| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 4, Number 3, September 2015, pages 271-274
Methyldopa Hepatotoxicity: A Paradoxical High-Risk Pregnancy
Onameyore Utuamaa, c, Omofolarin Fasuyia, Gregory Strayhorna, Karla Lorraine Bookerb
aDepartment of Family Medicine, Morehouse School of Medicine, Atlanta, GA, USA
bGwinnett Medical Center, Lawrenceville, GA, USA
cCorresponding Author: Onameyore Utuama, Department of Family Medicine, Morehouse School of Medicine, 1513 E. Cleveland Ave. Bldg. 100 Ste. 300-A, East Point, GA 30344, USA
Manuscript accepted for publication June 18, 2015
Short title: Methyldopa Hepatotoxicity in Pregnancy
doi: http://dx.doi.org/10.14740/jcgo343w
| Abstract | ▴Top |
Alpha methyldopa is one of the preferred medications for hypertension control in pregnant women because of its low teratogenic profile. However, when pregnant women on this medication develop an acute or chronic liver damage, methyldopa is not thought to be responsible. At 18 weeks gestation of a high-risk pregnancy, a patient with chronic hypertension controlled on methyldopa 500 mg three times daily, presented with jaundice, dark-colored urine and elevated liver function enzymes. Following worsening symptoms, inconclusive radiological and negative immunological laboratory investigations, she was hospitalized and a diagnosis of drug-induced hepatotoxicity was confirmed with a liver biopsy. Her symptoms resolved with withdrawal of the offending medication and prednisone taper, which led to diabetes mellitus in pregnancy. Paradoxically, following the diagnosis and discontinuation of methyldopa, the patients’ blood pressure remained controlled off antihypertensives.
Keywords: Methyldopa; Pregnancy; Hepatotoxicity
| Introduction | ▴Top |
A handful of case reports on methyldopa-induced liver injury during pregnancy have been published which are similar to our case presentation, yet no report exists with the development of steroid-induced diabetes mellitus in pregnancy as a complication of this hepatotoxicity treatment. It has been 45 years since the first case of hepatotoxicity with methyldopa in pregnancy was reported by Elkington Smith [1]. Despite that, the adverse effect of methyldopa on the liver is still of concern as there are no stated guidelines on the monitoring of liver enzymes in people, especially pregnant women taking the medication for blood pressure (BP) control in hypertension. Researchers and clinicians have made suggestions for liver enzyme monitoring on patients taking methyldopa; however, very little effort has been made to enforce it [1]. The adverse effect of methyldopa is uncommon. Research by Slim et al in 2010 stated four confirmed cases in pregnant women, and a prevalence of 2.5-10% among non-pregnant women [1-3]. Delay in diagnosis of methyldopa liver injury given the low index of suspicion, contributes to missed diagnosis and less reported cases than expected.
This case report highlights the fact that adverse drug reactions (ADRs) to methyldopa are still occurring and chronic complications which could have been avoided arise.
| Case Report | ▴Top |
Our setting was a Community Health Center providing primary obstetric (OB) care to many high-risk and some low-risk patients. A known patient of our center, 38-year-old G5P0130, presented at gestational age of 18 weeks 4 days for routine OB visit with complaints of yellowness of the eyes, diarrhea and dark-colored urine of 1 week duration. Patient had been on hypertensive medications, lisinopril and hydrochlorothiazide before her current pregnancy. These medications were discontinued and methyldopa was started 12 weeks prior to presentation. Her pregnancy was complicated by previous three mid-trimester losses, cervical incompetence with McDonald’s cerclage at 12 weeks, preterm contractions on 17-hydroxyprogesterone, and being Rhesus negative. Other medical conditions include morbid obesity on restricted diet, obstructive sleep apnea treated with continuous positive airway pressure (CPAP) and depression treated with fluoxetine. Methyldopa was increased at 6 weeks gestation from 500 mg twice daily to three times daily for better BP control. She was single, lived alone with no history of alcohol or tobacco use. Her examination revealed a BP of 120s/80s with BMI 45.4 kg/m2. She was deeply icteric, had right upper quadrant abdominal tenderness, and a negative Murphy’s sign.
Her laboratory values included total bilirubin 18.2, aspartate transaminase (AST) 1,289, alanine transaminase (ALT) 1,103, alkaline phosphatase (ALP) 122, amylase 33, lipase 10, deoxycholic acid 1.5, chenodeoxycholic acid 153.3, viral hepatitis panel negative, and clotting profile normal.
Radiological testing revealed normal obstetrical ultrasound. An abdominal duplex ultrasound revealed changes consistent with hepatitis. When immunological results came back negative, the patient was referred to a hepatologist, who hospitalized her for a trans-abdominal ultrasound guided liver biopsy. The biopsy revealed lymphoplasmacytic portal inflammation with moderate to severe interface activity, lobular inflammation with frequent acidophil bodies and mild cholestasis consistent with autoimmune hepatitis versus autoimmune hepatitis-like injury (Fig. 1-3).
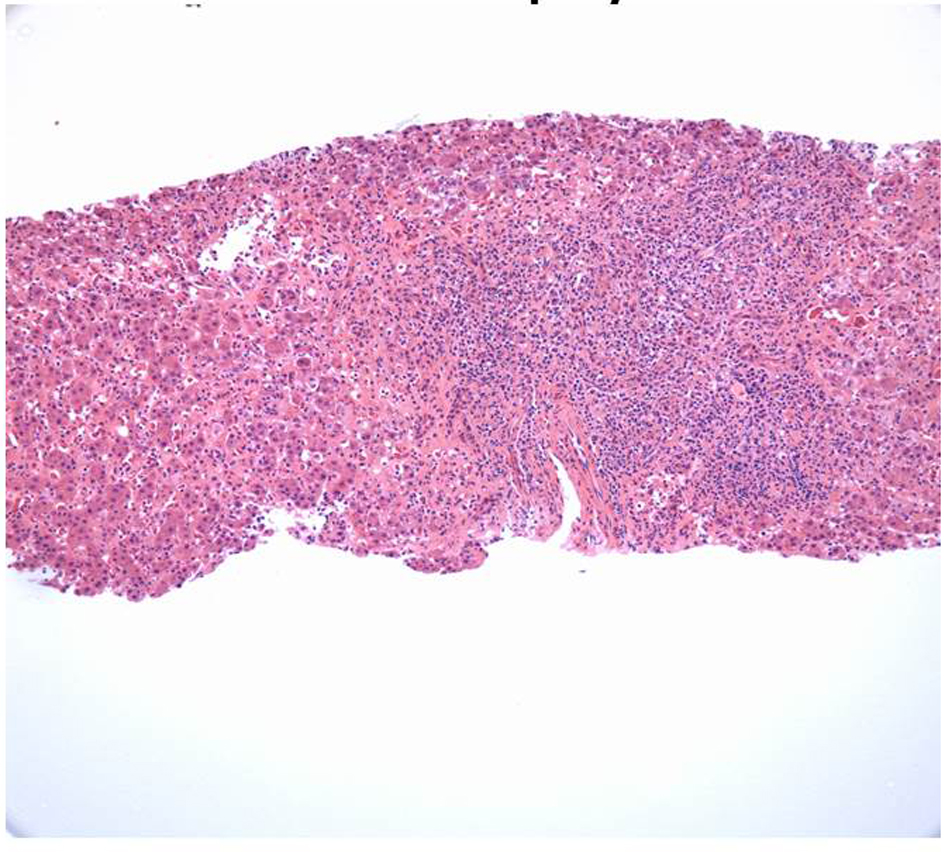 Click for large image | Figure 1. Liver biopsy (× 10 magnification): liver biopsy with heavy portal lymphoplasmacytic infiltrate, extensive interface hepatitis and hepatic lobular disarray. |
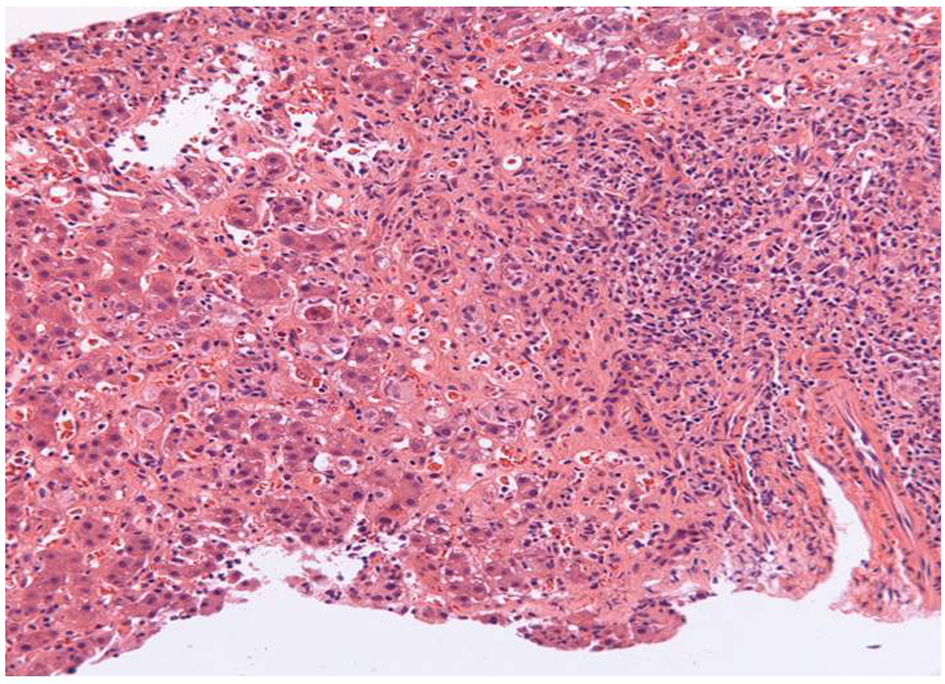 Click for large image | Figure 2. Liver biopsy (× 20 magnification): portal lymphoplasmacytic infiltrate, interface hepatitis with numerous plasma cells, scattered intralobular neutrophils and eosinophils, and extensive hepatocyte necrosis. |
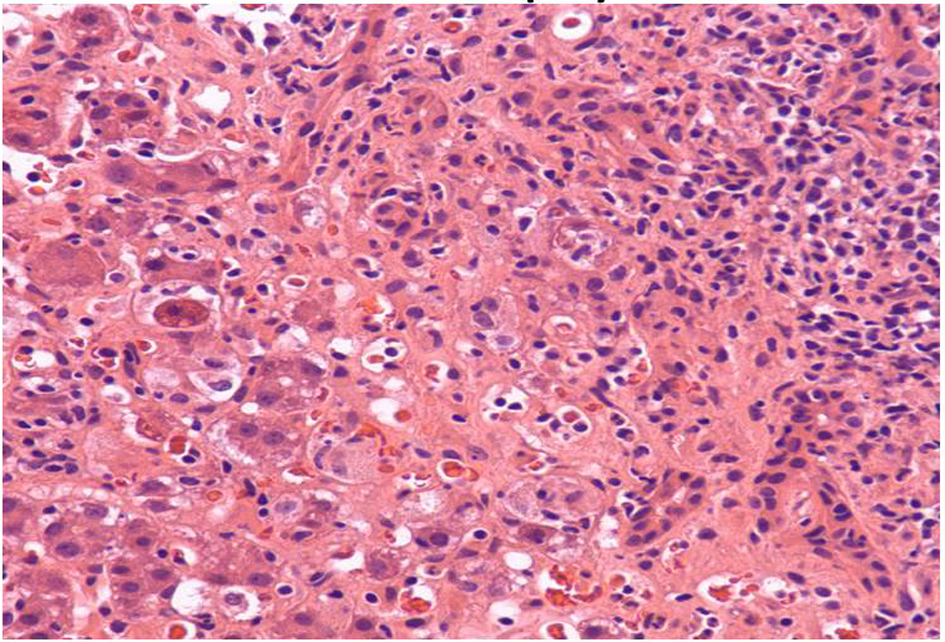 Click for large image | Figure 3. Liver biopsy (× 40 magnification): hepatocyte damage, perisinusoidal congestion and endothelial damage. |
Methyldopa was discontinued and a prednisone taper was started at 60 mg daily.
The patient’s symptoms resolved and the liver enzymes and bilirubin trended downward (Fig. 4, 5). Following prednisone taper, she developed steroid-induced diabetes mellitus and was started on subcutaneous insulin.
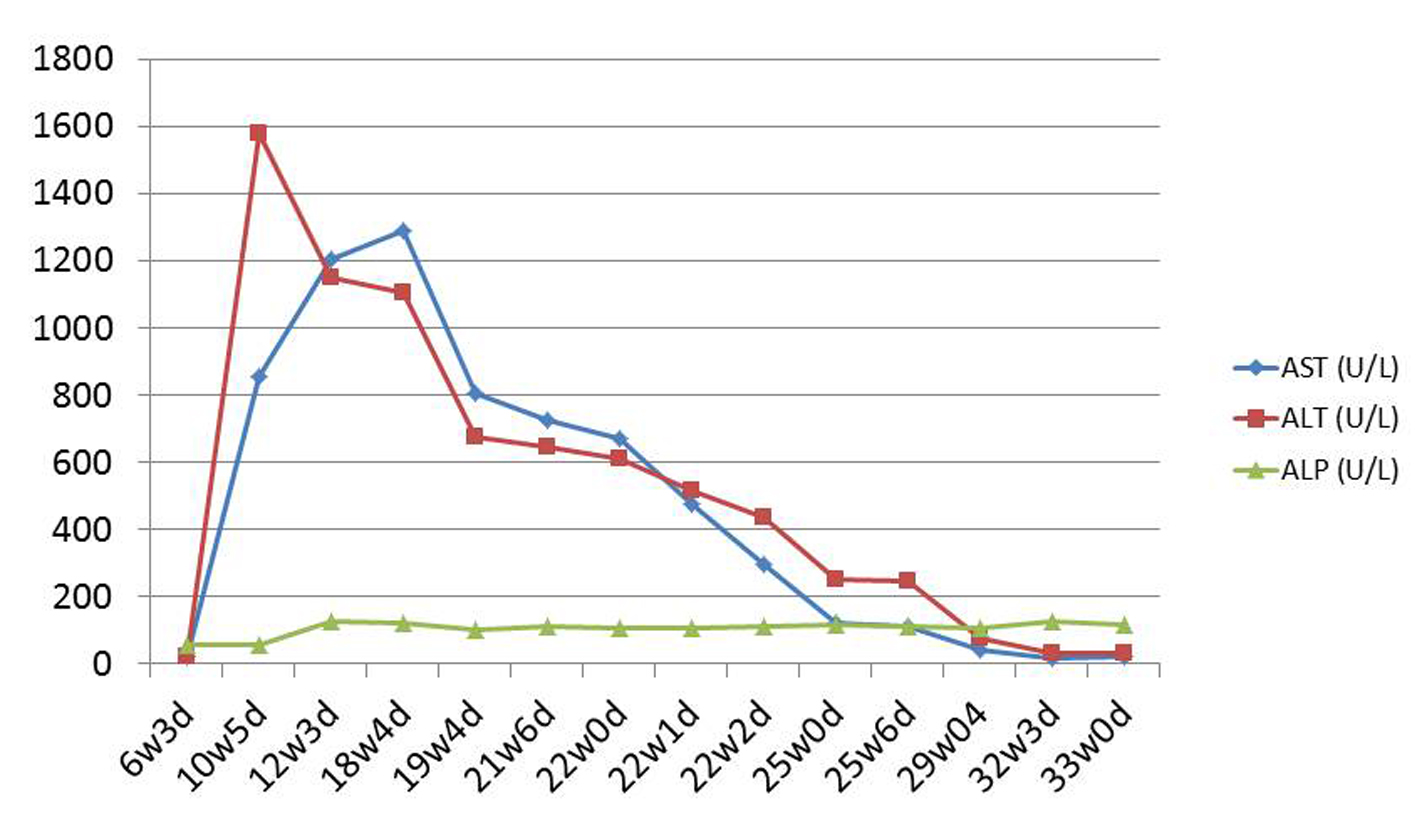 Click for large image | Figure 4. Liver enzyme trend. |
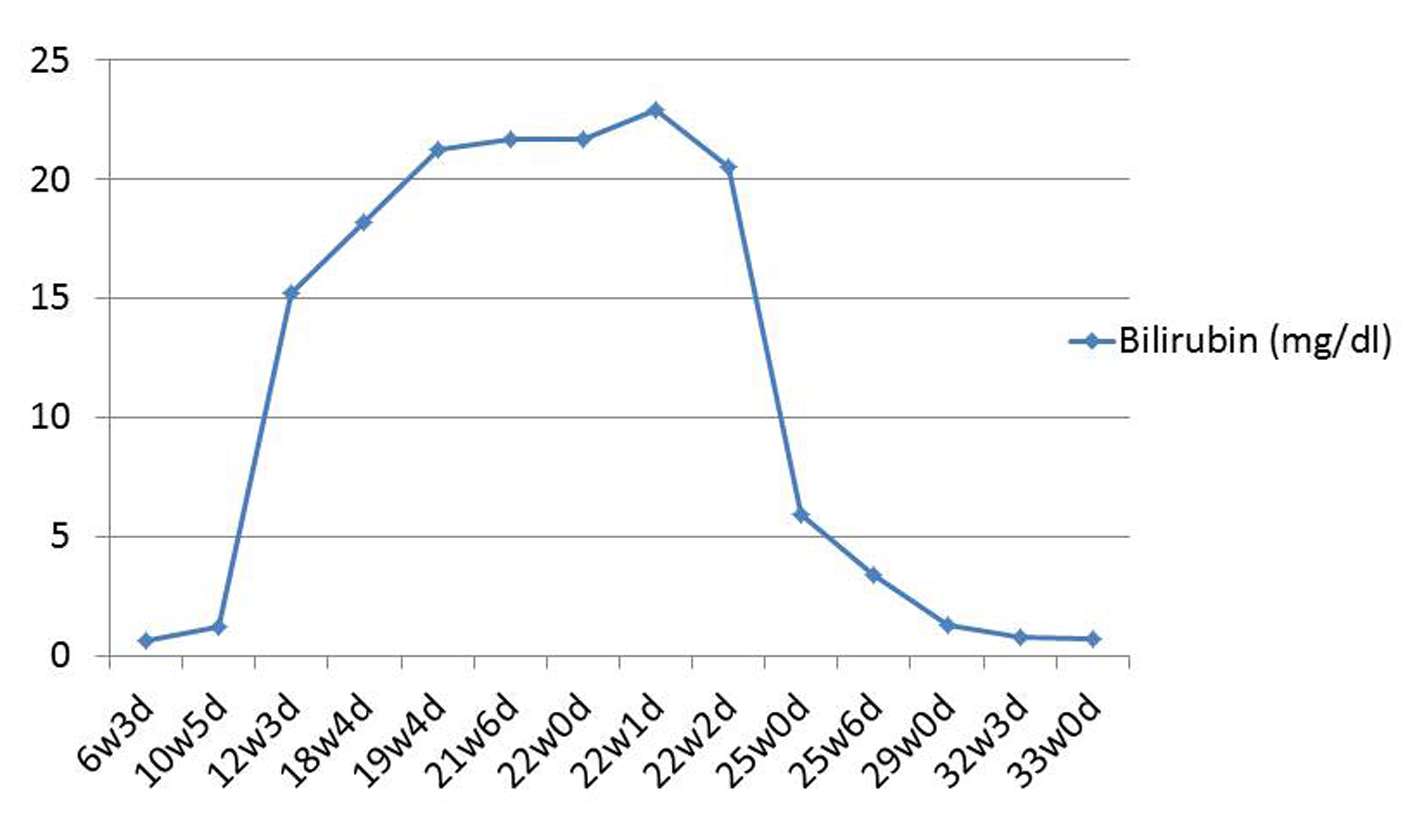 Click for large image | Figure 5. Bilirubin trend. |
The patient was discharged home on the 10th hospital day and her double boarded family physician obstetrician and the hepatologist managed her care. Her BP remained controlled without medication throughout the rest of the pregnancy. At 36 weeks gestation, the patient underwent a cesarean due to prolonged rupture of the membranes and poor labor progression. She delivered a live 2.66 kg male infant, and mother and baby were discharged home in good condition on the second post-operative day.
| Discussion | ▴Top |
Methyldopa is most commonly known for its use in treating hypertension in pregnancy, chronic or pregnancy induced. When compared to other antihypertensive medications, its effectiveness and lack of demonstrable risk to the fetus in the short and long term, makes methyldopa the drug of choice in controlling hypertension in pregnancy.
As a centrally mediating alpha-2 adrenergic agonist, it controls the BP by a reduction in peripheral vascular resistance with inhibition of sympathetic activity [4]. It is metabolized in the liver and intestine by dopamine-beta hydroxylase into the alpha methyl norepinephrine, the active metabolite which acts on the adrenergic receptors. It is mostly excreted as a sulfate conjugate in urine, with a small percentage excreted in breast milk. The mechanism of liver injury remains unclear but changes range from acute to chronic depending on duration of drug use [4]. There seems to be a strong autoimmune component; however, cases have been reported with negative autoantibodies (antinuclear and anti-smooth muscle), and injury is thought to be either due to toxins during metabolism or a hypersensitivity reaction [4, 5]. Hepatocellular and cholestatic damages are reflected by greater than five-fold increase in transferases and alkaline phosphatase respectively which can ultimately lead to fulminant hepatitis and death [6].
We presented a case of hepatotoxicity 12 weeks after the start of the antihypertensive medication, methyldopa in a closely monitored high-risk pregnancy. An adverse reaction to methyldopa was not initially considered given the time from methyldopa exposure to the 12 week onset of signs and symptoms. Previous research have reported onset ranging from as early as 3 days [2] to 7.5 months and rarely up to 3 years [7].
It is believed that the hepatotoxicity results from an autoimmune and hypersensitivity reaction that varies from time of exposure to liver damage [4, 5]. A negative hepatitis/immunological panel, abnormal liver function tests and the liver biopsy were critical in making a diagnosis of drug-induced liver injury (DILI), a diagnosis of exclusion. Using the Council for International Organizations of Medical Sciences (CIOMS) consensus, markedly elevated ALT levels (> twice normal or ALT/ALP ≥ 5) support an acute hepatocellular injury [7]. A post-diagnostic analysis of patients’ signs, symptoms, and laboratory tests using the Roussel Uclaf Causality Assessment Method (RUCAM), a score of 9 indicates a highly probable ADR to methyldopa [7].
Increasing the awareness of adverse effects with methyldopa use will heighten clinicians’ index of suspicion. Although monitoring of liver enzymes pre-diagnosis currently lacks evidence, this should be considered given the acuity and unpredictable nature of the liver injury [8]. As part of drug information, the pharmaceutical industry recommends liver function tests (LFT) in the first 6 - 12 weeks of methyldopa use, which might have been helpful in this patient. Treatment of hepatotoxicity with steroids led to diabetes, further complicating our patient’s pregnancy.
With the development of diabetes mellitus, strict diet and weight management was strongly emphasized. It is unclear if this contributed to patients’ BP control without hypertensive medications.
Conclusion
The dangers of hepatotoxic medications like methyldopa cannot be overemphasized. A high index of suspicion is critical in diagnosis for early management especially in pregnancy.
Acknowledgement
The authors are grateful to the patient for accepting to provide more awareness in the scientific community through her participation. We also acknowledge Dr. Samir Parekh and Dr. Yue Xue, both at Emory University for providing pathology support, and Dr. Olaoluwa Bode Omoleye at University of Texas Health Science Center for providing pathology support.
Conflict of Interest
Authors declare no conflict of interest.
| References | ▴Top |
- Ozsvar Z, Solymossi Z, Monostory K. [Methyldopa-induced acute reactive hepatitis in pregnancy, drug-metabolizing capacity of the liver]. Orv Hetil. 2010;151(11):457-461.
doi pubmed - Slim R, Ben Salem C, Hmouda H, Bouraoui K. Hepatotoxicity of alpha-methyldopa in pregnancy. J Clin Pharm Ther. 2010;35(3):361-363.
doi pubmed - Thomas LA, Cardwell MS. Acute reactive hepatitis in pregnancy induced by alpha-methyldopa. Obstet Gynecol. 1997;90(4 Pt 2):658-659.
doi - United States National Library of Medicine. Drug Record, Methyldopa. Clinical and Research Information on Drug-Induced Liver Injury (document on the internet). 1993 (Updated 2014 Nov 4; Cited 2014 July). Available from: http://livertox.nlm.nih.gov/Methyldopa.htm.
- Phadnis SV, Sangay MR, Sanusi FA. Alpha-methyldopa-induced acute hepatitis in pregnancy. Aust N Z J Obstet Gynaecol. 2006;46(3):256-257.
doi pubmed - Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62(6):481-492.
doi pubmed - Cameron IA, Achord JL, Bartee H. Severe hepatic necrosis associated with methyldopa. Can Fam Physician. 1981;27:675-680.
pubmed - Navarro VJ, Senior JR. Drug-related hepatotoxicity. N Engl J Med. 2006;354(7):731-739.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
