| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 2, June 2015, pages 212-216
A Study of Various Factors Influencing Fetal Scalp Lactate and Their Correlation With Composite Fetal and Neonatal Outcomes
Kylie Edwardsa, b, d, Alka Kotharib, c, Joel M. Dulhuntyb, c
aDepartment of Obstetrics and Gynaecology, Caboolture Hospital, Queensland, Australia
bSchool of Medicine, The University of Queensland, Brisbane, Queensland, Australia
cRedcliffe Hospital, Brisbane, Queensland, Australia
dCorresponding Author: Kylie Edwards, Department of Obstetrics and Gynaecology, Caboolture Hospital, McKean St, Caboolture, 4510, Queensland, Australia
Manuscript accepted for publication May 21, 2015
Short title: Factors Influencing Fetal Scalp Lactate
doi: http://dx.doi.org/10.14740/jcgo329w
| Abstract | ▴Top |
Background: Fetal scalp lactate has been shown to be as effective as fetal scalp pH in predicting neonatal outcomes. Maternal-fetal factors influencing variability in fetal scalp lactate have not been fully explored. This study aims to explore the association of gestational age with fetal scalp lactate and examine whether existing thresholds are predictive of adverse outcomes.
Methods: A retrospective study of all singleton births with a fetal scalp lactate taken during labor at a public teaching hospital between July 1, 2007 and June 1, 2013 was performed. Descriptive, bivariate and multivariate analysis was used to explore the association between fetal scalp lactate and other variables of interest.
Results: A total of 326 patients with fetal scalp lactate values during labor were studied. Fetal scalp lactate was not associated with gestational age (Spearman’s rho = -0.006, P = 0.92). A fetal scalp lactate ≥ 4.8 mmol/L was associated with maternal age (P = 0.049) and time in labor (P = 0.001). Fetal scalp lactate was strongly associated with a combined outcome variable that included emergency operative delivery (OR = 1.90; 95% CI: 1.44 - 2.51; P < 0.001). There was no significant association with a poor combined fetal and neonatal outcome when emergency intervention was excluded (OR = 1.11; 95% CI: 0.93 - 1.25; P = 0.092).
Conclusions: There was no significant correlation between fetal scalp lactate and gestational age; further exploration of the association with maternal age is warranted. A raised fetal scalp lactate is associated with progression to emergency operative delivery.
Keywords: Fetal academia; Fetal distress; Fetal scalp blood microsampling; Gestational age; labor
| Introduction | ▴Top |
Since its introduction in the 1960s, electronic fetal monitoring has played an important role in intrapartum monitoring of fetal wellbeing. It has long been established that while electronic fetal monitoring is highly sensitive, its specificity for fetal wellbeing is poor [1]. Measurement of pH by fetal blood sampling has been in use as a more specific measure of wellbeing since first described by Saling in 1962 [2]. However, obtaining fetal scalp pH requires a relatively large amount of blood (30 - 50 µL) making it technically challenging and prone to a high failure rate. In addition, fetal scalp pH cannot differentiate between metabolic acidosis and the more benign respiratory acidosis [3].
A number of studies have shown measurement of fetal scalp lactate to be a reliable alternative to scalp pH for predicting intrapartum hypoxia [4-8], including a recent multicentre randomized controlled trial [9] and Cochrane review [10]. These studies have shown no significant differences in neonatal outcomes when fetal monitoring with scalp pH and scalp lactate are compared. Requiring only 5 µL of blood, fetal scalp lactate is technically easier to perform than fetal scalp pH and has a lower sampling failure rate when the two methods are compared (1.2% vs. 10.4%, respectively) [9]. Use of scalp lactate in fetal surveillance is considered to be an acceptable alternative to scalp pH by the Royal Australian and New Zealand College of Obstetricians and Gynaecologists [1]. Standard threshold values suggestive of fetal hypoxia, ≥ 4.8 mmol/L for scalp lactate and ≤ 7.2 for scalp pH, are commonly used as an indication for operative delivery [11].
Despite the widespread use of fetal scalp lactate in fetal surveillance, the degree to which maternal-fetal factors influence variability in fetal scalp lactate has not been fully explored [10]. Most studies conducted so far have looked at lactate levels and outcomes of populations as a whole, with limited studies exploring potential factors that may be associated with lactate variability. One Swedish study has evaluated whether there is a difference in scalp lactate level by fetal size for gestational age and observed a higher median lactate concentration in small for gestational age fetuses, but no subgroup differences in outcomes for fetuses with a scalp lactate ≥ 4.8 mmol/L [12]. Nordstrom et al have compared fetal scalp lactate levels with duration of labor, demonstrating that scalp lactate rises in the second stage of labor by a mean of 0.032 mmol/L/min [13]. They did not, however, demonstrate a significant correlation between duration of the first stage of labor and fetal scalp lactate concentration [13].
Another possible factor that could influence fetal scalp lactate concentration is gestational age. It has been shown that umbilical cord lactate levels increase with increasing gestation [14]. A strong correlation between lactate levels in arterial cord blood and fetal scalp blood has also been demonstrated [15]. Given this association, it is likely that fetal scalp lactate would also increase with gestational age. However, so far, the relationship between fetal scalp lactate and gestational age has not been directly studied [10].
The aim of this study was to explore the association of factors such as maternal age, parity, birth weight, time in labor and gestational age with fetal scalp lactate in women who had fetal scalp lactate performed during labor for an abnormal cardiotocograph (CTG). A secondary goal was to explore whether a high fetal scalp using existing cut-off thresholds was predictive of common markers of adverse fetal or neonatal outcomes.
| Materials and Methods | ▴Top |
This study was conducted at Redcliffe Hospital, a 250-bed outer metropolitan public teaching hospital in Queensland, Australia, with 1,400 - 1,800 deliveries per year during the study period. A retrospective observational study was performed using data routinely collected in an obstetric clinical information system. All women who presented to Redcliffe Hospital in labor and had a fetal scalp lactate performed between July 1, 2007 and June 1, 2013 were included. If more than one lactate was performed, the last collected value measured was used. Women with multiple gestations or a non-cephalic presentation were excluded. The patient records were individually reviewed for any information not available via the clinical information system. Institutional ethics approval was obtained and the need for individual participant consent was waived.
The decision to obtain a fetal scalp lactate sample is based on abnormal CTG findings during labor. After obtaining verbal consent from the patient, she is placed in either lithotomy or left lateral position. An amnioscope is passed and the fetal scalp is cleaned to remove maternal blood or liquor. A small amount (5 µL) of fetal blood is obtained in a capillary tube then placed on a test strip and read by the Lactate Pro meter (Arkray, Kyoto, Japan). A lactate of 4.8 mmol/L or greater is considered an indication for intervention.
Gestational age was determined by the nuchal translucency scan, if available, or the earliest available dating ultrasound scan. Gestational age was recorded in completed weeks. Patients were categorized as nulliparous or multiparous. Maternal age, gestational age, birth weight and time in labor were recorded as a continuous variable. A dichotomous combined fetal outcome endpoint was defined as presence of one or more of the following: umbilical artery pH ≤ 7.20, base excess ≤ -12, Apgar score ≤ 7 at 1 or 5 min, presence of meconium, emergency operative delivery for fetal distress or special care nursery admission. Apgar scores at 1 and 5 min were rated by the attending pediatrician or midwife at birth. The midwife or obstetrician, who also determined the presence of meconium, collected paired umbilical artery and venous cord blood samples.
Statistical analysis
Descriptive, bivariate and multivariate analysis was used to explore the association between fetal scalp lactate and other variables of interest. A scatter plot and Spearman’s rank test were used to evaluate the association between fetal scalp lactate and continuous variables, such as gestational age and fetal scalp pH. The association of fetal scalp lactate with categorical variables of interest, such as parity, was evaluated using a Mann-Whitney U test or Kruskal-Wallis H test. Logistic regression was used to explore the association of fetal scalp lactate and other variables of interest with the combined outcome variable as the dependent variable. Backward stepwise regression was used to identify the simplest model. Goodness-of-fit was assessed by the Hosmer and Lemeshow statistic and the Nagelkerke R2 index. A two-sided P value < 0.05 was considered statistically significant in all analyses. An a priori sample size of 328 patients was estimated as being required to detect a difference of 0.6 mmol/L between groups using a non-parametric test with alpha of 0.05 and power of 80%. Statistical analysis was conducted using SPSS version 20 (IBM, Armonk, NY).
| Results | ▴Top |
Study population
Lactate values were obtained for 409 patients during the study period. Of these, 83 patients were excluded due to incomplete or missing arterial blood gas values (umbilical cord pH and/or base excess), resulting in 326 patients suitable for inclusion. There were no fetal scalp lactate measurements performed on patients with multiple gestations or non-cephalic presentations during the study period. Demographic characteristics are summarized in Table 1.
 Click to view | Table 1. Demographic Characteristics for Study Population |
Association between fetal scalp lactate and variables of interest
Fetal scalp lactate was associated with cord pH (Spearman’s rho = -0.13, P = 0.024), but not gestational age in bivariate analysis (Fig. 1; Spearman’s rho = -0.006, P = 0.92). The association of baseline variables of interest with scalp lactate ≥ 4.8 mmol/L is summarized in Table 1.
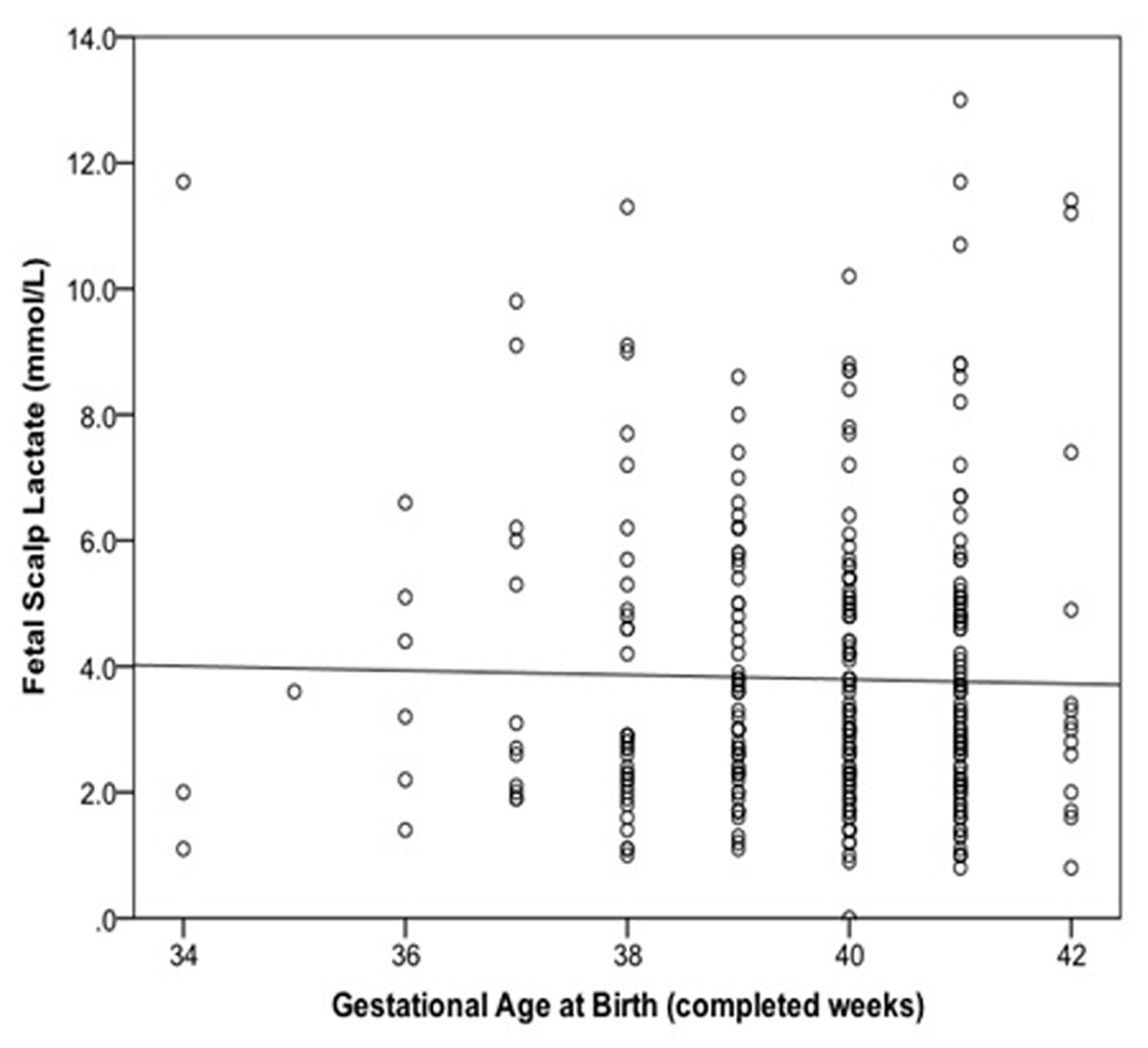 Click for large image | Figure 1. Relationship between fetal scalp lactate and gestational age at birth with linear regression line added. |
Association of fetal scalp lactate with negative fetal and neonatal outcomes
Table 2 shows the association of fetal scalp lactate with individual fetal and neonatal outcomes. Fetal scalp lactate was a significant predictor of the combined outcome variable in bivariate analysis (OR = 1.90; 95% CI: 1.44 - 2.51; P < 0.001). However, this association was not significant when operative delivery for fetal distress was excluded from the combined outcome measure (OR = 1.11; 95% CI: 0.93 - 1.25; P = 0.092).
 Click to view | Table 2. Association of Fetal Scalp Lactate With Fetal and Neonatal Outcomes |
Multivariate predictors of combined negative fetal and neonatal outcome
In multivariate analysis, fetal scalp lactate was an independent predictor of the combined outcome variable that included emergency operative delivery (Table 3). When this was excluded, fetal scalp lactate was no longer predictive of a combined negative fetal and neonatal outcome (Table 4). There were no significant predictors of the combined negative fetal and neonatal outcome when operative delivery for fetal distress was excluded.
 Click to view | Table 3. Logistic Regression Analysis With Combined Fetal and Neonatal Outcome as Dependent Variable |
 Click to view | Table 4. Logistic Regression Analysis With Combined Fetal and Neonatal Outcome (Excluding Operative Delivery for Fetal Distress) as Dependent Variable |
| Discussion | ▴Top |
This retrospective cohort study found a significant association between maternal age and fetal scalp lactate in fetuses with an abnormal CTG. Time in labor was also significantly associated with fetal scalp lactate; gestational age, birth weight and parity were not. While there was a significant correlation between the combined outcome variable and fetal scalp lactate, this appears to be primarily due to the strong association between operative delivery for fetal distress and fetal scalp lactate. The association between fetal scalp lactate and operative delivery confirms that local guidelines to proceed to an emergency operative intervention when scalp lactate is ≥ 4.8 mmol/L are followed.
The absence of correlation between fetal scalp lactate and gestational age suggests that the current threshold of 4.8 mmol/L is suitable for use in fetuses between the gestational ages of 34 - 42 completed weeks. It should, however, be noted that the study was limited to fetuses with an abnormal CTG. A larger sample of fetal scalp lactates from fetuses with and without CTG signs of distress would be required to determine if there was a general rise in scalp lactate with gestational age, similar to what has been seen with umbilical cord lactate [14]. Nevertheless, our results support the notion that there is no clinically significant rise in scalp lactate for gestational ages from 37 to 42 weeks, which comprised 77% of our population.
Comparison of other variables that may affect fetal scalp lactate showed a significant difference by time in labor, with a shorter labor time more likely to have a fetal scalp lactate ≥ 4.8 mmol/L (P = 0.001). This finding, however, may arise from high scalp lactate being an indication for prompt delivery. There was also a significant correlation between increased maternal age and fetal scalp lactate ≥ 4.8 mmol/L (P = 0.049). Poorer neonatal outcomes, such as preterm delivery, low birth weight and fetal or neonatal death, are known to be associated with increasing maternal age [16], which may have contributed to our finding. However, the lack of an association between maternal age and the combined outcome variable in multivariate analysis does not support this conclusion. To our knowledge, the relationship between fetal scalp lactate and maternal age has not been previously identified and warrants further investigation.
We found that lower (≤ 7) Apgar scores at 1 and 5 min were significantly associated with a fetal scalp lactate level ≥ 4.8 mmol/L (OR = 2.2 and 4.4, respectively). This supports the previous findings by Kruger et al [7]. In contrast, Ramanah et al [4] and Borruto et al [17] did not find a similar association; however, the total number of neonates with Apgar scores < 7 was low in both studies (1.5-6.4%).
Several limitations of this study deserve mention. This study was limited by its retrospective nature. The presence of unmeasured confounding factors may have influenced the study results. While the study was powered for a combined outcome variable, the small number of participants experiencing less common, but clinically significant, outcome events limits the ability to draw conclusions for individual outcomes. The association between umbilical artery lactate and fetal scalp lactate would have been useful to explore as umbilical artery lactate has been shown to increase with gestational age. However, umbilical artery lactate was not routinely tested during the study period.
Our results confirm that a raised fetal scalp lactate is clinically used to proceed to immediate operative intervention. Fetal scalp lactate levels in fetuses with abnormal CTGs are not significantly associated with gestational age, birth weight, parity or time in labor. Fetuses with scalp lactate levels ≥ 4.8 mmol/L are more likely to have Apgar scores of ≤ 7 at 1 and 5 min in this population. A possible relationship between maternal age and fetal scalp lactate has been identified and warrants further investigation.
Acknowledgement
The authors wish to thank Meredith Shallcross for her assistance with data collection.
Conflicts of Interest
The authors have no conflicts of interest to declare.
| References | ▴Top |
- RANZCOG Intrapartum Fetal Surveillance Clinical Guidelines. Second Edition. The Royal Australian and New Zealand College of Obstetricians and Gynaecologists.
- Bretscher J, Saling E. pH values in the human fetus during labor. Am J Obstet Gynecol. 1967;97(7):906-911.
pubmed - Nordstrom L. Fetal scalp and cord blood lactate. Best Pract Res Clin Obstet Gynaecol. 2004;18(3):467-476.
doi pubmed - Ramanah R, Martin A, Clement MC, Maillet R, Riethmuller D. Fetal scalp lactate microsampling for non-reassuring fetal status during labor: a prospective observational study. Fetal Diagn Ther. 2010;27(1):14-19.
doi pubmed - Heinis AM, Spaanderman ME, Gunnewiek JM, Lotgering FK. Scalp blood lactate for intra-partum assessment of fetal metabolic acidosis. Acta Obstet Gynecol Scand. 2011;90(10):1107-1114.
doi pubmed - Ridenour RV, Gada RP, Brost BC, Karon BS. Comparison and validation of point of care lactate meters as a replacement for fetal pH measurement. Clin Biochem. 2008;41(18):1461-1465.
doi pubmed - Kruger K, Hallberg B, Blennow M, Kublickas M, Westgren M. Predictive value of fetal scalp blood lactate concentration and pH as markers of neurologic disability. Am J Obstet Gynecol. 1999;181(5 Pt 1):1072-1078.
doi - Westgren M, Kruger K, Ek S, Grunevald C, Kublickas M, Naka K, Wolff K, et al. Lactate compared with pH analysis at fetal scalp blood sampling: a prospective randomised study. Br J Obstet Gynaecol. 1998;105(1):29-33.
doi pubmed - Wiberg-Itzel E, Lipponer C, Norman M, Herbst A, Prebensen D, Hansson A, Bryngelsson AL, et al. Determination of pH or lactate in fetal scalp blood in management of intrapartum fetal distress: randomised controlled multicentre trial. BMJ. 2008;336(7656):1284-1287.
doi pubmed - East CE, Leader LR, Sheehan P, Henshall NE, Colditz PB. Intrapartum fetal scalp lactate sampling for fetal assessment in the presence of a non-reassuring fetal heart rate trace. Cochrane Database Syst Rev. 2010;(3):CD006174.
doi - Intrapartum Fetal Surveillance, Queensland Maternity and Neonatal Clinical Guidelines. August 2010 MN10.15-V3-R15.
- Holzmann M, Cnattingius S, Nordstrom L. Lactate production as a response to intrapartum hypoxia in the growth-restricted fetus. BJOG. 2012;119(10):1265-1269.
doi pubmed - Nordstrom L, Achanna S, Naka K, Arulkumaran S. Fetal and maternal lactate increase during active second stage of labour. BJOG. 2001;108(3):263-268.
doi pubmed - Wiberg N, Kallen K, Herbst A, Aberg A, Olofsson P. Lactate concentration in umbilical cord blood is gestational age-dependent: a population-based study of 17 867 newborns. BJOG. 2008;115(6):704-709.
doi pubmed - Kruger K, Kublickas M, Westgren M. Lactate in scalp and cord blood from fetuses with ominous fetal heart rate patterns. Obstet Gynecol. 1998;92(6):918-922.
doi - Jacquemyn Y, Martens E, Martens G. Pregnancy at late premenopausal age: outcome of pregnancies at 45 years and older in Flanders, Belgium. J Obstet Gynaecol. 2014;34(6):479-481.
doi pubmed - Borruto F, Comparetto C, Treisser A. Prevention of cerebral palsy during labour: role of foetal lactate. Arch Gynecol Obstet. 2008;278(1):17-22.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
