| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 3, Number 3, September 2014, pages 108-113
A Rare Case of Histologic Benign Struma Ovarii With Distant Metastasis
Der-Wei Hwua, Shan-Yin Tsaib, Hon-Man Chanc, Yu-Wen Chend, Yu-Chieh Chene, Pi-Jung Hsiaoa, f
aDivision of Endocrinology and Metabolism, Kaohsiung Medical University Hospital, Taiwan
bDepartment of Pathology, Kaohsiung Medical University Hospital, Taiwan
cDepartment of Surgery, Kaohsiung Medical University Hospital, Taiwan
dDepartment of Nuclear Medicine, Kaohsiung Medical University Hospital, Taiwan
eDepartment of Obstetrics and Gynecology, Kaohsiung Medical University Hospital, Taiwan
fCorresponding Author: Pi-Jung Hsiao, 100 Tzyou 1st Rd, Kaohsiung 807, Taiwan
Manuscript accepted for publication July 31, 2014
Short title: Benign Struma Ovarii With Metastasis
doi: https://doi.org/10.14740/jcgo232w
| Abstract | ▴Top |
Struma ovarii (SO) is a rare variant of ovarian teratoma composed mainly of mature thyroid tissue. It is usually benign in histology. We present a case of benign SO with frequent recurrence and widespread extra-ovarian seeding. A 28-year-old woman was diagnosed initially with dermoid cysts over the right ovary. Over a 7-year period, frequent recurrence of ovarian tumor was accompanied by peritoneal strumosis and distant metastasis to liver and lung. Bilateral salpingo-oophorectomy (BSO), total thyroidectomy (TT) and radioactive iodine (RAI) therapy were performed with successfully declining serum thyroglobulin. The patient remains on scheduled follow-up in clinic. Histologic benign SO to behave like biological malignancy with frequent recurrence and distant metastasis is exceptional. Tumor enriched expression of the vascular endothelial growth factor (VEGF) and p53 protein may explain the risk of malignant behavior. There is still a lack of consensus on how to choose the optimal management for histologically benign SO with metastatic behavior. Based on our limited case experience, BSO is preferred. TT and RAI therapy is mandatory if persistence of elevated serum thyroglobulin and wide-seeding of the tumor was detected by I131 whole body scintigraphy or fluorodeoxyglucose (PET-FDG) scan.
Keywords: Histologic benign struma ovarii; Biologically malignant struma ovarii; Vascular endothelial growth factor; p53; Optimal surgical management
| Introduction | ▴Top |
Struma ovarii (SO) is a rare type (0.4%) of ovarian teratoma, which is diagnosed based on the presence of more than 50% of monodermal mature thyroid tissue. SO commonly occurs in females aged 50 - 60 years old. It usually manifests with a benign histology with exceptional rare (0.3-5%) malignant transformation or metastatic behavior [1, 2]. The optimal surgical management including the extent of pelvic resection, indications for total thyroidectomy (TT) and/or radioactive iodine (RAI) therapy remains undefined [3, 4].
Here, we present a case where a young woman who was diagnosed initially with unilateral dermoid cysts progressed to benign bilateral SO with multiple distant metastases within 7 years.
| Case Report | ▴Top |
A 28-year-old female patient visited our gynecological outpatient clinic complaining of intermittent dull pain over the lower abdomen for 6 months. Her past history included one myomectomy for uterine myoma and enucleation of the right ovary for multiple dermoid cysts at a local hospital in 2006. Two years later, she was diagnosed with recurrent ovarian dermoid cysts over the right ovary and she underwent laparoscopic enucleation of the ovarian dermoid cyst and partial myomectomy again in another local hospital in 2008. The histology of the excised ovary tissue showed hair and skin but was absent of thyroid tissues. However, she still felt persistent lower abdominal pain until 2010. Recurrent right ovarian tumor was suspected and an exploratory operation was performed. During this operation, bilateral multiple ovarian tumors were observed with multiple seeding tumors over the cul-de-sac, anterior surface of the uterus and rectum, spreading to the left infra-diaphragm. Her third operation involved total enucleation of the both ovaries and wide excision of the seeding tumors. The pathology definitely revealed that the entire ovarian tumor was replaced by mature thyroid tissues and SO with peritoneal strumosis was diagnosed.
The patient visited our clinic for treatment of persistent abdominal pain in 2012. A series of image studies was arranged that showed recurrent bilateral ovarian tumors by pelvic ultrasound. An abdominal computerized tomography (CT) showed bilateral ovarian tumors with wide-spreading multiple hyper-vascular tumors over the liver and peritoneal space. The fourth operation included left salpingo-oophorectomy and wide excision of the peritoneal strumosis (Fig. 1). The histology was still reported as benign thyroid tissues only. The liver biopsy also disclosed benign thyroid tissues with positive staining for thyroglobulin (Fig. 2). Ultrasonography of the thyroid showed a normal thyroid gland. Positron emission tomography with fluorodeoxyglucose (PET-FDG) and single-photon emission computed tomography (SPECT) after a test dose of I131 scintillation scan showed extensive intense uptake over the uterus, adnexa, liver and lung (Fig. 3). TT was followed by radioactive iodine (RAI) therapy (150 mCi) prepared with recombinant human TSH (rhTSH, Thyrogen) by injection in an attempt to eradicate all metastatic tumor seeding. The I131 therapy combined with post-therapy scintillation scan and SPECT clearly revealed intense uptake over the right lower lung, bilateral hepatic lobes, uterus and pelvic adnexa. The molecular mechanism of metastatic behavior of SO was further investigated by immunohistochemical stains for vascular endothelial growth factor (VEGF) by rabbit anti-human VEGF (A-20, sc-152) polyclonal antibody (Santa Cruz Biotechnology, Inc., Europe) and p53 protein by monoclonal mouse anti-human p53 protein (clone DO-7, Dako., Denmark). The widespread expressions of VEGF and p53 protein were significantly increased over ovary, peritoneal and liver seedings compared with negative in normal thyroid tissue (Fig. 4).
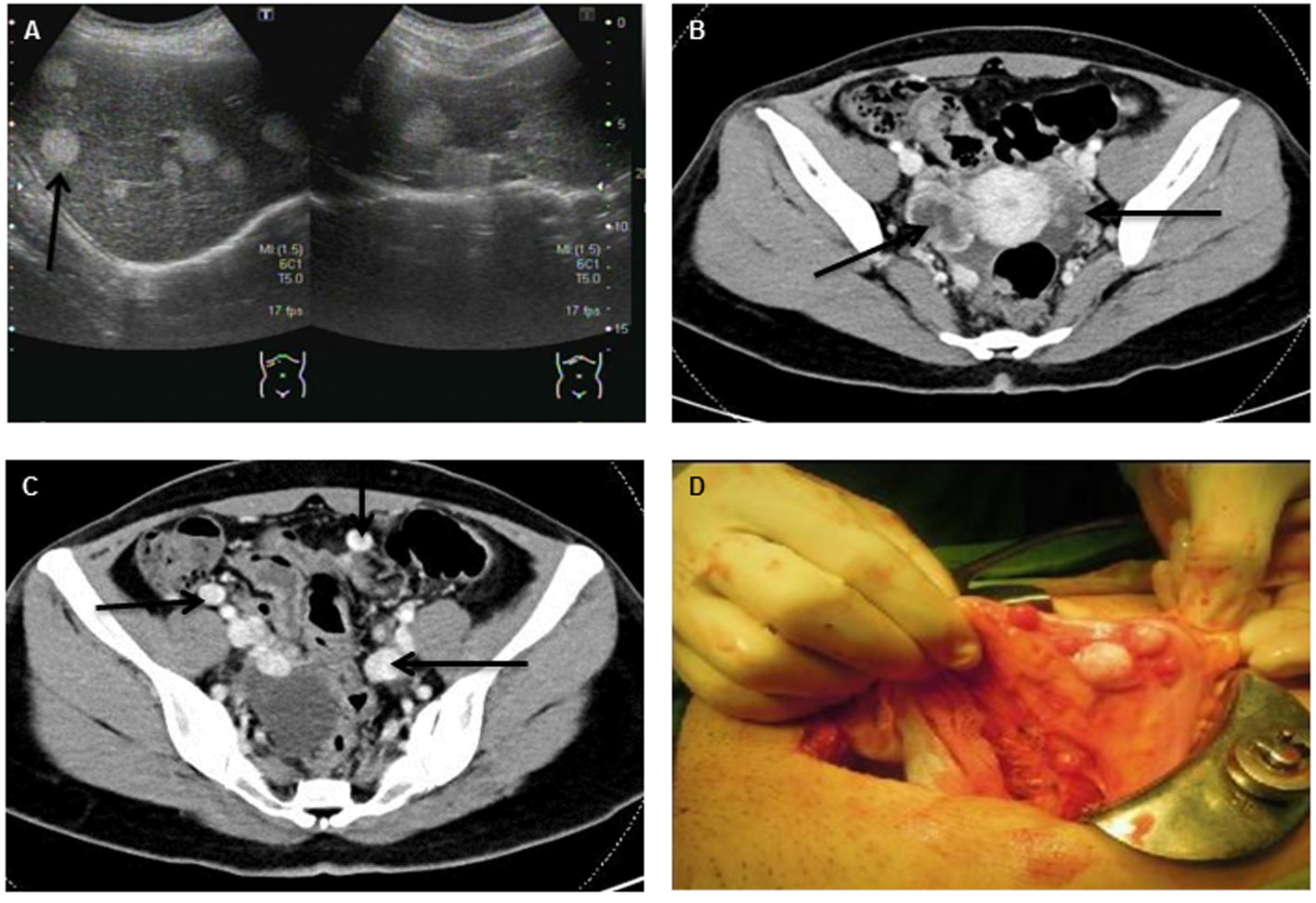 Click for large image | Figure 1. Abdominal ultrasonography (A) and abdominal CT with contrast enhancement (B, C) revealed multiple mixed cystic lesions over bilateral ovaries and multiple hyper-vascular tumors widespread to peritoneum, liver and lung (indicated by arrowhead). (D) Disseminated tumor seeding to peritoneum and colon (peritoneal strumosis) was demonstrated during the operation. |
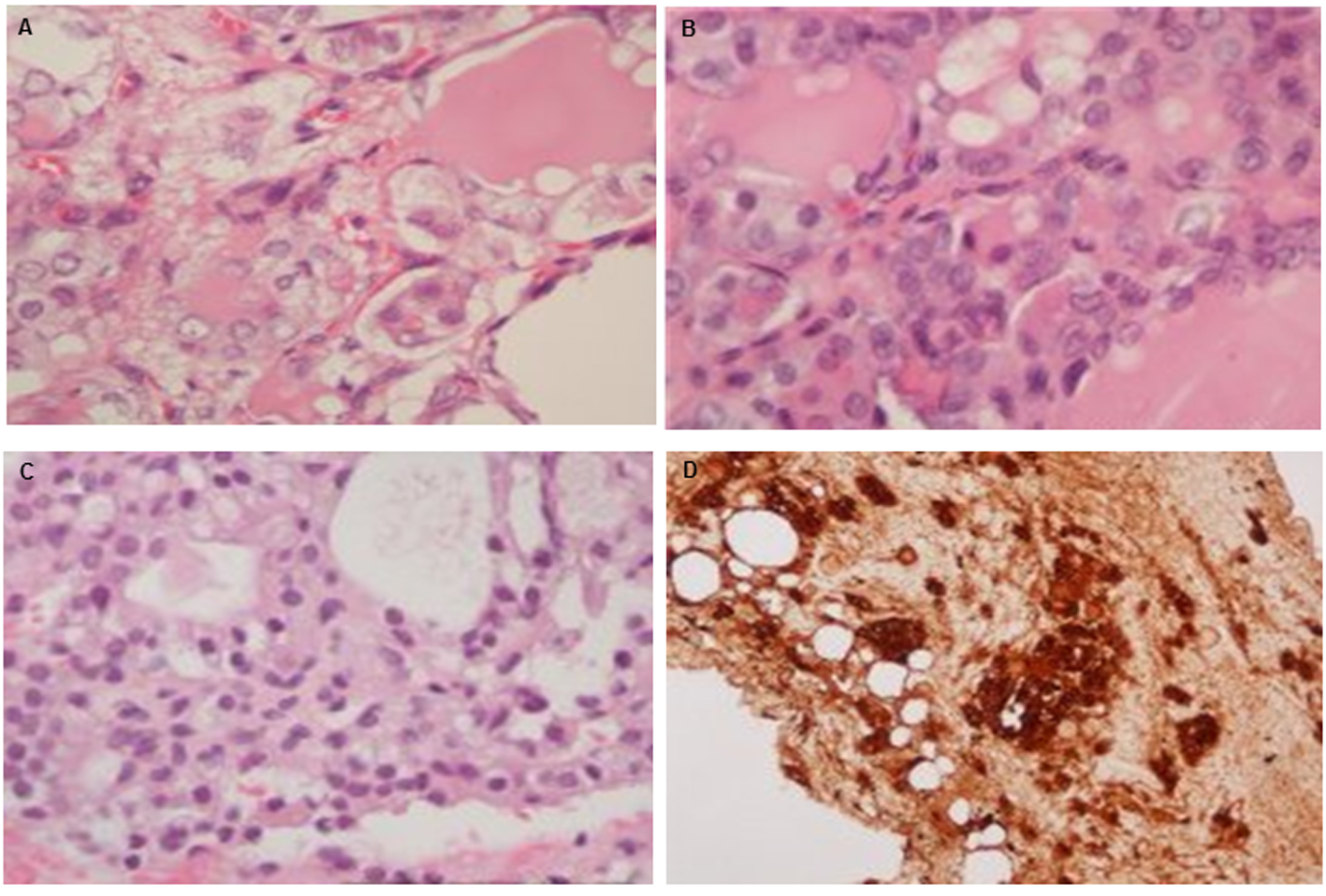 Click for large image | Figure 2. Histology of ovary and excised tumor from peritoneum and liver: (A) H&E stain (× 400) in ovarian tissue; (B) peritoneal tumor seeding; (C) liver seeding; and (D) thyroglobulin stain of liver seeding. |
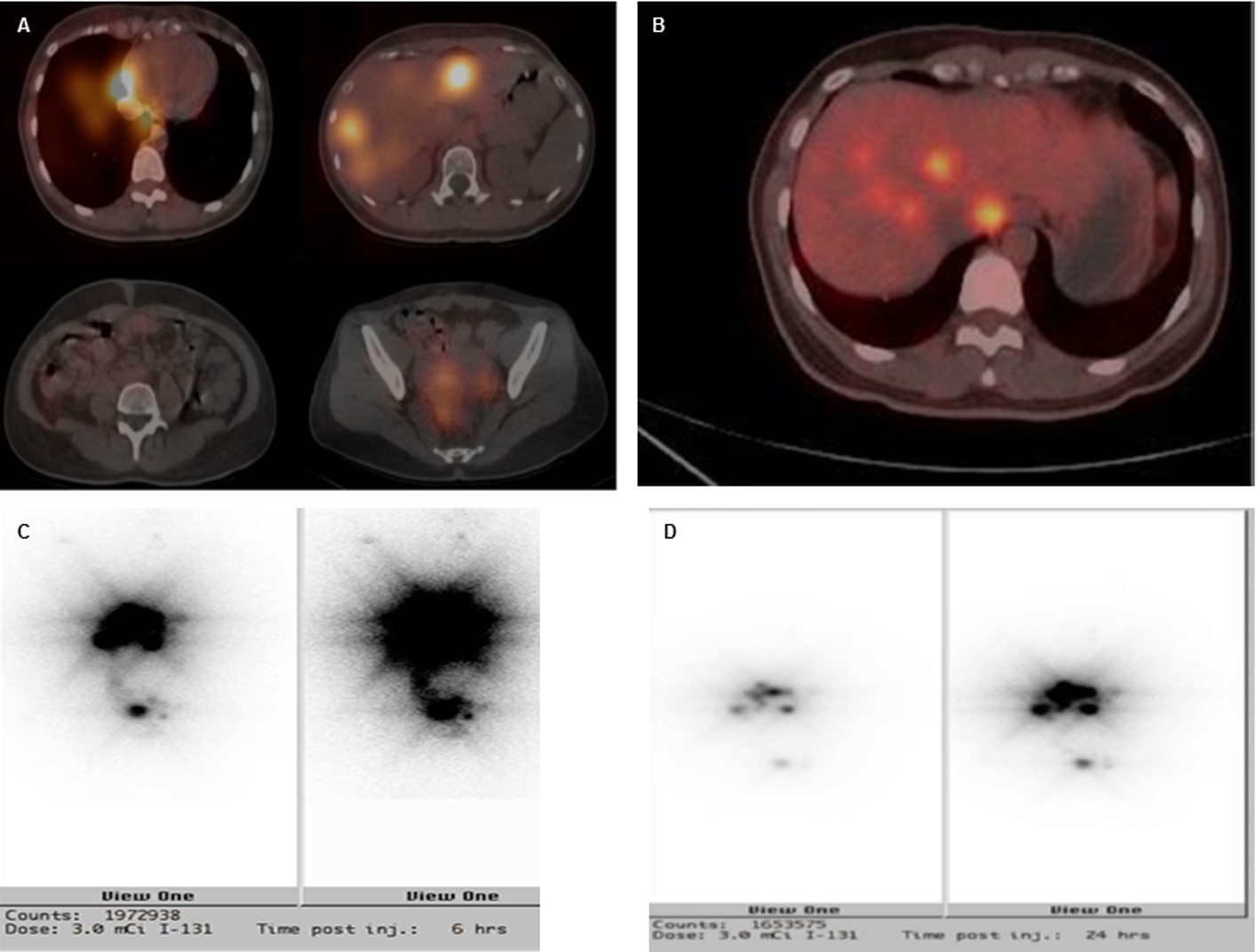 Click for large image | Figure 3. Imaging studies of SPECT (A), PET-FDG (B) and I131 (3 mCi) pre-treatment scan of 6 h (C) and 24 h (D). There were multiple sites of intense radiotracer uptake accumulation over the right lower lung, liver and pelvis. Relatively less intense uptake was seen over the left lower lung, right peritoneal cavity and uterine adnexa. |
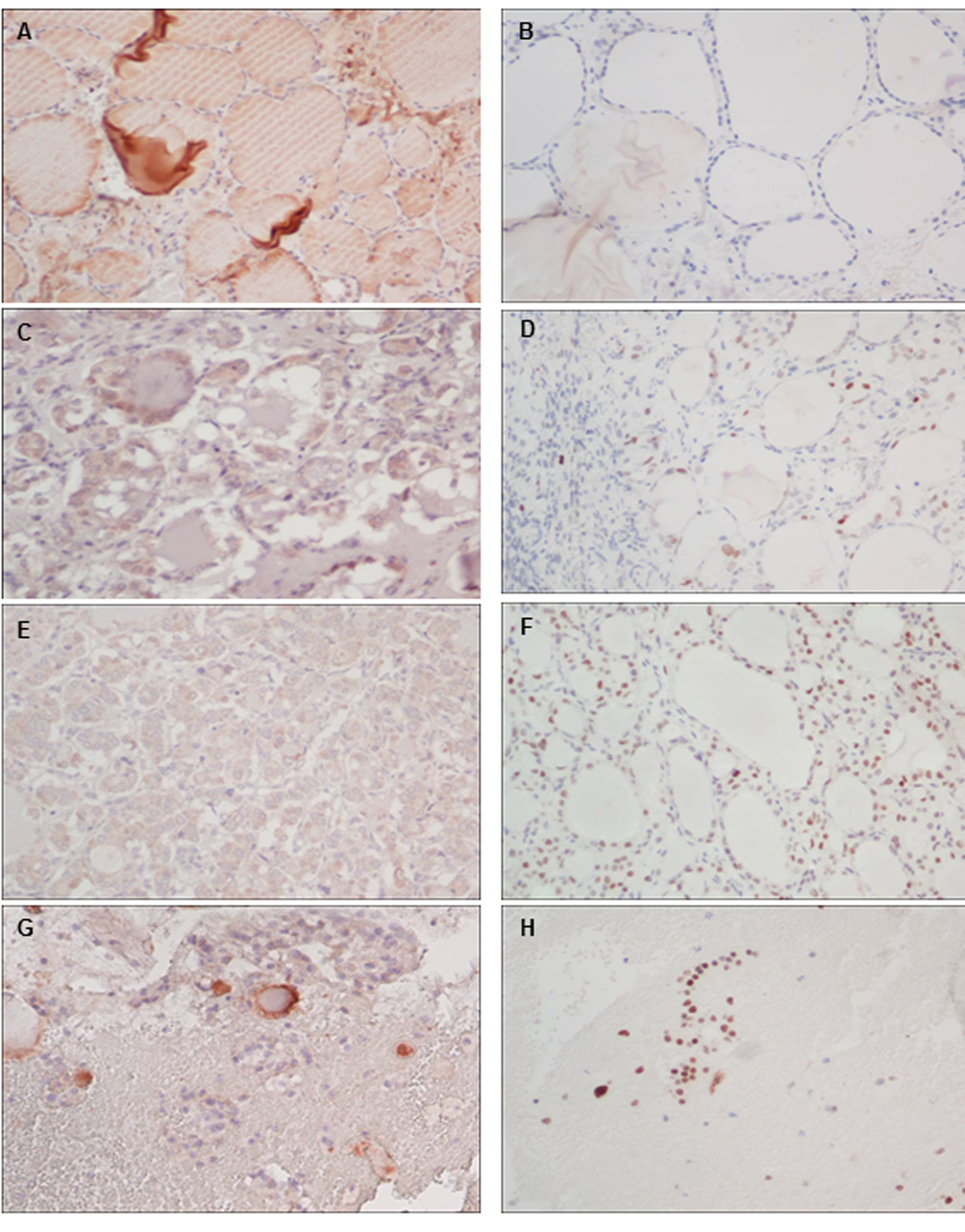 Click for large image | Figure 4. Immunohistochemistry (× 200) of the expressions of VEGF (left panel) and p53 (right panel) for normal thyroid (A, B), excised ovary(C, D), peritoneal (E, F) and liver seedings (G, H). |
In a thorough follow-up survey, her thyroid function and serum CA-125 11.71 U/mL (normal reference 2.4 - 36.3) were within normal limits. The initial serum thyroglobulin level was highly elevated to 1,573.4 ng/mL (normal reference < 50) but dropped to 497.47 ng/mL after TT in euthyroid status (TSH 0.84 mIU/mL). However, her serum thyroglobulin rapidly rose to 1,292.1 ng/mL in a hypothyroid status just before RAI therapy. Fortunately, her serum thyroglobulin declined significantly to 123.2 ng/mL at 2 months and 76.14 ng/mL at 4 months after her RAI therapy. She maintains a regular follow-up schedule in our hospital.
| Discussion | ▴Top |
Though the first case was described by Von Kalden in 1895, SO is still extremely rare in clinical practice. The common manifestation is abdominal pain or pelvic mass. Histologically, SO is usually composed of benign thyroid tissues, including hyperplasia, thyroiditis or adenoma. However, 5-10% of SO may contain thyroid carcinoma or progress to malignant transformation [1, 2, 5]. There is no consistent definition for “malignant SO”. The term “malignant SO” basically refers to SO with histological malignancy similar to thyroid cancer, with papillary thyroid carcinoma being the most common histologic type (44%), followed by follicular thyroid carcinoma (30%) and a follicular variant of papillary carcinoma (26%) [1]. Some literature also categorizes SO with no evidence of histologically malignant change that behaves aggressively with extra-ovarian and distant metastatic spreading as “biologically malignant SO” [5, 6].
The treatment strategy for SO is quite challenging. Diverse clinical entities and prognosis have been reported from a limited number of case experiences. There is still no guideline or consensus for the optimal treatment for SO. Based on a literature review, the optimal management should consider factors including the patient’s age, preservation of fertility, histologic malignancy, tumor size and peritoneal strumosis or metastasis [3, 4, 7-9]. For SO composed of thyroid carcinoma, there is controversy over the extent of surgical exploration and the choice of combinations for unilateral salpingo-oophorectomy (USO)/bilateral salpingo-oophorectomy (BSO)/total hysterectomy (TH)/TT/RAI ablation [6, 9-11]. Risk stratification for histologically malignant SO was suggested to guide optimal surgical management by Marti et al. Low-risk patients were defined as having a primary tumor < 2 cm in size confined to ovary without aggressive histologic or biological features, who were indicated for USO and thyroxin suppression therapy. Otherwise, the high-risk patients were indicated for additional TT and RAI to eradicate extra-ovarian metastasis and tumor burden. The prognosis for low-risk patients is reported to be quite good, with a low recurrence rate of about 7.5% over 25 years in a case series of 57 patients [3]. The average time to recurrence was found to be around 6 years in a review of 59 case series of malignant SO by Jean et al [12]. Serum thyroglobulin is usually used to evaluate treatment effects and recurrence of thyroid carcinoma. Therefore, serum thyroglobulin is regarded as a tumor marker to follow for at least 10 years [13]. One lethal case reported by Marcy et al presented as low-risk SO initially but the patient died about 3 years later after delayed TT with rapidly advanced peritoneal carcinomatosis and liver metastasis [14]. Generally speaking, the survival rate for malignant SO is favorable and similar to thyroid carcinoma, about 89% at 10 years and 84% at 25 years in an 88 case series reported by Robboy et al [6].
Angiogenesis with high expression of the VEGF is believed to be an early event in tumorigenesis and may facilitate tumor metastasis [15, 16]. Wild-type p53 protein functions as a tumor suppressor but presents only very short time with minute amounts below the detection level of immunocytochemical methods. The mutant p53 proteins are much more stable to easily accumulate and behave as oncogene to facilitate DNA replication and tumor growth [17, 18]. Though tumor enriched with VEGF or p53 mutation may explain some of the mechanisms of the metastatic behavior for thyroid carcinoma, why this histologically benign tumor behaves like a malignancy with distant spreading remains enigmatic. No reports ever discuss the mechanism of biologic metastasis in a histologic benign SO. Nevertheless, the abundant expressions of the VEGF and p53 proteins are speculated contributing to the malignant behavior in our case.
For benign SO without extra-ovarian seeding, tumor resection is the treatment option favored by most authors. However, there is no consensus on the best treatment for biologically malignant SO such as our case. A case report by Massimo et al described a patient who presented with benign SO at the age of 12 but progressed to malignant transformation to papillary carcinoma that spread to the peritoneum and liver. To preserve her fertility, gonadotropin-releasing hormone analogs and ovarian tissue cryopreservation were used before extensive BSO, TH, TT and RAI therapy [7]. Considering the frequent recurrences, we doubted the adequacy or suitability of repeated conservative tumor resection. Further research is needed to shed light on when to employ aggressive surgical intervention, how the patient’s fertility can be preserved and how to evaluate extra-ovarian spreading.
In our case experience, the extra-ovarian tumor seeding was well detected by PET-FDG and an I131 scintillation scan simultaneously. We also found an extremely high level of serum thyroglobulin. The positive uptake of PET-FDG for thyroid carcinoma usually represents poorly differentiated thyroid cancer cells, dedifferentiated cells without thyroglobulin expression or resistance to RAI therapy [19]. However, the SO tissue of this case demonstrated the iodine avid activity, elevated thyroglobulin as well as apparent uptake of PET-FDG, which is quite distinctive to the behavior of thyroid cancers. To avoid frequent surgical intervention and tumor recurrence, we highly recommend using serum thyroglobulin as a biochemical index to evaluate tumor burden during follow-up. Image studies using I131 scintillation scan and PET-FDG to localize the extent of tumor seeding may help guide decisions for optimal surgical management and indications for RAI therapy.
Based on our limited case experience, we recommended close follow-up of serum thyroglobulin and image study by I131 or PET-FDG scan for cases presenting with recurrent or “biologically malignant” SO. More aggressive treatment options may include BSO, TT and RAI ablation therapy to eradicate the tumor completely.
Conflict of Interest
The authors have disclosed no any competing financial interests and conflict of interests at all.
| References | ▴Top |
- Gunasekaran S, Kopecka E, Maung KH, England RJ. Struma ovarii and the thyroid surgeon. J Laryngol Otol. 2012;126(8):858-860.
doi pubmed - Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS. Clinical characteristics of struma ovarii. J Gynecol Oncol. 2008;19(2):135-138.
pubmed - Marti JL, Clark VE, Harper H, Chhieng DC, Sosa JA, Roman SA. Optimal surgical management of well-differentiated thyroid cancer arising in struma ovarii: a series of 4 patients and a review of 53 reported cases. Thyroid. 2012;22(4):400-406.
doi pubmed - Shrimali RK, Shaikh G, Reed NS. Malignant struma ovarii: the west of Scotland experience and review of literature with focus on postoperative management. J Med Imaging Radiat Oncol. 2012;56(4):478-482.
doi pubmed - Shaco-Levy R, Bean SM, Bentley RC, Robboy SJ. Natural history of biologically malignant struma ovarii: analysis of 27 cases with extraovarian spread. Int J Gynecol Pathol. 2010;29(3):212-227.
doi pubmed - Robboy SJ, Shaco-Levy R, Peng RY, Snyder MJ, Donahue J, Bentley RC, Bean S, et al. Malignant struma ovarii: an analysis of 88 cases, including 27 with extraovarian spread. Int J Gynecol Pathol. 2009;28(5):405-422.
doi pubmed - Salvatori M, Dambra DP, D'Angelo G, Conte LL, Locantore P, Zannoni G, Campo V, et al. A case of metastatic struma ovarii treated with 131I therapy: focus on preservation of fertility and selected review of the literature. Gynecol Endocrinol. 2008;24(6):312-319.
doi pubmed - Roth LM, Miller AW, 3rd, Talerman A. Typical thyroid-type carcinoma arising in struma ovarii: a report of 4 cases and review of the literature. Int J Gynecol Pathol. 2008;27(4):496-506.
doi pubmed - McGill JF, Sturgeon C, Angelos P. Metastatic struma ovarii treated with total thyroidectomy and radioiodine ablation. Endocr Pract. 2009;15(2):167-173.
doi pubmed - Wirtz ED, Bothwell N, Klem C. Role of the otolaryngologist in the treatment of struma ovarii. Laryngoscope. 2010;120(2):259-260.
pubmed - Wolff EF, Hughes M, Merino MJ, Reynolds JC, Davis JL, Cochran CS, Celi FS. Expression of benign and malignant thyroid tissue in ovarian teratomas and the importance of multimodal management as illustrated by a BRAF-positive follicular variant of papillary thyroid cancer. Thyroid. 2010;20(9):981-987.
doi pubmed - Jean S, Tanyi JL, Montone K, McGrath C, Lage-Alvarez MM, Chu CS. Papillary thyroid cancer arising in struma ovarii. J Obstet Gynaecol. 2012;32(3):222-226.
doi pubmed - Kim D, Cho HC, Park JW, Lee WA, Kim YM, Chung PS, Park SG, et al. Struma ovarii and peritoneal strumosis with thyrotoxicosis. Thyroid. 2009;19(3):305-308.
doi pubmed - Marcy PY, Thariat J, Benisvy D, Azuar P. Lethal, malignant, metastatic struma ovarii. Thyroid. 2010;20(9):1037-1040.
doi pubmed - Cao Y, E G, Wang E, Pal K, Dutta SK, Bar-Sagi D, Mukhopadhyay D. VEGF exerts an angiogenesis-independent function in cancer cells to promote their malignant progression. Cancer Res. 2012;72(16):3912-3918.
doi pubmed - Salajegheh A, Pakneshan S, Rahman A, Dolan-Evans E, Zhang S, Kwong E, Gopalan V, et al. Co-regulatory potential of vascular endothelial growth factor-A and vascular endothelial growth factor-C in thyroid carcinoma. Hum Pathol. 2013;44(10):2204-2212.
doi pubmed - Marcello MA, Morari EC, Cunha LL, De Nadai Silva AC, Carraro DM, Carvalho AL, Soares FA, et al. P53 and expression of immunological markers may identify early stage thyroid tumors. Clin Dev Immunol. 2013;2013:846584.
doi pubmed - Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih Ie M, Kurman RJ. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24(9):1248-1253.
doi pubmed - Mosci C, Iagaru A. PET/CT imaging of thyroid cancer. Clin Nucl Med. 2011;36(12):e180-185.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
