| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 1, March 2015, pages 170-176
Polymorphisms of Renin Angiotensin System Genes in Uterine Leiomyomas Among Egyptian Females
Salwa H. Gomaaa, d, Ahmed M. Zakia, Eman A. El-Attara, Mohamed M. Mokhtarb, Manal S. Swelemc
aDepartment of Chemical Pathology, Medical Research Institute, Alexandria University, Alex, Egypt
bDepartment of Genetics, Medical Research Institute, Alexandria University, Alex, Egypt
cDepartment of Obstetrics and Gynaecology, Faculty of Medicine, Alexandria University, Alex, Egypt
dCorresponding Author: Salwa Hamdi Gomaa, Department of Chemical pathology, Medical Research Institute, Alexandria University, 12 Warda elyazgi street. Alexandria, Egypt
Manuscript accepted for publication November 25, 2014
Short title: Polymorphisms of RAS Genes
doi: http://dx.doi.org/10.14740/jcgo300w
| Abstract | ▴Top |
Background: Uterine leiomyomas are the most common gynecological benign myometrial neoplasms. They have been associated with infertility and recurrent abortion as well as obstructed labor and post-partum hemorrhage. They are the most important indication for hysterectomy. The renin angiotensin system (RAS), in particular angiotensin type 1 receptor (AT1R) and to a lesser extent angiotensin II, angiotensin converting enzyme (ACE) and angiotensin type 2 receptor (AT2R), are often upregulated during the progression from normal to malignant phenotypes indicating a possible correlation between RAS and tumor progression. The present study investigated the association of A1166C single nucleotide polymorphism (SNP) of AT1R gene and insertion deletion (I/D) polymorphism of ACE gene with uterine leiomyomas in Egyptian females.
Method: The study was carried out on 124 Egyptian females divided into 70 patients diagnosed as having uterine leiomyomas and 54 matched control females. DNA extraction from peripheral blood leucocytes was done using commercial kits for detection of the presence of the (I) and (D) alleles in the ACE gene by polymerase chain reaction (PCR) amplification using specific primers and detection of A1166C gene polymorphism using polymerase chain reaction and restriction fragment length polymorphism (PCR/RFLP) technique.
Results: The genotype distribution patterns of A1166C polymorphism of AT1R gene among leiomyoma patients were statistically significantly different (P = 0.028) from the control group where patients had a higher frequency of CC genotype than controls (8.6% versus 0%), a higher frequency of AC than controls (35.7% versus 25.9%) and a lower frequency of AA than controls (55.7% versus 74.1%). However, ACE I/D polymorphism was not found to be associated with uterine leiomyoma.
Conclusions: We found a significant association of A1166C polymorphism in AT1R gene with uterine leiomyoma among Egyptian females suggesting that its potential regulatory function warrants further investigation.
Keywords: Leiomyomas; Angiotensin II; Angiotensin II type I receptor (A1166C) gene polymorphism; ACE gene polymorphism
| Introduction | ▴Top |
Uterine leiomyomas, known as fibroids, are the most common pelvic tumors. A total of 20-25% of reproductive age women have clinically symptomatic fibroids [1, 2]. They commonly cause severe symptoms that can seriously impact women’s health. Fibroids have also been associated with infertility and recurrent abortion and can cause obstructed labor and post-partum hemorrhage. Uterine leiomyoma is the most important indication for hysterectomy [3].
Angiogenesis and vascular-related factors are involved in the pathogenesis and growth of leiomyomas [4, 5]. Growth factors could preferentially promote the angiogenesis of leiomyoma cells compared with myometrial cells [6].
Independent tissue renin angiotensin systems (RASs) have been demonstrated for many organs including the kidney, brain, the uteroplacental unit, adrenal glands, vasculature and heart [7]. The angiotensin peptides bind and signal through their cognate angiotensin receptors, angiotensin type 1 receptor (AT1R), angiotensin type 2 receptor (AT2R), insulin-regulated aminopeptidase (IRAP) and Mas1 oncogene receptor (MaSR) to elicit various biological responses [8].
The genetic aspects of uterine leiomyoma development have been previously investigated. Roth et al [9] screened several genes in uterine leiomyoma and matched unaffected myometrium using microarray-based hybridization. Their data suggest that several genes are selectively overexpressed in leiomyomas compared with normal myometrium. These include growth factor genes as pleitropin, insulin-like growth factor-2 receptor, and insulin-like growth factor binding protein. However, they found the angiotensinogen gene to be downregulated. This may point out that not all aspects of the RAS may be overexpressed in uterine fibroids.
ACE inhibition as well as AT1R blockade have been shown to inhibit tumor angiogenesis, tumor growth, reduced tumor volume, vascular density, mitotic index and cell proliferation as well as reduce metastasis [10]. These findings highlight the role of ACE and AT1R as parts of RAS in tumor development and progression. They also highlight the importance of studying polymorphisms of the RAS in association with tumors. Since the effects of these polymorphisms could be reversed or antagonized through medical treatment, they could be used to prevent or reduce tumor development in susceptible subjects bearing the risky genotypes. This type of study could bring the concept of personalized tumor therapy a step closer towards reality [11].
AT1R protein is expressed in benign conditions as ovarian cystadenomas and is involved in tumor progression and angiogenesis. It is also expressed in several cancers such as the breast [12], gastric [13], bladder [14], prostate [15], pancreatic [16], endometrial [17] and renal cancer as well as ovarian carcinoma [18]. AT1R is often upregulated during the progression from normal to malignant phenotypes, indicating at the very least a correlation between the RAS and tumor progression.
Bonnardeaux et al [19] first described a nucleotide substitution (A/C in position 1166) in the exon 5 of the gene AT1R. They reported an increased prevalence of the C allele in hypertensive patients, a single base pair change from adenine to cytosine at the 1166 position in the 3’-UTR of the AT1R. The A allele is the wild allele while the C allele is the minor or the mutant allele and therefore the homozygous (CC) would be the least common genotype (NCBI, dbSNP: rs5186) [20]. The A1166C substitution in the AT1R receptor was found to reduce breast cancer risk [21] and to increase the risk of benign prostatic hyperplasia as an example of benign tumors [22].
In 1990, Rigat et al [23] observed a polymorphism in ACE gene involving insertion of 287 bp sequence in intron 16 (NCBI, dbSNP: rs1799752) resulting in insertion (I) allele, whereas deletion (D) allele is present in the absence of insertion. The D allele is the wild allele while the I allele is the minor or the mutant allele. This polymorphism is responsible for the ACE activity level, which increases two-fold in homozygous deletion carriers (D/D), as compared to homozygous insertion carriers (I/I). I/D carriers show intermediate ACE activity.
When ACE I/D polymorphism was examined as a cancer risk marker, it was also associated with endometrial [24] and prostate cancers [25]. This polymorphism may play a role in cancer risk by affecting the level of ACE produced in vivo. If ACE is increased in the plasma, there is an increase in the production of angiotensin II (Ang II) which is thought to have a role in tumor carcinogenesis via the binding to its receptor AT1R [12].
Ang II-induced proliferation of leiomyoma cells has been shown [26]. Experimental findings in vitro also highlight the potential role of Ang II in the proliferation of leiomyoma cells [27]. Ang II is not only a vasoactive peptide, but it also stimulates several growth factors including vascular endothelial growth factor (VEGF), angiopoietin 2, basic fibroblast growth factor (b-FGF) and platelet-derived growth factor (PDGF) inducing effects such as hypertrophy and hyperplasia. Ang II is also a mitogen for smooth muscle cells and fibroblasts [28]. Ang II-induced leiomyoma cell proliferation may thus play a crucial role in development of uterine leiomyoma.
The present study aims at studying any possible association between angiotensin converting enzyme (I/D) and angiotensin II type I receptor (A1166C) gene polymorphisms and uterine leiomyomas among Egyptian females.
| Material and Methods | ▴Top |
The study was carried out on 124 Egyptian females divided into 70 patients diagnosed as having uterine leiomyomas and 54 matched control females. To all studied subjects, detailed history taking, thorough physical examination, preoperative pelvic ultrasonography and postoperative histopathological examination of the tumor tissue were done for the patient group only.
The study was conducted after the acceptance of the Ethical Committee of the Medical Research Institute, University of Alexandria; an informed consent was taken from all subjects included in this study before its start.
Molecular studies for the detection of I/D polymorphism of ACE gene and A1166C polymorphism in AT1R gene
For molecular studies, whole blood samples were collected in sterile tripotassium ethylene diamine tetraacetic acid 3 (K + EDTA) vacutainer tubes [29, 30]. DNA was extracted from peripheral blood leucocytes from the freshly withdrawn whole blood, using GeneJET™ Genomic DNA Purification Kit, Fermentas [31]. The purity and concentration of extracted genomic DNA were determined using the Thermo Scientific NanoDrop™ 1000 Spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA).
Genotyping for the A1166C gene polymorphism of AT1R gene
Polymerase chain reaction and restriction fragment length polymorphism (PCR/RFLP) technique was used to detect A1166C gene polymorphism of AT1R gene. The specific primers used were from AccuOligo®, Bioneer, Korea [32, 33]: forward primer: (5’-AAT GCT TGT AGC CAA AGT CAC CT-3’) and reverse primer: (5’-GGC TTT GCT TTG TCT TGT TG -3’).
PCR amplification of the extracted DNA was done on the S-96 thermal cycler (Quanta Biotech, UK) according to the protocol shown in Table 1.
 Click to view | Table 1. Protocol of PCR Amplification for Genotyping of the A1166C Gene |
The PCR product was digested using 0.2 μL (2 U) of a restriction endonuclease, Dde I, at 37 °C (Dde I restriction endonuclease (cat. #R6291), 200 units (10 U/µL), Promega, USA) for 1 - 4 h, which cuts the product into two pieces, 600 bp and 250 bp long [34]. An additional DdeI recognition site is created in the C-type variant at nucleotide 1166, which is located within the 250 bp fragment. Digestion products were resolved by electrophoresis in a 2% agarose gel to detect (A) and (C) alleles (Fig. 1). Thus, the homozygote CC variant produced three bands (600, 140 and 110 bp long), the homozygote AA variant produced two bands (600 and 250 bp long), and the heterozygote AC variant produced all four bands (Fig. 2).
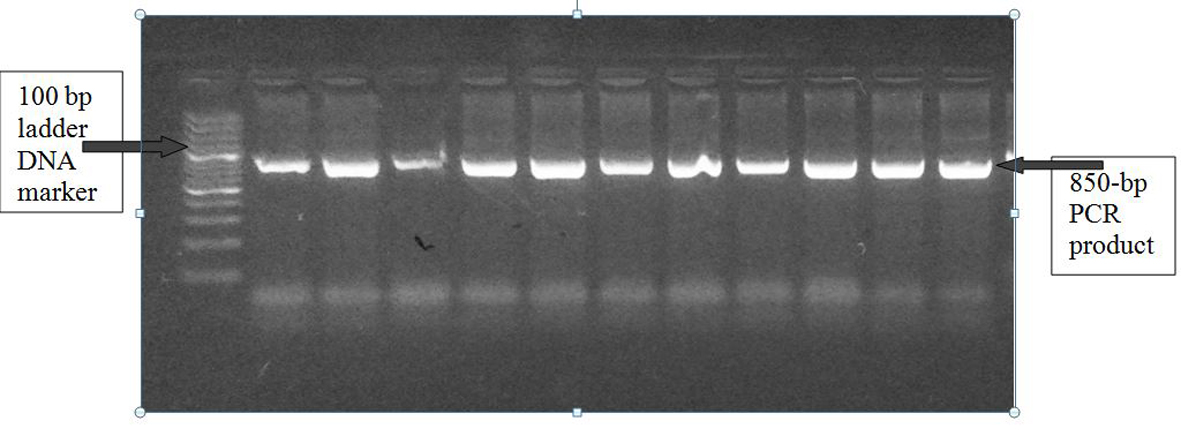 Click for large image | Figure 1. A1166C polymorphism of AGTR1 gene: PCR product on 2% agarose gel at 850 bp with a 1,000 bp ladder DNA marker. |
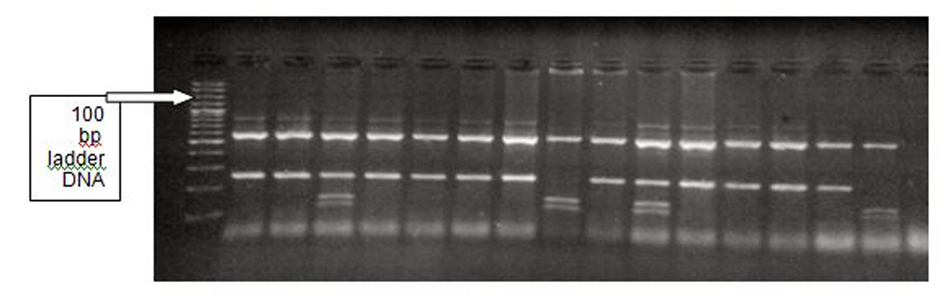 Click for large image | Figure 2. A1166C polymorphism of AGTR1 gene: Dde I digestion of the PCR product. Left to right: lane 1 (DNA ladder), lanes 2-3 (AA), lane 4 (AC), lanes 5-8 (AA), lane 9 (CC), lane 10 (AA), lane 11 (AC), lanes 12-15 (AA), lane 16 (CC). |
Genotyping for I/D polymorphism of ACE gene
A set of primers flanking the insertion region were used for the detection of I/D polymorphism in intron 16 of ACE gene through PCR [35, 36]: forward primer: (5’-CTG GAG ACC ACT CCC ATC CTT TCT-3’) and reverse primer: (5’-GAT GTG GCC ATC ACA TTC GTC AGA T-3’) from AccuOligo®, Bioneer, Korea.
PCR was done on the S-96 thermal cycler (Quanta Biotech, UK) according to the protocol shown in Table 2.
The above method has a 5-10% mistyping error, causing preferred amplification of the D allele depicting I/D heterozygotes as D/D carriers. Therefore, all samples found to be DD after amplification with the conventional primers were reamplified using primer pair recognized insertion-specific sequences from AccuOligo®, Bioneer, Korea: forward primer: (5’-TGG GAC CAC AGC GCC CGC CAC TAC-3’) and reverse primer: (5’-TCG CCA GCC CTC CCA TGC CCA TAA-3’).
 Click to view | Table 2. Protocol of PCR Amplification for Genotyping of I/D Polymorphism of ACE Gene |
The PCR products were applied on a 2% agarose gel. Bands were detected using ethidium bromide staining under UV light. A 190 bp fragment was found in the absence of an insertion (D) and a 490 bp fragment in the presence of insertion (I). Thus, the homozygote DD produced one band (190 bp long), the homozygote II produced one band (480 bp long), and the heterozygote ID produced both bands (190 bp and 480 bp long) (Fig. 3).
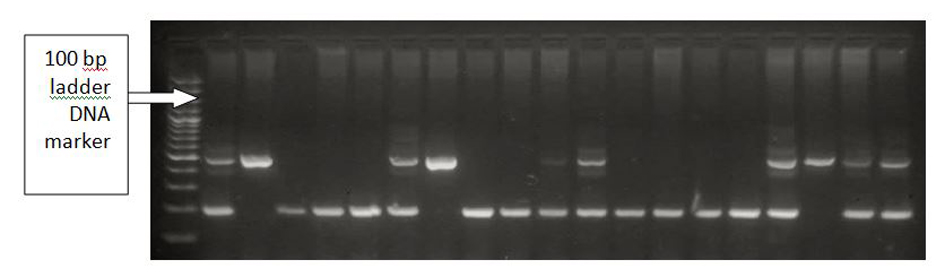 Click for large image | Figure 3. Insertion/deletion polymorphism run on 2% agarose. Left to right: lane 1 (DNA ladder), lane 2 (ID), lane 3 (II), lanes 4-6 (DD), lane 7 (ID), lane 8 (II), lane 7 (AA), lanes 9-11 (DD), lane 12 (ID), lanes 13-16 (DD), lane 17 (ID), lane 19 (II), lanes 20-21 (ID). |
The Chi-square test (χ2 test) with a Monte Carlo estimate of the exact P value was used to compare proportions of nominal clinical data variables between patient and control groups. Odds ratio (OR) was used to measure the effect of +A1166C AT1R SNP and ACE I/D polymorphism genotypes or alleles on the risk of developing fibroids.
| Results | ▴Top |
Genotype and allele frequencies of the A1166C polymorphism of the AT1R gene
The genotype distribution patterns of +A1166C SNP of AT1R gene among control group (AA = 40, AC = 14, CC = 0) and among fibroid patient group (AA = 39, AC = 25, CC = 6) were both in agreement with Hardy-Weinberg equilibrium (P = 0.273 and P = 0.494 respectively).
The difference in the genotype frequencies of +A1166C SNP of AT1R gene in both groups revealed a statistically significant difference (P = 0.028) between fibroid patients and controls, where the fibroid patients had a higher frequency of the homo-mutant genotype CC than controls (six patients (8.6%) versus nil among control subjects (0%)), a higher frequency of the hetero-mutant genotype “AC” controls (35.7% versus 25.9%) and a lower frequency of the Homo-wild genotype “AA” than controls (55.7% versus 74.1%) (Table 3).
 Click to view | Table 3. Comparison of AT1R +A1166C SNP Genotype Frequencies Between Fibroid Patients and Controls |
There was also a statistically significant difference (P = 0.011) in the allelic frequencies of human AT1R gene +A1166C between fibroid patients and controls (Table 4), where the patients had a higher frequency of the mutant allele “C” than controls (26.4% versus 13%), and a lower frequency of the wild allele “A” than controls (73.6% versus 87%), resulting in an overall OR of 2.227 (95% CI: 1.149 - 4.319).
From these results, it could be concluded that the mutant allele “C” was associated with a statistically significant increased risk of developing uterine fibroids.
 Click to view | Table 4. Comparison of AT1R +A1166C SNP Allele Frequencies Between Fibroid Patients and Controls |
Genotype and allele frequencies of the I/D polymorphism of ACE gene
Regarding I/D polymorphism of ACE gene, the control group, patient group as well as the overall sample were all in agreement with Hardy-Weinberg equilibrium (P = 0.25, P = 0.25 and P = 0.10).
Comparing I/D polymorphism of ACE gene genotype frequencies in both groups revealed absence of a statistically significant difference (P = 0.752) between fibroid patients and controls (Table 5). Over and above, there was also no statistically significant difference (P = 0.4978) in the allelic frequencies of human ACE gene I/D between fibroid patients and controls (Table 6, Fig. 4).
 Click to view | Table 5. Comparison of ACE I/D Polymorphism Genotype Frequencies Between Fibroid Patients and Controls |
 Click to view | Table 6. Comparison of ACE I/D Polymorphism Allele Frequencies Between Fibroid Patients and Controls |
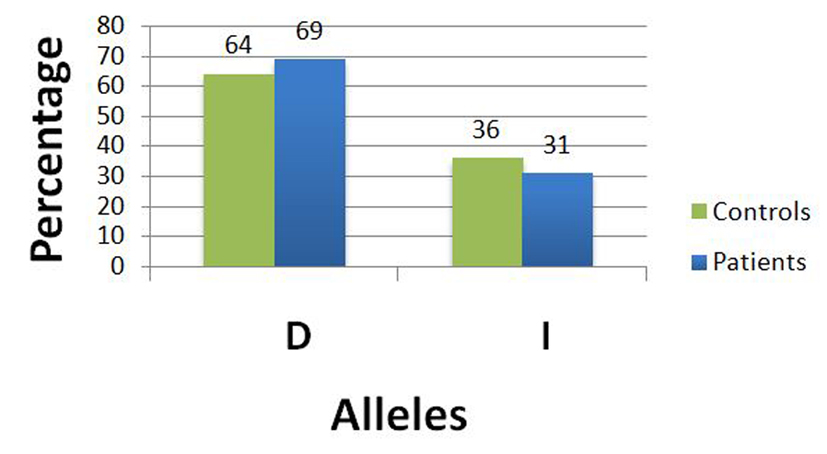 Click for large image | Figure 4. Frequency of both alleles of I/D polymorphism in controls and patients. |
| Discussion | ▴Top |
Regarding the +A1166C SNP of AT1R gene, we found in our study that both genotype and allelic frequencies revealed a statistically significant difference (P = 0.028, P = 0.011 respectively) between fibroid patients and controls (Table 3, 4).
In 2007, Isobe et al [26] investigated the role of Ang II in the proliferation of rat ELT-3 leiomyoma cells (Eker rat uterine leiomyoma-derived smooth muscle cells) in vitro. They found that Ang II significantly induced ELT-3 leiomyoma cell proliferation and the expression of AT1R and AT2R mRNA and protein was confirmed. These experimental findings in vitro highlight the potential role of Ang II, through AT1R in the proliferation of leiomyoma cells.
In 2010, Isobe et al reinvestigated the presence of complex regulation of Ang II and aldosterone-induced leiomyoma cell proliferation. They found that aldosterone as well could potentiate the Ang II signaling pathway through upregulating levels of AT1R mRNA [37].
These data highlight the importance of AT1R in the development of uterine fibroids and justify the above results of the present study. Since the +A1166C SNP of the AT1R gene is known to increase the expression of AT1R, it is reasonable to conclude that it is involved in the proliferation of uterine leiomyoma. Accordingly, the mutant allele “C” which is associated with increased transcription of AT1R is associated with an increased risk of developing uterine fibroids. To our knowledge, this is the first study to investigate the association between this polymorphism and uterine fibroids.
Comparing I/D polymorphism of ACE gene, both genotype and allelic frequencies revealed absence of a statistically significant difference between fibroid patients and controls (P = 0.752, P = 0.4978, respectively).
Hsieh et al [38] studied the association of ACE I/D polymorphism with uterine fibroids. Their results were contradictory to the present study. They concluded that ACE I/D gene polymorphism is associated with leiomyoma susceptibility. The II genotype was significantly higher in fibroid patients while the ID and DD genotypes were lower in the controls (P = 0.08). The I allele was significantly increased in fibroid patients while the D allele was higher in controls than the fibroid patients (P = 0.048). The contradiction with the present study may be attributed to differences in ethnic groups, environmental factors, sample size, recruitment procedures of the study population as well as exclusion criteria. Also, they could not explain how the “I” allele which is related to lower levels of ACE could be involved in the pathogenesis of uterine fibroids.
In light of the above, The A1166C polymorphism, which finally leads to increased expression of the receptor AT1R, was found to be associated with uterine fibroids. However, ACE I/D polymorphism, which leads to increased levels of ACE and subsequently increased levels of Ang II, was not found to be associated with uterine fibroids. This could be partly explained through “ligand independent AT1R activation” phenomenon. As previously explained, Ang II is not essential for the AT1R activation which could occur in spite of low levels of Ang II. Therefore, it could be suggested that the A1166C polymorphism of AT1R could be more important for uterine fibroid development than ACE I/D polymorphism.
| References | ▴Top |
- Wegienka G, Baird DD, Hertz-Picciotto I, Harlow SD, Steege JF, Hill MC, Schectman JM, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101(3):431-437.
doi - Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Hum Reprod Update. 2008;14(3):259-274.
doi pubmed - Somigliana E, Vercellini P, Daguati R, Pasin R, De Giorgi O, Crosignani PG. Fibroids and female reproduction: a critical analysis of the evidence. Hum Reprod Update. 2007;13(5):465-476.
doi pubmed - Di Lieto A, De Falco M, Pollio F, Mansueto G, Salvatore G, Somma P, Ciociola F, et al. Clinical response, vascular change, and angiogenesis in gonadotropin-releasing hormone analogue-treated women with uterine myomas. J Soc Gynecol Investig. 2005;12(2):123-128.
doi pubmed - Boehm KD, Daimon M, Gorodeski IG, Sheean LA, Utian WH, Ilan J. Expression of the insulin-like and platelet-derived growth factor genes in human uterine tissues. Mol Reprod Dev. 1990;27(2):93-101.
doi pubmed - Strawn EY, Jr., Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172(6):1837-1843; discussion 1843-1834.
- Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251-287.
doi pubmed - Elton TS, Sansom SE, Martin MM. Cardiovascular Disease, Single Nucleotide Polymorphisms; and the Renin Angiotensin System: Is There a MicroRNA Connection? Int J Hypertens. 2010;2010.
- Roth TM, Klett C, Cowan BD. Expression profile of several genes in human myometrium and uterine leiomyoma. Fertil Steril. 2007;87(3):635-641.
doi pubmed - Fujita M, Hayashi I, Yamashina S, Itoman M, Majima M. Blockade of angiotensin AT1a receptor signaling reduces tumor growth, angiogenesis, and metastasis. Biochem Biophys Res Commun. 2002;294(2):441-447.
doi - Hortobagyi GN. Toward individualized breast cancer therapy: translating biological concepts to the bedside. Oncologist. 2012;17(4):577-584.
doi pubmed - Herr D, Rodewald M, Fraser HM, Hack G, Konrad R, Kreienberg R, Wulff C. Potential role of Renin-Angiotensin-system for tumor angiogenesis in receptor negative breast cancer. Gynecol Oncol. 2008;109(3):418-425.
doi pubmed - Rocken C, Rohl FW, Diebler E, Lendeckel U, Pross M, Carl-McGrath S, Ebert MP. The angiotensin II/angiotensin II receptor system correlates with nodal spread in intestinal type gastric cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1206-1212.
doi pubmed - Kosugi M, Miyajima A, Kikuchi E, Horiguchi Y, Murai M. Angiotensin II type 1 receptor antagonist candesartan as an angiogenic inhibitor in a xenograft model of bladder cancer. Clin Cancer Res. 2006;12(9):2888-2893.
doi pubmed - Uemura H, Hasumi H, Ishiguro H, Teranishi J, Miyoshi Y, Kubota Y. Renin-angiotensin system is an important factor in hormone refractory prostate cancer. Prostate. 2006;66(8):822-830.
doi pubmed - Amaya K, Ohta T, Kitagawa H, Kayahara M, Takamura H, Fujimura T, Nishimura G, et al. Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int J Oncol. 2004;25(4):849-856.
pubmed - Watanabe Y, Shibata K, Kikkawa F, Kajiyama H, Ino K, Hattori A, Tsujimoto M, et al. Adipocyte-derived leucine aminopeptidase suppresses angiogenesis in human endometrial carcinoma via renin-angiotensin system. Clin Cancer Res. 2003;9(17):6497-6503.
pubmed - Suganuma T, Ino K, Shibata K, Kajiyama H, Nagasaka T, Mizutani S, Kikkawa F. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11(7):2686-2694.
doi pubmed - Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E, Tiret L, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24(1):63-69.
doi pubmed - Tiret L, Bonnardeaux A, Poirier O, Ricard S, Marques-Vidal P, Evans A, Arveiler D, et al. Synergistic effects of angiotensin-converting enzyme and angiotensin-II type 1 receptor gene polymorphisms on risk of myocardial infarction. Lancet. 1994;344(8927):910-913.
doi - Mendizabal-Ruiz AP, Morales J, Castro Martinez X, Gutierrez Rubio SA, Valdez L, Vasquez-Camacho JG, Sanchez Corona J, et al. RAS polymorphisms in cancerous and benign breast tissue. J Renin Angiotensin Aldosterone Syst. 2011;12(2):85-92.
doi pubmed - Sierra Diaz E, Sanchez Corona J, Rosales Gomez RC, Gutierrez Rubio SA, Vazquez Camacho JG, Solano Moreno H, Moran Moguel MC. Angiotensin-converting enzyme insertion/deletion and angiotensin type 1 receptor A1166C polymorphisms as genetic risk factors in benign prostatic hyperplasia and prostate cancer. J Renin Angiotensin Aldosterone Syst. 2009;10(4):241-246.
doi pubmed - Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86(4):1343-1346.
doi pubmed - Freitas-Silva M, Pereira D, Coelho C, Bicho M, Lopes C, Medeiros R. Angiotensin I-converting enzyme gene insertion/deletion polymorphism and endometrial human cancer in normotensive and hypertensive women. Cancer Genet Cytogenet. 2004;155(1):42-46.
doi pubmed - Medeiros R, Vasconcelos A, Costa S, Pinto D, Lobo F, Morais A, Oliveira J, et al. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J Pathol. 2004;202(3):330-335.
doi pubmed - Isobe A, Takeda T, Sakata M, Miyake A, Yamamoto T, Minekawa R, Nishimoto F, et al. Dual repressive effect of angiotensin II-type 1 receptor blocker telmisartan on angiotensin II-induced and estradiol-induced uterine leiomyoma cell proliferation. Hum Reprod. 2008;23(2):440-446.
doi pubmed - Boynton-Jarrett R, Rich-Edwards J, Malspeis S, Missmer SA, Wright R. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol. 2005;161(7):628-638.
doi pubmed - Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29(9):1675-1684.
doi pubmed - Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. Int J Oral Maxillofac Surg. 2007;36(5):391-394.
doi pubmed - Sinorita H, Madiyan M, Pramono RB, Purnama LB, Ikhsan MR, Asdie AH. ACE gene insertion/deletion polymorphism among patients with type 2 diabetes, and its relationship with metabolic syndrome at Sardjito Hospital Yogyakarta, Indonesia. Acta Med Indones. 2010;42(1):12-16.
pubmed - Lee JH, Park Y, Choi JR, Lee EK, Kim HS. Comparisons of three automated systems for genomic DNA extraction in a clinical diagnostic laboratory. Yonsei Med J. 2010;51(1):104-110.
doi pubmed - Antonio de Araujo M, Menezes BS, Lourenco C, Elisangela Rosa Cordeiro, Renata Rispoli Gatti, Luiz Ricardo Goulart. The A1166C Polymorphism of the Angiotensin II Type-1 Receptor in Acute Myocardial Infarction. Arquivos Brasileiros de Cardiologia. 2004;83(5):409-413.
- Lapierre AV, Arce ME, Lopez JR, Ciuffo GM. Angiotensin II type 1 receptor A1166C gene polymorphism and essential hypertension in San Luis. Biocell. 2006;30(3):447-455.
pubmed - Frishberg Y, Becker-Cohen R, Halle D, Feigin E, Eisenstein B, Halevy R, Lotan D, et al. Genetic polymorphisms of the renin-angiotensin system and the outcome of focal segmental glomerulosclerosis in children. Kidney Int. 1998;54(6):1843-1849.
doi pubmed - Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Kelten C, Erdem E. Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J Exp Med. 2006;210(2):109-116.
doi pubmed - Shanmugam V, Sell KW, Saha BK. Mistyping ACE heterozygotes. PCR Methods Appl. 1993;3(2):120-121.
doi pubmed - Isobe A, Takeda T, Wakabayashi A, Tsuiji K, Li B, Sakata M, Yaegashi N, et al. Aldosterone stimulates the proliferation of uterine leiomyoma cells. Gynecol Endocrinol. 2010;26(5):372-377.
doi pubmed - Hsieh YY, Lee CC, Chang CC, Wang YK, Yeh LS, Lin CS. Angiotensin I-converting enzyme insertion-related genotypes and allele are associated with higher susceptibility of endometriosis and leiomyoma. Mol Reprod Dev. 2007;74(7):808-814.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
