| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 1, March 2015, pages 164-169
Normalized Relative Contrast Improves the Power of Pre-Therapy Contrast-Enhanced MRI to Predict the Prognosis of Uterine Leiomyoma Treated With Uterine Artery Embolization
Kejia Caia, b, c, Karen Xiea, Winnie Mara, Alison Palumboa, Frederick C. Damena, Yi Suib, Yang Lua, Xiaohong J. Zhoua, b, Grace M. Knuttinena, c
aDepartment of Radiology, University of Illinois Hospital & Health Sciences System, Chicago, IL, USA
b3T Program of the Center of Magnetic Resonance Research, University of Illinois Hospital & Health Sciences System, Chicago, IL, USA
cCorresponding Authors: Kejia Cai, Department of Radiology, University of Illinois Hospital & Health Sciences System, Chicago, IL, USA. Grace M. Knuttinen, Department of Radiology, University of Illinois Hospital & Health Sciences System, Chicago, IL, USA
Manuscript accepted for publication March 18, 2015
Short title: Pre-Therapy MRI in Uterine Embolization
doi: http://dx.doi.org/10.14740/jcgo279w
| Abstract | ▴Top |
Background: Uterine artery embolization (UAE) has emerged as an effective treatment option for women with symptomatic uterine leiomyomas, the most common benign tumor of the female reproductive system. Assessing factors that aid in predicting treatment outcomes is critical for patient selection, procedure planning and post-procedural follow-up. Previous studies have demonstrated variable correlations between MRI predictors and response to UAE. In this study, we investigated if the relative tumor to intratumor myometrium contrast may improve the predictive power of pre-therapy contrast-enhanced MRI.
Methods: A retrospective study of a total of 42 uterine leiomyomas treated with UAE was performed. Treated tumors were categorized as either fully or not fully responsive based on if they became completely necrotic 3 - 6 months post-UAE.
Results: There was no significant difference (P = 0.34) in the pre-UAE contrast to noise ratio (CNR) between fully responsive (64.6 ± 38.6) and not fully responsive (74.2 ± 24.8) tumors. On the other hand, the pre-UAE relative contrast of not fully responsive tumors was significantly higher than the fully responsive tumors (1.6 ± 0.4 vs. 1.0 ± 0.4, P < 0.05). Pre-UAE tumor relative contrast was found to correctly predict 7/9 not fully responsive and 30/33 fully responsive tumors at a threshold of 1.3. Larger area under the receiver operating characteristic (ROC) curve based on relative contrast than that based on CNR also indicated that relative contrast improved the predictive power of pre-therapy contrast-enhanced MRI.
Conclusion: Upon further validation with large studies, pre-UAE relative contrast may prove to be a useful tool to predict UAE treatment outcome of leiomyomas and improve the clinical management of uterine leiomyoma.
Keywords: Uterine artery embolization; Uterine leiomyomas; Contrast-enhanced MRI; Treatment response
| Introduction | ▴Top |
Uterine leiomyomas or uterine fibroids are the most common uterine and gynecologic neoplasm, occurring in over 20% of women older than 30 years [1]. It is a common cause of significant, sometimes even disabling, pelvic-related symptoms in women. Symptomatic leiomyomata cost the US health care system more than $1 billion per year in direct costs [2, 3]. In addition, the estimated mortality is estimated at 5 - 10 million person-days, with close to 1 million hospital admissions per year, more than that attributed to breast cancer [4].
A few options are available for the treatment of symptomatic uterine myomata. Traditional first-line medical management includes the use of anti-hormonal agents, and the traditional use of non-steroidal anti-inflammatory drugs. For those women whose symptoms are not relieved by these medical approaches, invasive surgical procedures such as hysterectomy or myomectomy can be typically recommended. As a result, over 150,000 hysterectomies and 35,000 myomectomies each year are performed in the US to relieve symptoms from uterine fibroids.
Uterine artery embolization (UAE) was first proposed by Ravina et al in 1995 [5]. Since then, multiple studies have shown this minimally invasive technique to be a safe and effective alternative treatment option for symptomatic uterine fibroids [6-8]. It has been demonstrated that UAE provides a 95-98% of technical success rate [9], rapid recovery, only 1-5% perioperative complication rate [10], and a sustained patient satisfaction and symptomatic improvement in the majority of patients [11].
Although the efficacy of UAE remains high, treatment failures can occur in the setting of uterine adenomyosis, the presence of spasm in the uterine arteries, or other anatomic variation in the uterine blood supply [12, 13]. Predicting UAE outcome before the actual treatment is hence critical for determining whether other treatment options, including surgical intervention, would be otherwise recommended. MRI techniques such as T1-, T2-weighted, diffusion and contrast-enhanced MRI have been routinely obtained to evaluate leiomyoma distributions and characteristics. Previous studies show pre-UAE MRI has variable predictive power for UAE prognosis [14-17]. For example, pre-UAE apparent diffusion coefficient (ADC) generated by diffusion MRI was found to moderately correlate tumor volume reduction due to UAE [14, 15]. T1, T2 and contrast-enhanced MRI also showed value [14-17].
Given that both contrast-enhanced MRI and UAE treatment are intrinsically linked to tumor vasculature, the purpose of this study was to assess if alternative quantification strategies may improve the predictive power of pre-UAE contrast-enhanced MRI for UAE prognosis.
| Methods | ▴Top |
This retrospective study was conducted under an approved IRB protocol. After pre-UAE MRI, UAE treatment was performed according to standardized procedures [6-8]. Post-UAE MRI at 3 - 6 months was performed to check the treatment response.
MRI
Eight patients with cumulative 42 tumors completed pre- and 3 - 6 months post-UAE contrast-enhanced MRI of the pelvis on a 3T GE Discovery MR 750 scanner with a 32 channel cardiac coil. For each contrast-enhanced MRI, a 3D fat-suppressed, T1-weighted, gradient-echo sequence was performed pre- and post-administration of contrast agent with a dose containing gadolinium of 0.03 mmol/kg body weight (gadofosveset trisodium). Spectral inversion at lipids (SPIR) was used for fat suppression with 5 ms inversion time. One hundred to 200 axial slices were acquired with slice thickness of 5 mm, TR/TE of 5.2/2.5 ms, field of view (FOV) of 380 × 380 mm2, and matrix size of 512 × 256 interpolated into 512 × 512 during image reconstruction, yielding an in-plane resolution < 1 × 1 mm2. To achieve high resolution, a portion of anatomy along the left-right direction was truncated in the acquired FOV with the placement of phase encoding along the posterior-anterior direction. The total imaging time for each 3D contrast-enhanced MRI takes about 45 s.
Image processing
Tumor contrast to noise ratio (CNR) and relative contrast were quantified. As a commonly used imaging index, CNR was quantified as in Equation (1).
(1) CNR = ΔF/std(N)
where ΔF is the tumor signal difference due to contrast agent, and std(N) is the standard deviation of noise (N).
Tumor relative contrast is quantified as in Equation (2).
(2) Relative Contrast = ΔF/ΔM
where ΔM is the signal difference of intratumoral healthy myometrium tissue due to contrast agent.
Categorization of tumor responses
UAE treatment blocks tumor vascular supply, which leads to tumor necrosis. Depending on if tumor becomes completely necrotic a few months post-UAE, we divided treated tumors into two categories. Leiomyomas that showed no post-contrast enhancement were completely necrotic and were considered to be fully responsive (group A). The remaining leiomyomas that were partially or persistent enhancing were considered to be not fully or incompletely responsive (group B).
Statistics
Two-tailed unpaired Student’s t-tests were performed to compare fully and not fully responsive tumors. Differences were considered to be significant at P < 0.05. Values were reported as mean ± standard deviation.
To assess how CNR or the relative contrast can correctly predict whether a tumor is incompletely responsive, receiver operating characteristic (ROC) curve analysis was performed. True positive and false positive rates are calculated at different thresholds of relative contrast from 0 to 2.5 at an increment of 0.1, or CNR from 0 to 180 at an increment of 10. ROC curve is the plot of the true positive rate (y axis) vs. the false positive rate (x axis). The area under the curve reflects the predictive performance of contrast-enhanced MRI based on either tumor CNR or the relative contrast.
Here, “positive” means correctly predicting the not fully responsive tumors. The true positive rate is the percentage of correctly predicted not fully responsive tumors over the entire not-responsive tumors. On the other hand, the false positive rate represents the percentage of falsely predicted positive over the entire negative pool, or the falsely predicted not fully responsive cases divided by the number of fully responsive tumors.
| Results | ▴Top |
After UAE treatment, 33/42 leiomyomas were found to be completely non-enhancing and considered to be fully responsive (group A). The remaining 9/42 leiomyomas showed partial or no necrosis (group B). Figure 1 demonstrated a patient with both fully responsive and not fully responsive tumors (arrows). From the post-UAE post-contrast images, we found that fully responsive group A tumors showed hypointense signal, indicating necrosis, while not fully responsive group B tumors were hyperintense due to contrast enhancement. Comparing the pre- and post-UAE maps of CNR, tumors with higher pre-therapy enhancement were the group B tumors that were not fully responsive to therapy. On the other hand, tumors with less pre-UAE enhancement turned out to be group A tumors that were fully responsive.
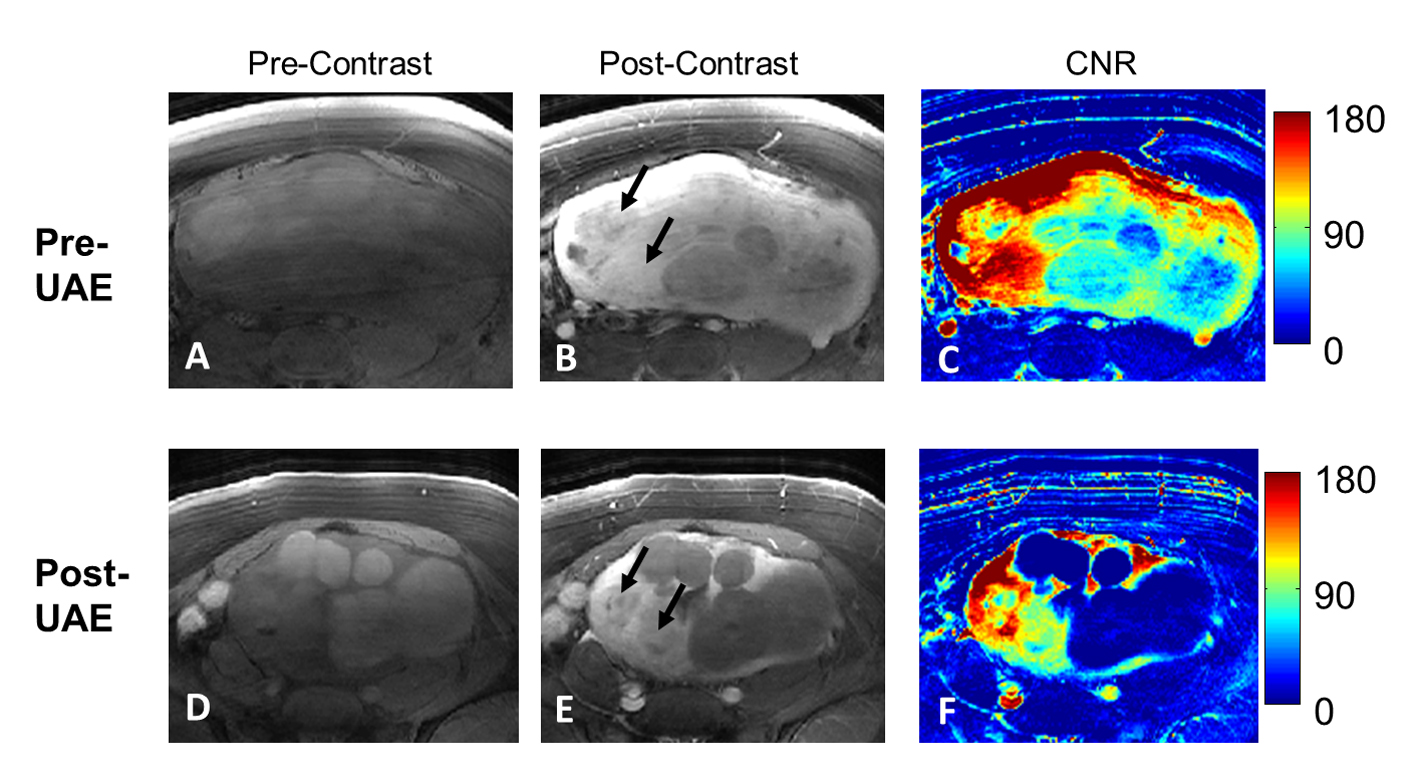 Click for large image | Figure 1. A patient with both group A and group B tumors (arrows). From left to right columns, cropped pre- and post-contrast images and the corresponding CNR maps are shown in A-C (pre-UAE) and D-F (3-6 months post-UAE). The two tumors (arrows in B) with the highest contrast enhancements (as shown in C) are the ones not fully responsive from UAE treatment as shown in the E (arrows) and F. |
As summarized in Figure 2, while the CNRs of the two tumor groups were not significantly different (74.2 ± 24.8 vs. 64.6 ± 38.6, P = 0.34). Tumors in group B exhibited significantly higher relative contrast than those in group A (1.6 ± 0.4 vs. 1.0 ± 0.4, P < 0.05). Tumor CNR correctly predicted 7/9 not fully responsive tumors and 22/33 fully responsive tumors at the manually set optimal threshold of 70 (Fig. 2C). On the other hand, Figure 2D shows that using an heuristically optimal threshold of 1.3, pre-UAE tumor relative contrast correctly predicted 7/9 not fully responsive tumors and 30/33 fully responsive tumors.
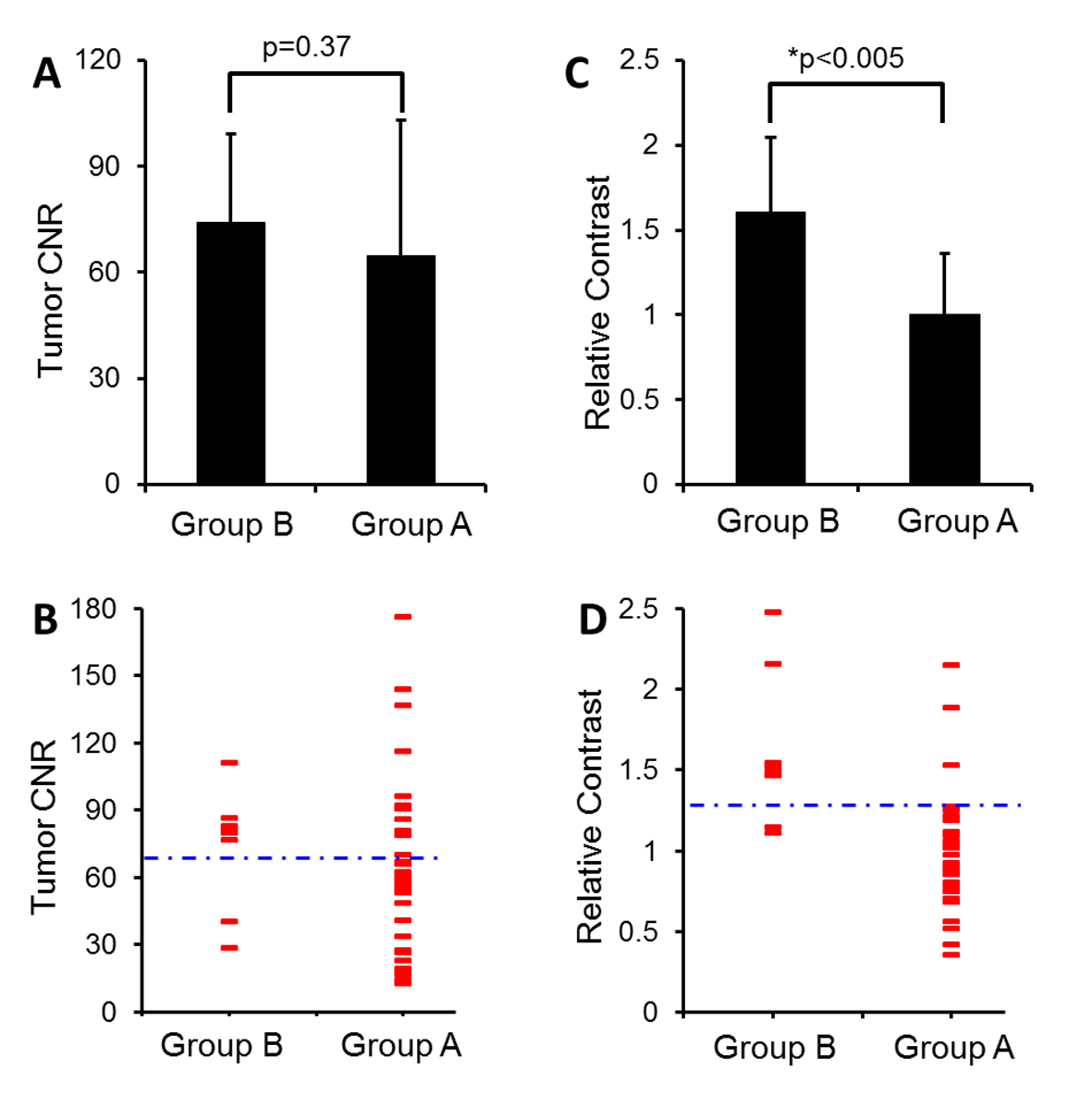 Click for large image | Figure 2. Summarized results of pre-UAE CNR (A-B) and relative contrast (C-D) in group A and B tumors. (A) CNR of group B tumors is not significantly higher than group A tumors (P = 0.37). (B) A dotted blue line crossing at CNR 70 was heuristically prescribed to differentiate tumor responsiveness. Each red dash represents an individual case. (C) Relative contrast of group B tumors is significantly higher than group A tumors (*P < 0.05). (D) Relative contrast of 1.3 (a dotted blue line) separates most group A from group B tumors. |
Relative contrast appears to have higher predictive power than CNR, probably due to the large standard deviations of group CNRs. To understand this variation, we hence investigated the stability of noise taken for CNR quantification. Figure 3 shows how noise selection may affect CNR quantification in our study. Four representative regions of interests (ROI) beyond the anatomy were selected as noise regions, the corresponding tumor CNRs were calculated, and the CNR maps were generated. As a result, we found that tumor CNR can be greatly affected by selecting different noise regions. This is a major issue particularly when most areas beyond anatomy were affected by either ghosting or motion artifacts.
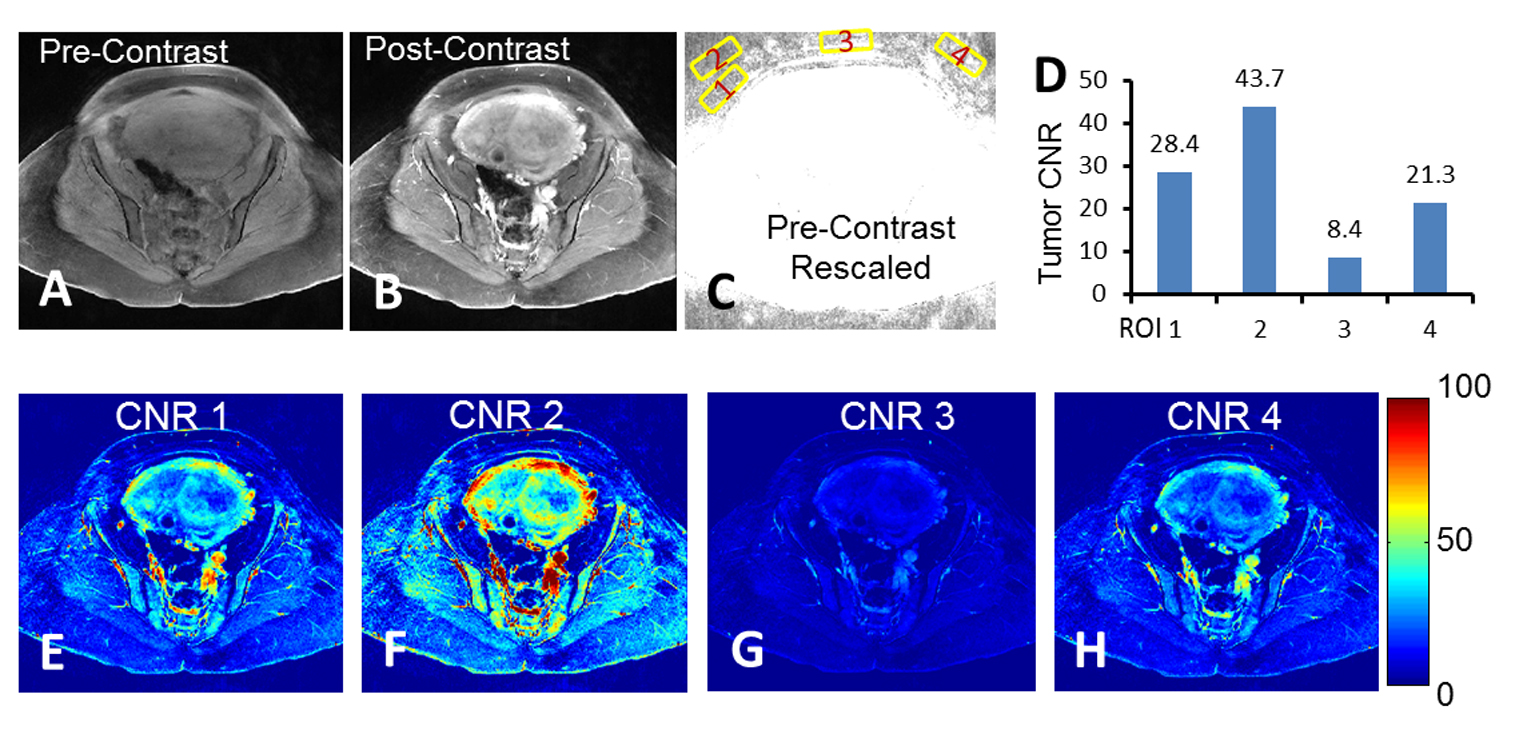 Click for large image | Figure 3. Quantification challenges regarding to noise ROI selection. (A-B) The pre- and post-contrast images with acquired full FOV. (C) Rescaled pre-contrast image to show background noise. Numbered yellow squares were the regions selected for tumor CNR analysis. (D) Tumor CNR calculated based on the noise ROIs shown in (C). (E-H) Individual CNR maps with false-color overlay corresponding to noise ROIs shown in (C). |
The performance of using either the pre-UAE relative contrast or CNR for predicting UAE treatment responses was analyzed with ROC curves (Fig. 4). Larger area was observed from the ROC curve based on the relative contrast than that based on CNR.
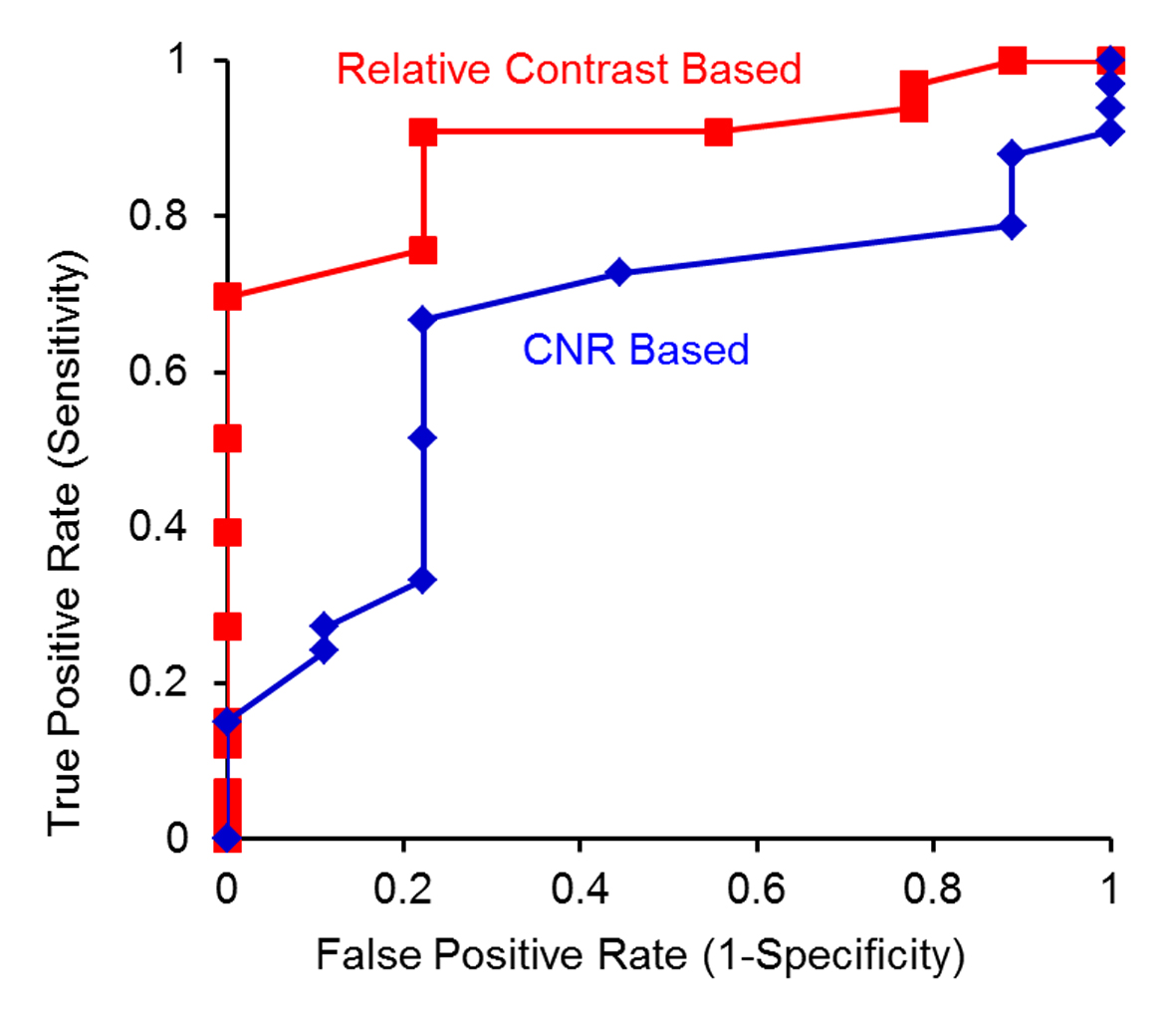 Click for large image | Figure 4. ROC curve analysis to test whether a tumor is not fully responsive using either relative contrast (red) or CNR (blue) as the predictor. |
| Discussion | ▴Top |
MRI for predicting UAE treatment responses has been previously investigated. In our study, a few changes in methodology have been made. First of all, we simplified the categorization of treatment responses simply based on whether tumors became fully necrotic after treatment. In the literature, however, UAE treatment response was typically quantified based on the percentage of tumor volume reduction [14, 15]. Because there are typically multiple tumors per patient and it is difficult to co-register tumors pre- and post-treatment, accurate tumor volume quantification is time-consuming and challenging. In addition, volume reduction may not be the most informative and quantitative index for treatment efficacy since tumor volumes are highly time-dependent. After a few months post-treatment, unresponsive tumor tissue may keep on growing while UAE-induced necrotic tissues may undergo a continuous shrinking process. Hence, tumor volume reduction post-UAE may be affected by multiple factors, including the time gap for post-UAE MRI, tumor growth rate and the clearance rate for necrotic tissues. To avoid these complications, we utilized a simplified and descriptive categorization method for UAE treatment responses in our study.
The second change we made was to avoid using noise in our quantification of tumor response. Noise standard deviation is typically a very useful denominator and is widely used for quantitative MRI processing. However, caution should be taken with the presence of motion artifacts, eddy current induced artifacts, etc. Noise ROI selection becomes more challenging if a small FOV is covered as in this study for the purpose of increasing spatial resolution and reducing imaging time. We demonstrated that noise ROI selection greatly affected the noise-based CNR quantitation, preventing CNR for being a reliable predictor. Instead, relative tumor contrast normalized to intratumor tissue was proposed and utilized.
In addition, though further validation is needed, we believe that relative contrast may be less affected by the contrast dynamics or the timing of the perfusion of contrast agents. Variation of both injection timing and doses between patients may be attenuated by using the contrast dynamics of tissue surrounding the tumors as the denominator, since normal tissues around tumors presumably share the similar contrast dynamics.
As a result, we show that the relative contrast has better predictive power than the noise-based CNR with higher accuracy of differentiating fully responsive tumors from the not fully responsive ones.
Intrinsically, UAE and contrast-enhanced MRI are related, as both involve tumor vasculature. UAE is based on the blockage of vasculature supplied to tumor using microspheres. Contrast enhancement in MRI, on the other hand, is primarily due to the perfusion of gadolinium-based agents in the tumor vasculature pool. It is well known that tumor vasculature develops via angiogenesis and is characterized by immature, fragile, and chaotic structures [18]. Because of this abnormal structure, it is presumably harder for UAE microspheres to completely block the vasculature supply in highly vascularized tumors (with high contrast enhancement), leading to poor responses for those tumors.
It is interesting to note that there are other factors that affect UAE treatment, such as the presence of uterine adenomyosis, vessel spasm and collateral vessels [12, 19]. New angiogenic vessels developing post-UAE may also prevent tumors from complete necrosis even though the original tumor vascular supply is completely blocked by UAE.
There are a few limitations in this study. First of all, it remains a challenge to co-register the tumors pre- and post-UAE given the tumor volumes and anatomies are changed greatly during the months’ post-UAE period of time. Our co-registration is based on our best knowledge and validated by radiologists. Secondly, we have a limited sample size accumulated so far with a total of 42 tumors studied. Validation with large scale studies in the future is expected.
Conclusions
We demonstrate that leiomyomas with high pre-UAE post-contrast enhancement were found more likely to have poor responses to UAE. Upon further validation with a large sample size, pre-UAE relative contrast may help to predict UAE treatment outcome. Clinically, this may help to determine whether other treatment options including surgical intervention would be otherwise beneficial.
Conflicts of Interest
The authors disclose no potential conflicts of interest.
| References | ▴Top |
- Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90(6):967-973.
doi - Gross CP, Anderson GF, Powe NR. The relation between funding by the National Institutes of Health and the burden of disease. N Engl J Med. 1999;340(24):1881-1887.
doi pubmed - Greenberg MD, Kazamel TI. Medical and socioeconomic impact of uterine fibroids. Obstet Gynecol Clin North Am. 1995;22(4):625-636.
pubmed - Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915-921.
doi - Ravina JH, Herbreteau D, Ciraru-Vigneron N, Bouret JM, Houdart E, Aymard A, Merland JJ. Arterial embolisation to treat uterine myomata. Lancet. 1995;346(8976):671-672.
doi - Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79(1):120-127.
doi - Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109(11):1262-1272.
doi - Worthington-Kirsch RL, Popky GL, Hutchins FL, Jr. Uterine arterial embolization for the management of leiomyomas: quality-of-life assessment and clinical response. Radiology. 1998;208(3):625-629.
doi pubmed - Pron G, Bennett J, Common A, Sniderman K, Asch M, Bell S, Kozak R, et al. Technical results and effects of operator experience on uterine artery embolization for fibroids: the Ontario Uterine Fibroid Embolization Trial. J Vasc Interv Radiol. 2003;14(5):545-554.
doi pubmed - Goodwin SC, Spies JB, Worthington-Kirsch R, Peterson E, Pron G, Li S, Myers ER. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22-33.
doi pubmed - Spies JB, Myers ER, Worthington-Kirsch R, Mulgund J, Goodwin S, Mauro M. The FIBROID Registry: symptom and quality-of-life status 1 year after therapy. Obstet Gynecol. 2005;106(6):1309-1318.
doi pubmed - Matson M, Nicholson A, Belli AM. Anastomoses of the ovarian and uterine arteries: a potential pitfall and cause of failure of uterine embolization. Cardiovasc Intervent Radiol. 2000;23(5):393-396.
doi pubmed - Smith SJ, Sewall LE, Handelsman A. A clinical failure of uterine fibroid embolization due to adenomyosis. J Vasc Interv Radiol. 1999;10(9):1171-1174.
doi - Hecht EM, Do RK, Kang SK, Bennett GL, Babb JS, Clark TW. Diffusion-weighted imaging for prediction of volumetric response of leiomyomas following uterine artery embolization: a preliminary study. J Magn Reson Imaging. 2011;33(3):641-646.
doi pubmed - Ananthakrishnan G, Macnaught G, Hinksman L, Gilmour H, Forbes KP, Moss JG. Diffusion-weighted imaging in uterine artery embolisation: do findings correlate with contrast enhancement and volume reduction? Br J Radiol. 2012;85(1019):e1046-1050.
doi pubmed - Jha RC, Ascher SM, Imaoka I, Spies JB. Symptomatic fibroleiomyomata: MR imaging of the uterus before and after uterine arterial embolization. Radiology. 2000;217(1):228-235.
doi pubmed - Cura M, Cura A, Bugnone A. Role of magnetic resonance imaging in patient selection for uterine artery embolization. Acta Radiol. 2006;47(10):1105-1114.
doi pubmed - Cai K, Shore A, Singh A, Haris M, Hiraki T, Waghray P, Reddy D, et al. Blood oxygen level dependent angiography (BOLDangio) and its potential applications in cancer research. NMR Biomed. 2012;25(10):1125-1132.
doi pubmed - Spies JB. Uterine artery embolization for fibroids: understanding the technical causes of failure. J Vasc Interv Radiol. 2003;14(1):11-14.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
