weeks after curettage. Transvaginal ultrasonography revealed a 26 × 17 mm hyperechoic mass in the uterus (Fig. 1), and the level of hCG was 3,457 mIU/mL. The results of thoracoabdominal computed tomography (CT) and head magnetic resonance imaging (MRI) showed no evidence of metastases. Because she did not select fertility-sparing therapy, total hysterectomy with bilateral salpingectomy was performed 13 weeks after curettage. The tumor was completely resected, so we omitted adjuvant chemotherapy. Five weeks after the surgery, the level of hCG decreased below the detection limit. She has been in a good course without recurrence for more than 3 years after the treatment.
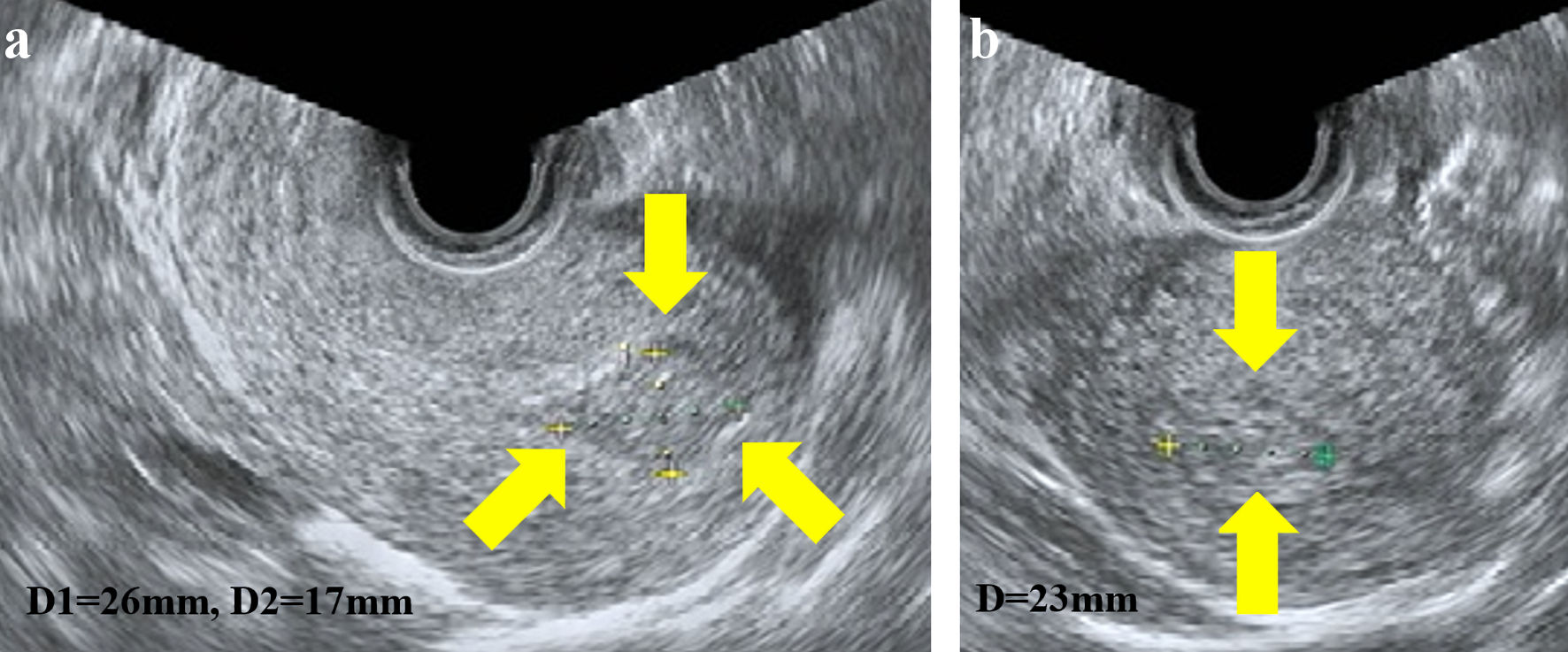 Click for large image | Figure 1. Transvaginal ultrasonography showed a 26 × 17 mm hyperechoic mass in the uterus; sagittal plane (a) and transverse plane (b). |
Pathological findings
Macroscopic examination of the surgical specimen showed a 2-cm dark red nodule protruding into the uterine cavity from the fundus (Fig. 2).
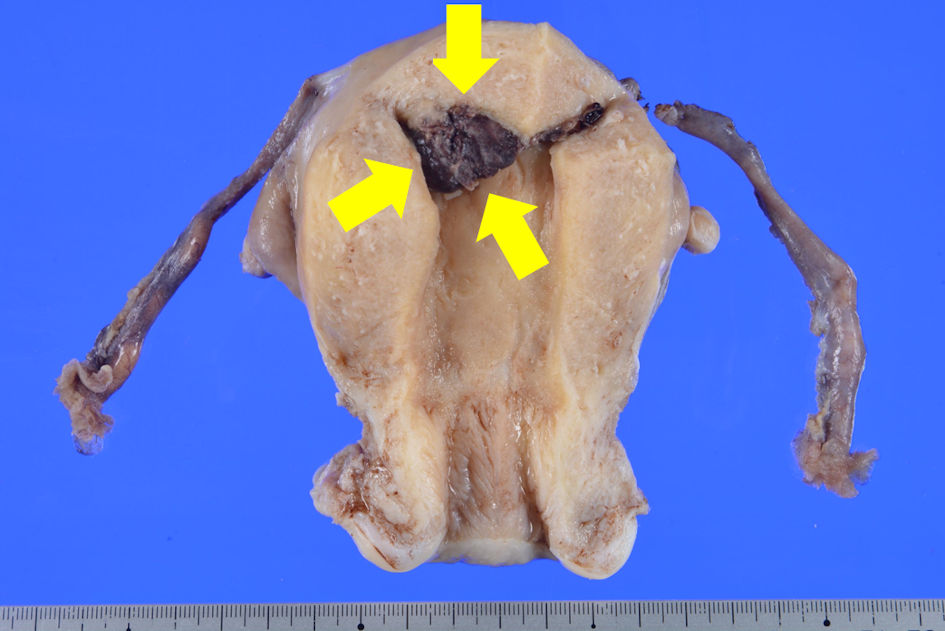 Click for large image | Figure 2. Macroscopic findings revealed a 2-cm dark red nodule protruding into the uterine cavity from the fundus. |
Histological examination showed biphasic proliferation with pleomorphic syncytiotrophoblast-like cells and proliferative cytotrophoblast-like cells (Fig. 3). The chorionic villi were not identified in the tumor. These atypical trophoblast cells infiltrated the muscle layer in a sheet-like fashion with areas of hemorrhage and necrosis. The tumor had a high mitotic count with 15 - 17 mitoses/10 high-power fields (HPFs) and showed a high Ki-67 index in 50-90% of trophoblasts. Immunohistochemical staining for atypical trophoblast cells revealed positivity for CAM5.2 and weak positivity in most and strong in partial for hCG (Fig. 4). These cells were positive for human placental lactogen (hPL) and negative for p63. Sal-like protein 4 (SALL4), which is considered a new useful marker for distinguishing choriocarcinoma from PSTT and ETT, was mostly negative in tumors. The immunohistochemical results rather likely suggested PSTT. However, hematoxylin and eosin stain (H&E) findings, such as biphasic proliferation with hemorrhage and necrosis, severe nuclear atypia, and a high Ki-67 index, tended to be consistent with choriocarcinoma. Although this case had difficulty distinguishing between choriocarcinoma and PSTT, we finally decided to treat it as a malignant trophoblast tumor with features of choriocarcinoma rather than PSTT.
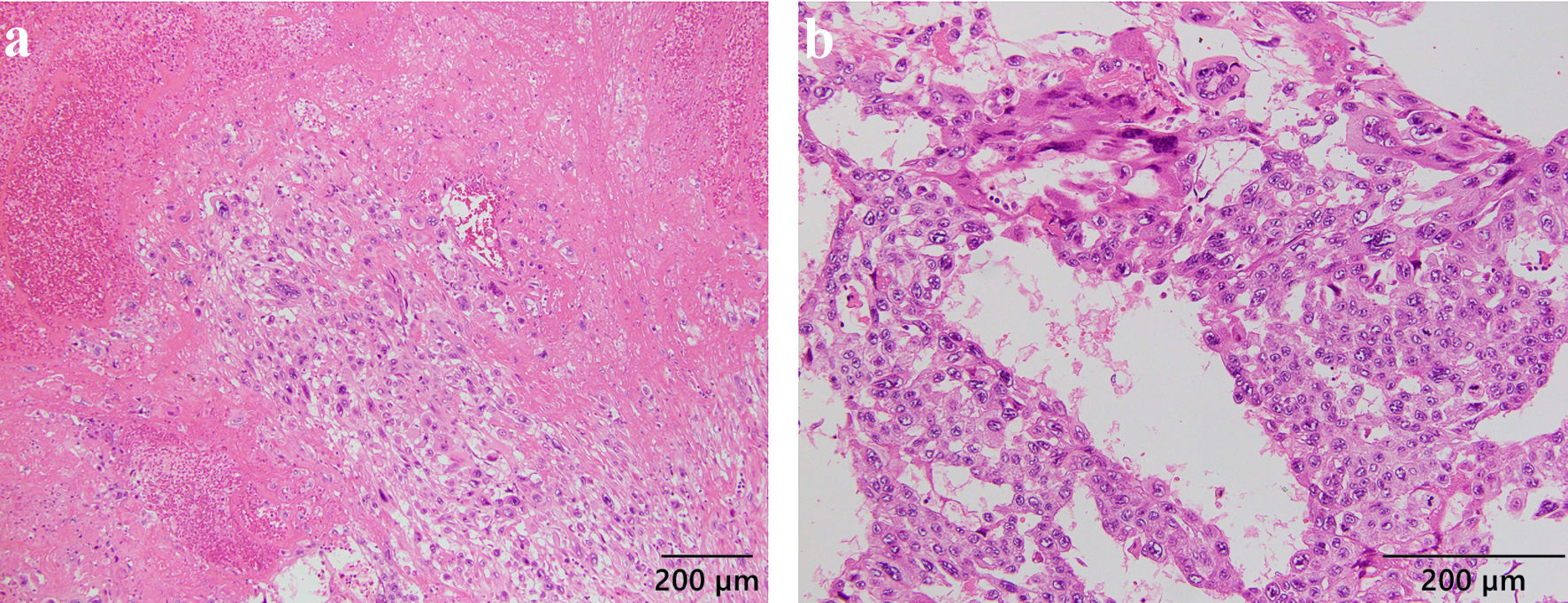 Click for large image | Figure 3. Hematoxylin and eosin staining showed prominent atypical cells arranged in biphasic proliferation of syncytiotrophoblasts and cytotrophoblasts, infiltrating the muscle layer in a sheet-like form, with hemorrhage and necrosis. Tumor cells showed marked cytologic atypia, with a high mitotic count of 15 - 17 mitoses/10 high-power fields (HPFs). |
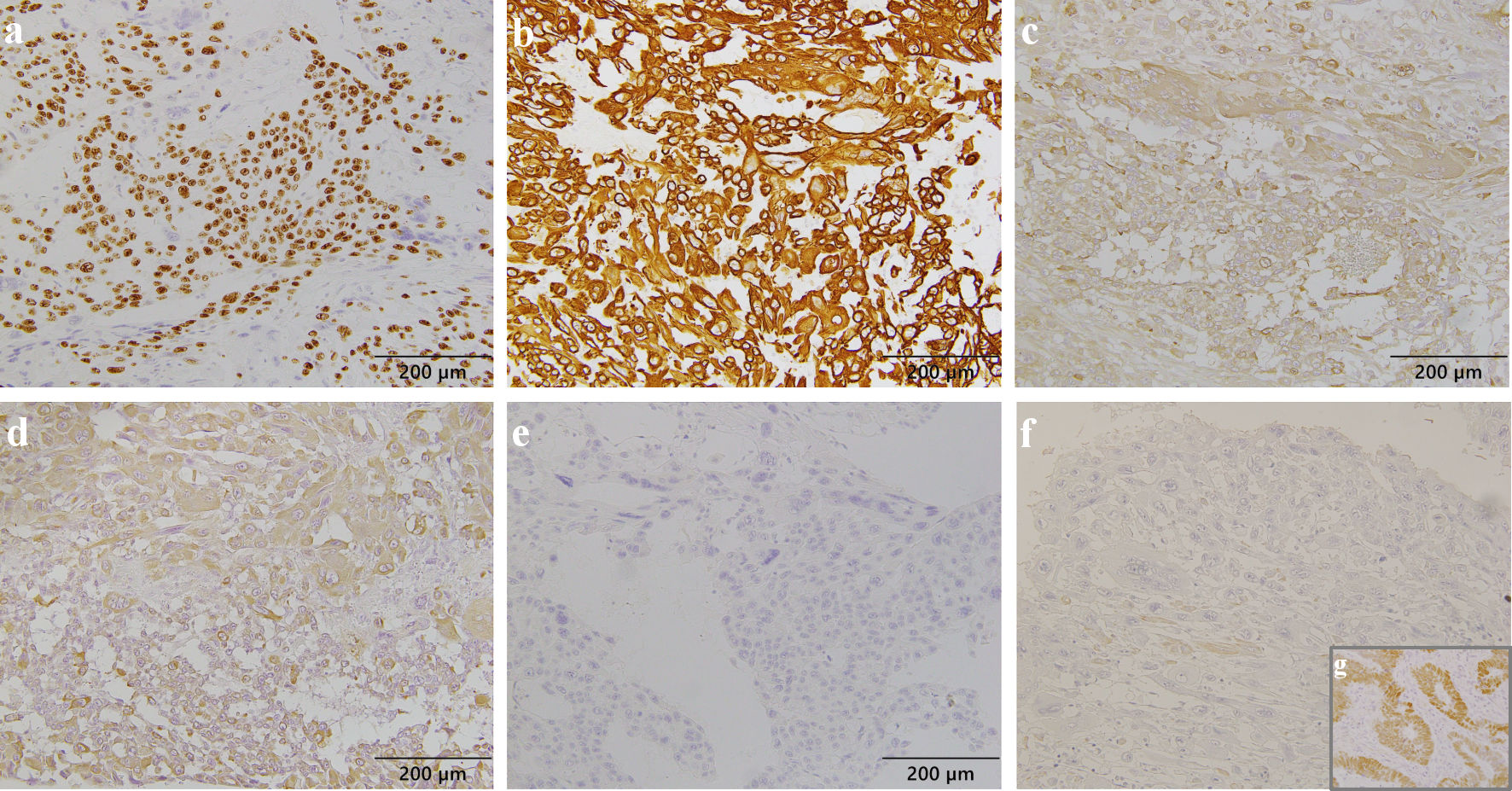 Click for large image | Figure 4. Immunohistochemical staining of atypical trophoblast cells. Ki-67 was expressed in 50-90% of tumor cells (a). Tumor cells were positive for CAM5.2 (b), weakly diffusely and strongly positive in partial for hCG (c), positive for hPL (d), negative for p63 (e), and negative for SALL4 (f); positive control for SALL4 in a case of adenocarcinoma with enteroblastic differentiation (g). hCG: human chorionic gonadotropin; hPL: human placental lactogen; SALL4: Sal-like protein 4. |
| Discussion | ▴Top |
Choriocarcinoma typically presents as bulky and destructive masses in the uterus with extensive hemorrhage and necrosis. Deep myometrial invasion is often seen and occasionally leads to uterine perforation. Histologically, choriocarcinoma is distinguished from invasive mole by the absence of villi. Choriocarcinoma reveals a biphasic to triphasic pattern, with sheets or cords of mononuclear tumor cells that are large, intermediate trophoblasts with abundant amphophilic to eosinophilic cytoplasm and/or smaller cytotrophoblasts, rimmed by layers of multinuclear syncytiotrophoblast cells. It is associated with rapid mitotic activity, nuclear atypia, pleomorphism, extensive necrosis, and hemorrhage [1, 13, 14].
On the other hand, PSTT typically invades the myometrium as nodular, round, solid masses ranging from 1 to 10 cm in size. Deep myometrial invasion and focal hemorrhage and necrosis are seen in nearly half of the cases. Histologically, the villi are also not seen in PSTT. A monomorphic population of extravillous cytotrophoblast-like cells from the implantation site makes up most PSTT. The tumor cells form cords, nests, and sheets. In the myometrium, where the extravillous cytotrophoblast invades, causing a distinct separation of the muscle fibers, PSTT presents intense vascular invasion, in which malignant cells and fibrinoid material take the place of blood vessel walls, with little necrosis or inflammation within the lesions [1, 13, 14]. The tumor cells have a low mitotic count, with 2 - 4 mitoses/10 HPF [8]. PSTT can be differentiated from choriocarcinoma by the absence of a two-cell pattern, relatively low mitotic activity, and low tendency to hemorrhagic necrosis.
Choriocarcinoma must be distinguished from PSTT because they require different treatments, based on chemotherapy and surgery for localized tumors, respectively. However, the differential diagnosis is not easy to address, even though the cellular components of the various trophoblastic tumors have characteristic morphologic features. With the increasing prevalence of specific tissue biomarkers, an immunostaining algorithm using a panel of markers consisting of hCG, hPL and p63 has been proposed for the differential diagnosis of GTN [15, 16]. The neoplastic syncytiotrophoblastic cells show strong and diffuse positivity for hCG, and hCG is known as a major marker for GTN. Choriocarcinoma expresses strong immunoreactivity for hCG, whereas PSTT is negative or weakly positive for hCG. The other differential marker is hPL, and hPL is expressed in the implantation site intermediate trophoblast and syncytiotrophoblast of the placenta. hPL is recognized as a reliable marker of PSTT but is also observed at lower expression levels in choriocarcinoma. P63 is detected diffusely in ETT but in the cytotrophoblasts of choriocarcinomas as well. Thus, these markers are useful, but regretfully, none of them are completely specific to choriocarcinoma, PSTT, or ETT [15-17]. Mitotic activity may be a marker of choriocarcinoma, as a high Ki-67 proliferative index of greater than 90% is typically observed in choriocarcinoma, while it is 10-30% in PSTT [18].
SALL4 is a zinc-finger transcription factor associated with embryonic cell pluripotency [19]. In the fetus, SALL4 was detected in gonadal germ cells, renal and intestinal epithelia, and hepatocytes. However, the SALL4 gene is switched off during development and is expressed only in germ cells until adulthood, although its distribution in human tissues is incompletely characterized. SALL4 has been identified as a reliable immunohistochemical marker for germ cell tumors, showing perfect sensitivity [20]. In a recent report, SALL4 was expressed in 100% of choriocarcinomas and was not detected in PSTT and ETT [17]. Since choriocarcinoma can be considered the most primitive type of GTN, it may particularly express the stem cell marker SALL4. SALL4 would be useful in distinguishing choriocarcinoma from PSTT and ETT. However, SALL4 positivity could not be induced by tumor stem cells but might simply reflect the low level of differentiation of choriocarcinoma compared to PSTT and ETT. Previous reports have indicated that SALL4 plays an important role in tumorigenesis and tumor cell invasiveness through its correlation with TGF-β signaling genes [21]. Furthermore, SALL4 has been reported to be detected in poorly differentiated tumors in many cases; ovarian serous carcinomas, urothelial and gastric carcinomas, cholangiocarcinomas and small cell carcinomas [20].
In our case, trophoblastic tumor cells revealed a biphasic pattern, invading the myometrium with hemorrhage and necrosis. The mitotic count and Ki-67 index were high. These results indicated choriocarcinoma rather than PSTT. However, immunohistochemical staining of the tumor cells showed weak diffusion for hCG, positivity for hPL, and negativity for p63, suggesting that more PSTT was more likely than choriocarcinoma. We added SALL4 staining, which was negative, indicating the probability of PSTT. It was difficult to explain this discrepancy, but we finally decided to treat this case as a malignant trophoblastic tumor closer to choriocarcinoma than PSTT because of the histological features and the reason why the usual immunohistochemistry markers could not be completely specific.
However, assuming that this case is a malignant trophoblast tumor with clinical features of PSTT, more careful follow-up is needed. Several risk factors associated with the prognosis of PSTT have been reported, including metastases beyond the uterus, more than 4 years after the prior pregnancy, FIGO stage III - IV, age > 40 years, and histological features such as deep invasion of the myometrium (> 50%), high mitotic count (> 5/10 HPF), clear cytoplasm, coagulative necrosis or invasion into the vascular space [22, 23]. Fortunately, our case was successfully treated with only surgery. However, it can also be said that PSTT with histological findings of choriocarcinoma may have a poor prognosis and the course of this case should be monitored more carefully.
Conclusions
GTN is a rare malignant tumor. Furthermore, choriocarcinoma is often treated with chemotherapy, so there are few reports of histological evaluation. Here, we report a case of a SALL4-negative malignant trophoblastic tumor that could not be classified as either choriocarcinoma or PSTT. This rare and valuable case may suggest the presence of malignant trophoblastic tumors with variable features that cannot be categorized by known diagnostic methods. The accumulation of such cases, which do not fit into the current diagnostic concepts, is expected to be useful for the accurate diagnosis of GTN in the future.
Learning points
A recent report identified SALL4 as a reliable immunohistochemical marker with perfect sensitivity, since SALL4 was expressed in 100% of choriocarcinomas and was not detected in PSTT. In our case, H&E findings and a high Ki-67 index tended to be indicative of choriocarcinoma, but immunohistochemical staining of SALL4 was negative for trophoblastic tumor cells. This case may suggest the presence of malignant trophoblastic tumors with variable features that cannot be categorized by known diagnostic methods.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Informed consent was obtained from the patient for publication of this case report and accompanying images.
Author Contributions
CK reviewed the literature and was responsible for manuscript drafting. RY and SA evaluated the histological findings, and YO contributed to the revision of the histological evaluation. MM and KE provided clinical information. YU contributed to the revision and final approval of the manuscript. All authors have approved the submitted version of the manuscript and agreed to be accountable for any part of the work.
Data Availability
The data supporting the conclusions of this article is available from the corresponding author.
Abbreviations
GTN: gestational trophoblastic neoplasia; PSTT: placental site trophoblastic tumor; ETT: epithelioid trophoblastic tumor; hCG: human chorionic gonadotropin; hPL: human placental lactogen; SALL4: Sal-like protein 4
| References | ▴Top |
- Silva A, Monteiro KDN, Sun SY, Borbely AU. Gestational trophoblastic neoplasia: Novelties and challenges. Placenta. 2021;116:38-42.
doi pubmed - Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematol Oncol Clin North Am. 2012;26(1):111-131.
doi pubmed - Sheridan MA, Zhao X, Fernando RC, Gardner L, Perez-Garcia V, Li Q, Marsh SGE, et al. Characterization of primary models of human trophoblast. Development. 2021;148(21):dev199749.
doi pubmed pmc - Altieri A, Franceschi S, Ferlay J, Smith J, La Vecchia C. Epidemiology and aetiology of gestational trophoblastic diseases. Lancet Oncol. 2003;4(11):670-678.
doi pubmed - Shih Ie M. Gestational trophoblastic neoplasia—pathogenesis and potential therapeutic targets. Lancet Oncol. 2007;8(7):642-650.
doi pubmed - Horowitz NS, Eskander RN, Adelman MR, Burke W. Epidemiology, diagnosis, and treatment of gestational trophoblastic disease: A Society of Gynecologic Oncology evidenced-based review and recommendation. Gynecol Oncol. 2021;163(3):605-613.
doi pubmed - Ning F, Hou H, Morse AN, Lash GE. Understanding and management of gestational trophoblastic disease. F1000Res. 2019;8:F1000 Faculty Rev-428.
doi pubmed pmc - Hui P. Gestational trophoblastic tumors: a timely review of diagnostic pathology. Arch Pathol Lab Med. 2019;143(1):65-74.
doi pubmed - Chaves MM, Maia T, Cunha TM, Veiga VF. Placental site trophoblastic tumour: the rarest subtype of gestational trophoblastic disease. BMJ Case Rep. 2020;13(10):e235756.
doi pubmed pmc - Gadducci A, Carinelli S, Guerrieri ME, Aletti GD. Placental site trophoblastic tumor and epithelioid trophoblastic tumor: Clinical and pathological features, prognostic variables and treatment strategy. Gynecol Oncol. 2019;153(3):684-693.
doi pubmed - Cavoretto P, Cioffi R, Mangili G, Petrone M, Bergamini A, Rabaiotti E, Valsecchi L, et al. A pictorial ultrasound essay of gestational trophoblastic disease. J Ultrasound Med. 2020;39(3):597-613.
doi pubmed - Cavoretto P, Gentile C, Mangili G, Garavaglia E, Valsecchi L, Spagnolo D, Montoli S, et al. Transvaginal ultrasound predicts delayed response to chemotherapy and drug resistance in stage I low-risk trophoblastic neoplasia. Ultrasound Obstet Gynecol. 2012;40(1):99-105.
doi pubmed - Mahendra INB, Brahmantara BN, Setiawan WA. A review of current management of gestational trophoblastic disease. European Journal of Medical and Health Sciences. 2022;4:7-10.
- Lurain JR. Gestational trophoblastic disease I: epidemiology, pathology, clinical presentation and diagnosis of gestational trophoblastic disease, and management of hydatidiform mole. Am J Obstet Gynecol. 2010;203(6):531-539.
doi pubmed - Shih IM, Kurman RJ. p63 expression is useful in the distinction of epithelioid trophoblastic and placental site trophoblastic tumors by profiling trophoblastic subpopulations. Am J Surg Pathol. 2004;28(9):1177-1183.
doi pubmed - Kalhor N, Ramirez PT, Deavers MT, Malpica A, Silva EG. Immunohistochemical studies of trophoblastic tumors. Am J Surg Pathol. 2009;33(4):633-638.
doi pubmed - Stichelbout M, Devisme L, Franquet-Ansart H, Massardier J, Vinatier D, Renaud F, Kerdraon O. SALL4 expression in gestational trophoblastic tumors: a useful tool to distinguish choriocarcinoma from placental site trophoblastic tumor and epithelioid trophoblastic tumor. Hum Pathol. 2016;54:121-126.
doi pubmed - Shih Ie M. Trophogram, an immunohistochemistry-based algorithmic approach, in the differential diagnosis of trophoblastic tumors and tumorlike lesions. Ann Diagn Pathol. 2007;11(3):228-234.
doi pubmed - Yang J, Chai L, Fowles TC, Alipio Z, Xu D, Fink LM, Ward DC, et al. Genome-wide analysis reveals Sall4 to be a major regulator of pluripotency in murine-embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105(50):19756-19761.
doi pubmed pmc - Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, Lasota J, et al. SALL4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38(3):410-420.
doi pubmed pmc - Collet C, Lopez J, Battail C, Allias F, Devouassoux-Shisheboran M, Patrier S, Lemaitre N, et al. Transcriptomic characterization of postmolar gestational choriocarcinoma. Biomedicines. 2021;9(10):1474.
doi pubmed pmc - Zeng X, Liu X, Tian Q, Xue Y, An R. Placental site trophoblastic tumor: A case report and literature review. Intractable Rare Dis Res. 2015;4(3):147-151.
doi pubmed pmc - Rey Valzacchi GM, Odetto D, Chacon CB, Wernicke A, Xiang Y. Placental site trophoblastic disease. Int J Gynecol Cancer. 2020;30(1):144-149.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
