| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 13, Number 1, March 2024, pages 18-22
Spontaneous Adrenal Hemorrhage in Pregnancy
Priyanka Desaia, c, Bidhya Pandeya, Henry Navaa, Anil KCb, Richard Trestera
aDepartment of Obstetrics & Gynecology, Mount Sinai Hospital, Chicago, IL 60608, USA
bMayo Clinic, Rochester, MN 55902, USA
cCorresponding Author: Priyanka Desai, Department of Obstetrics & Gynecology, Mount Sinai Hospital, Chicago, IL 60608, USA
Manuscript submitted January 17, 2024, accepted February 27, 2024, published online March 26, 2024
Short title: Spontaneous Adrenal Hemorrhage in Pregnancy
doi: https://doi.org/10.14740/jcgo949
| Abstract | ▴Top |
Spontaneous adrenal hemorrhage (SAH) is a serious medical condition that occurs due to acute damage to the adrenal gland or chronic atrophy of the adrenal gland. Although pregnancy is a known risk factor for adrenal hemorrhage, its etiology and incidence are unknown, causing it to be a rare and poorly understood disorder in obstetric patients. For this reason, the risk of SAH being misdiagnosed is severe and may pose significant life-threatening consequences to the patient if left untreated. This report describes the clinical signs and symptoms, diagnostic testing and management of SAH by following a patient in the third trimester of pregnancy that developed a spontaneous unilateral adrenal hemorrhage. Fortunately, through the early recognition of SAH, the patient was successfully treated with complete resolution of her symptoms and was able to deliver at full term without any obstetric complications.
Keywords: Adrenal; Hematoma; Hemorrhage; Pregnancy; Obstetric; Third trimester; Spontaneous adrenal hemorrhage
| Introduction | ▴Top |
Damage to the adrenal cortex causes deficiency in three essential hormones: aldosterone, cortisol and androgens, while damage to the adrenal medulla causes decreased secretion and release of catecholamine hormones such as epinephrine and norepinephrine. These hormones are essential for maintaining bodily homeostasis as well as regulating both maternal and fetal hemodynamic states. For this reason, it is critical that disorders causing damage to this vital structure, such as adrenal hemorrhage, not be missed when establishing differential diagnoses.
Adrenal hemorrhage is an uncommon disorder, with a reported incidence of 0.14-1.1% on post-mortem studies [1]. Although the causes of adrenal hemorrhage during pregnancy specifically are still unknown [2], common causes of spontaneous adrenal hemorrhage (SAH) outside of pregnancy include sepsis, trauma, disseminated intravascular coagulation (DIC), anticoagulation, venous thromboembolism or adrenal neoplastic disease.
While some patients with adrenal hemorrhage may be asymptomatic, the common presentation includes non-specific pain located in the epigastrium, flank, upper or lower back, and pelvis in 65-85% of cases [3, 4]. Symptoms of adrenal insufficiency, such as fatigue, weakness, dizziness, anorexia, nausea, vomiting, myalgia, and diarrhea, are present in approximately 50% of extensive, bilateral adrenal hemorrhage cases [2].
In severe cases, damage to the adrenal gland may lead to a life-threatening medical condition known as adrenal crisis or Addison’s disease. When this condition is left untreated, patients can develop symptoms of hypotension, tachycardia, shock and in extreme cases death of both fetus and mother.
| Case Report | ▴Top |
Investigations
A 25-year-old G2P1001 previously healthy female at 33 weeks of gestation presented with a 3-day history of abdominal pain radiating to the left side along with symptoms of nausea. The pain was localized to the left anterior chest in the inframammary region, extending to the right upper abdomen and epigastric region. She described the pain as a constant pressure-like sensation with burning that worsened with deep inspiration and palpation. The patient also endorsed eight episodes of emesis since the onset of her symptoms. She denied fever, chills, headache, vision changes and swelling of her face or extremities. She had no significant medical or surgical history and no history of coagulopathy or trauma.
At the time of admission, the patient’s complete blood count (CBC) was significant for hemoglobin of 11.2 mg/dL, hematocrit of 34.8%, white blood cell (WBC) of 9,300 mg/dL, random blood sugar of 102 mg/dL, platelet of 333,000 µL, blood urea nitrogen (BUN) of 5 mg/dL and creatinine of 0.6 mg/dL. A comprehensive metabolic panel (CMP) was significant for sodium of 137 mg/dL, bicarbonate of 18 mEq/L, partial pressure of carbon dioxide (PCO2) of 18 mm Hg and magnesium of 1.4 mEq/L. Physical exam revealed a blood pressure of 117/80 mm Hg and fetal heart rate (FHR) was 143 beats per minute (bpm) with a category one fetal heart rate tracing (FHRT).
Chest X-ray and pelvic ultrasound scans were unremarkable. Electrocardiogram (EKG) showed no ischemic changes and normal troponin levels, suggesting that the patient’s chest pain was unlikely to be cardiac in etiology. The patient was started on Protonix 20 mg intravenous (IV), Zofran 4 mg IV, morphine 2 mg IV, ondansetron 4 mg IV and sequential compression devices (SCDs) for deep venous thrombosis (DVT) prophylaxis.
Three hours post-admission, a code blue was called due to the acute onset of severe chest pain and shortness of breath. Vitals at the time reflected a blood pressure of 116/69 mm Hg, heart rate of 100 bpm, and oxygen saturation of 100% on 2 L of oxygen. FHRT remained at category one with FHR of 160 bpm.
Diagnosis
A non-contrast abdominal computed tomography (CT) for suspected pulmonary embolism was negative, however demonstrated a left adrenal gland nodule measuring 4.4 × 3.2 cm on incidental finding (Figs. 1, 2). Maternal-fetal medicine and surgical departments were consulted, and both suggested no embolization or surgical intervention was warranted due to lack of active extravasation or disruption to surrounding structures.
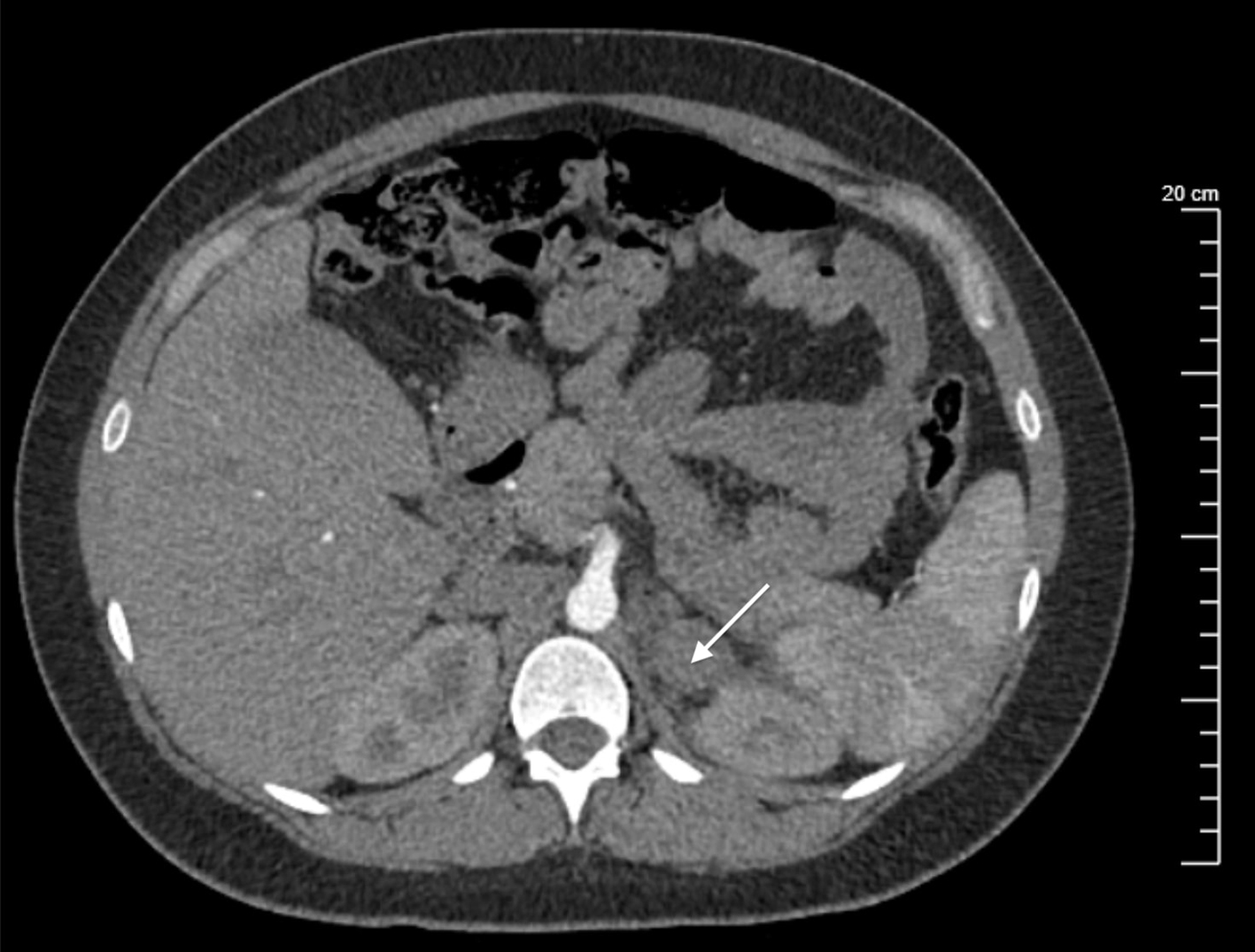 Click for large image | Figure 1. Transverse cross-section of a non-contrast abdominal CT scan reflecting hyperattenuation of the left adrenal gland measuring 4.4 × 3.2 cm (arrow). CT: computed tomography. |
 Click for large image | Figure 2. Coronal cross-section of a non-contrast CT scan reflecting hyperattenuation of the left adrenal gland measuring 4.4 × 3.2 cm (arrow). CT: computed tomography. |
The patient’s pain was addressed with acetaminophen 975 mg four times daily and fentanyl citrate 100 µg as needed, but her pain remained refractory to pharmaceutical management. Pain management with hydromorphone 0.4 mg as per patient-controlled analgesia (PCA) pump in addition to her current pharmacotherapy regimen was successful in controlling the patients’ symptoms.
Repeat CT of abdomen 2 days later demonstrated a stable left hyperdense structure measuring 5 cm with no acute extravasation (Fig. 3). Given the rapid interval of development of the adrenal hematoma compared to previous CT imaging, the patient was diagnosed with an acute adrenal hemorrhage.
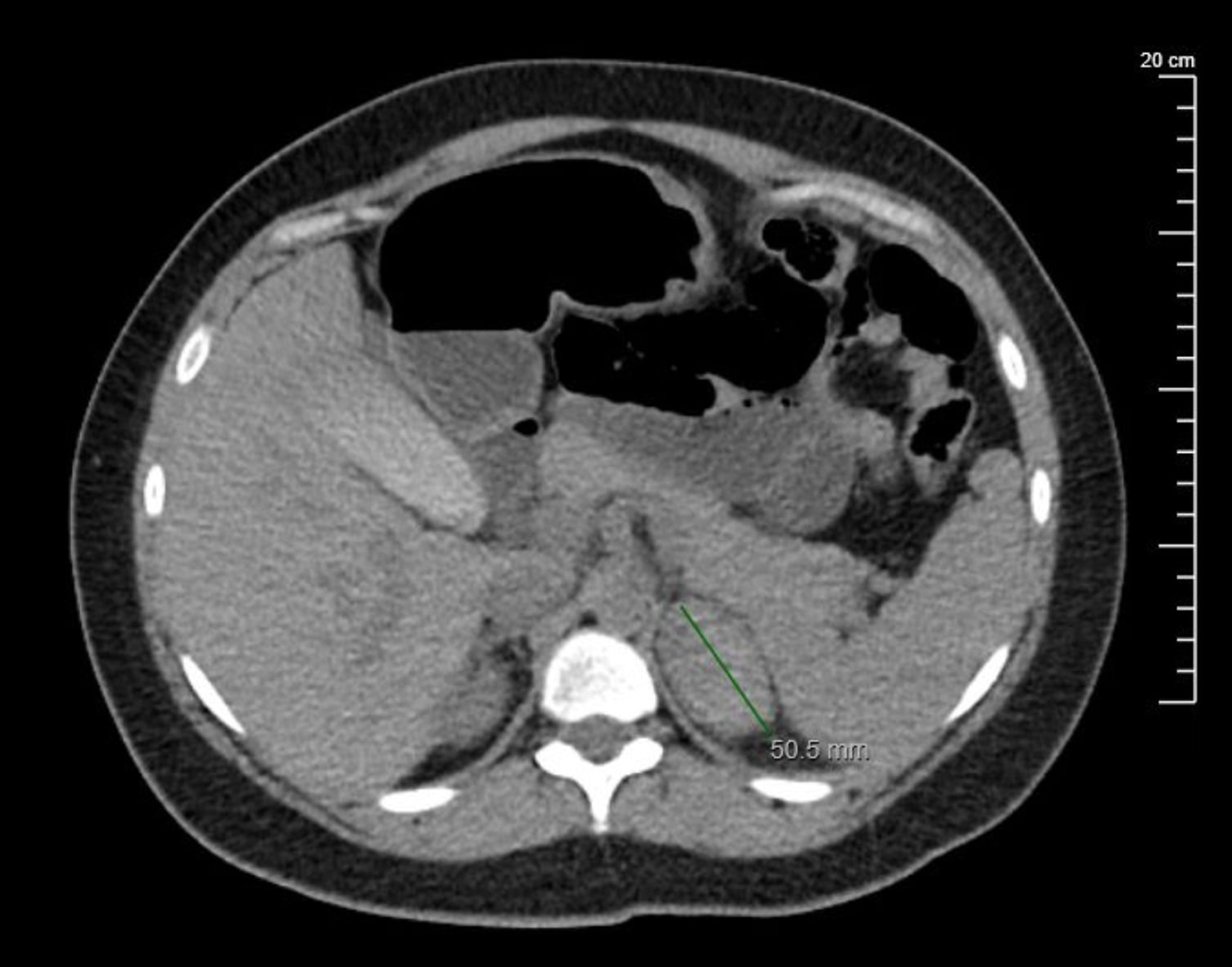 Click for large image | Figure 3. Transverse cross-section of a non-contrast abdominal CT scan reflecting hyperattenuation of the left adrenal gland measuring 5 cm, consistent with a diagnosis of acute unilateral adrenal hemorrhage (arrow). CT: computed tomography. |
Treatment
Endocrinology was consulted and laboratory evaluation showed a morning cortisol of 12.5 µg/dL, random cortisol of 19.1 µg/dL, dehydroepiandrosterone (DHEA) of 49.5 µg/dL, adrenocorticotropic hormone (ACTH) of 13 pg/nL, potassium of 3.9 mEq/L and sodium of 134 mEq/L.
Due to high suspicion of adrenal insufficiency, the patient was given hydrocortisone 100 mg and started on a maintenance dose of hydrocortisone 15 mg in the morning and hydrocortisone 10 mg in the afternoon. The patient remained hemodynamically stable with a plan to administer a stress dose of hydrocortisone 100 mg IV if the patient became hemodynamically unstable.
On day 7 post-admission, repeat laboratory examination reflected hemoglobin of 10.8 mg/dL, platelet of 333,000 µL, WBC of 9,300 mg/dL, sodium of 138 mg/dL, potassium of 3.9 mg/dL, BUN of 4 mg/dL and creatinine of 0.6 mg/dL. The patient was successfully weaned from the PCA pump and transitioned to oral pain medication of Tylenol 1,000 mg four times daily. The patient remained hemodynamically stable with vitals reflecting a temperature 97.5 °F, heart rate 82 bpm, respiratory rate 18 bpm and blood pressure 108/52 mm Hg. FHRT remained at a category one with moderate variability and presence of accelerations, but no associated decelerations while FHR was 140 bpm.
Follow-up and outcomes
The patient was discharged with a maintenance dose of hydrocortisone 15 and 10 mg in the morning and afternoon, respectively. The patient was advised to take a triple dose of hydrocortisone upon onset of acute symptoms as well as a cortisol (Solu-Cortef Act-O-Vial) 100 mg dose intramuscularly in case of emergency. At the time of discharge, the patient reported no symptoms of nausea, vomiting, fever, blurry vision, headache, epigastric pain or lower extremity edema.
The patient subsequently returned at 39 weeks of gestation for a scheduled cesarean section. Scheduled cesarean section was chosen as the modality of delivery due to the patient’s history of prior low-transverse cesarean section, as well as the increased risk of adrenal hemorrhage exacerbation and adrenal insufficiency in the presence of increased intra-abdominal pressure associated with vaginal delivery. The delivery was successful with no complications or emergency intervention required. The patient was advised to follow up with endocrinology and maternal-fetal medicine at an outpatient clinic upon postpartum discharge.
| Discussion | ▴Top |
SAH is a condition that is difficult to recognize clinically. Firstly, due to its low incidence, SAH is often missed when developing a list of differential diagnoses [1, 5]. Secondly, the presentation of SAH is often non-specific and varies in severity, particularly in obstetric patients or patients with other concurrent illnesses. The inability to recognize SAH clinically makes it a condition prone to being overlooked. This is especially concerning as SAH is associated with a high mortality rate. If this condition is not recognized immediately or treated appropriately, it can lead to severe complications such as acute adrenal crisis, shock, or maternal and fetal demise [6].
Due to the gravity of the complications associated with SAH, clinicians should be prompted to place a greater significance on objective findings such as imaging, laboratory work and endocrine studies. The initial abdominal imaging of choice in pregnancy is an ultrasound; however, its sonographic features are nonspecific in identifying SAH. Thus, the gold standard imaging modality is magnetic resonance imaging (MRI) which is the most sensitive and specific in confirming a suprarenal adrenal hemorrhage in pregnancy and evaluating the underlying etiology [2].
When there is a high suspicion for SAH, but imaging studies are inconclusive, clinicians should defer to laboratory findings. A CMP in a patient with SAH typically reflects hyponatremia, hyperkalemia, hypercalcemia, normal anion gap metabolic acidosis, hypoglycemia, with elevated creatinine and BUN levels. In addition to imaging and laboratory examination, endocrine panels may be ordered to assess a patient’s morning cortisol and morning ACTH levels, with the gold standard being a cosyntropin or standard-dose ACTH stimulation test.
In hemodynamically stable patients, conservative management should focus on steroid therapy, fluid resuscitation, pain management, maintaining hemodynamic stability and correction of underlying coagulopathies or comorbidities that may exaggerate symptoms [6]. In hemodynamically unstable patients, surgery may be indicated to maintain adrenal sufficiency and prevent circulatory collapse [7]. In severe cases, such as in patients with severe hemorrhage, arterial embolization to block blood flow and catalyze resorption of hematoma may be considered [8].
Learning points
SAH is a rare but serious medical anomaly. SAH has the ability to cause life-threatening consequences if the condition is misdiagnosed or there is a delay in initiating appropriate care. Due to its low incidence, high mortality rate and non-specific symptoms, especially in the setting of an obstetric patient, SAH has a high susceptibility to be overlooked as a credible differential diagnosis. Physicians should be aware of its presentation to ensure prompt management and successful treatment of the patient.
Acknowledgments
This undertaking of this case report submission would not have been possible without the exceptional support of the wonderful physicians, nurses and technicians at Mount Sinai Hospital, Chicago, Illinois.
Financial Disclosure
The authors or the institution responsible for this case report did not at any time receive payment or services from a third party (government, commercial, private foundation, etc.) for any aspect of the submitted work (such as grants, data monitoring board, study design, manuscript preparation, statistical analysis, etc.).
Conflict of Interest
None to declare.
Informed Consent
Consent was obtained by all human participants in this clinical study. IRB/ethics committee approval was obtained prior to submission of this case report.
Author Contributions
All authors involved in this submission provided significant contribution to the acquisition, analysis and interpretation of data involved in this case report. All authors offered intellectual insight and aided in the drafting, editing and critical review of this works.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACTH: adrenocorticotropic hormone; BUN: blood urea nitrogen; CBC: complete blood count; CMP: comprehensive metabolic panel; CT: computed tomography; DHEA: dehydroepiandrosterone; DIC: disseminated intravascular coagulation; DVT: deep venous thrombosis; EKG: electrocardiogram; FHR: fetal heart rate; FHRT: fetal heart rate tracing; IV: intravenous; MRI: magnetic resonance imaging; PCA: patient-controlled analgesia; PCO2: carbon dioxide; SAH: spontaneous adrenal hemorrhage; SCD: sequential compression device; WBC: white blood cell
| References | ▴Top |
- Ali A, Singh G, Balasubramanian SP. Acute non-traumatic adrenal haemorrhage-management, pathology and clinical outcomes. Gland Surg. 2018;7(5):428-432.
doi pubmed pmc - Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: a review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
doi pubmed - Imga NN, Tutuncu Y, Tuna MM, Dogan BA, Berker D, Guler S. Idiopathic spontaneous adrenal hemorrhage in the third trimester of pregnancy. Case Rep Med. 2013;2013:912494.
doi pubmed pmc - Bockorny B, Posteraro A, Bilgrami S. Bilateral spontaneous adrenal hemorrhage during pregnancy. Obstet Gynecol. 2012;120(2 Pt 1):377-381.
doi pubmed - Wani MS, Naikoo ZA, Malik MA, Bhat AH, Wani MA, Qadri SA. Spontaneous adrenal hemorrhage during pregnancy: review of literature and case report of successful conservative management. J Turk Ger Gynecol Assoc. 2011;12(4):263-265.
doi pubmed pmc - Kadhem S, Ebrahem R, Munguti C, Mortada R. Spontaneous unilateral adrenal hemorrhage in pregnancy. Cureus. 2017;9(1):e977.
doi pubmed pmc - Patel A, Downing R, Vijay S. Spontaneous rupture of the adrenal artery successfully treated using the endovascular approach: a report of 2 cases. Vasc Endovascular Surg. 2013;47(2):124-127.
doi pubmed - Almutairi W, Alibrahim A, Alanazi M. A successful conservative management of spontaneous adrenal hemorrhage (SAH) in pregnancy: a case report. Cureus. 2022;14(5):e24989.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
