| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 4, Number 3, September 2015, pages 237-250
Urogenital Biomolecular and Physical Measures in Pre- and Post-Menopausal Women
Miranda A. Faragea, c, Ken Wehmeyera, Gina Fadayela, Stacey Carpentera, Richard Chenga, Baiyang Wanga, William J. Ledgerb
aThe Procter and Gamble Company, Cincinnati and Mason, OH, USA
bDepartment of Obstetrics and Gynecology, Weill-Cornell Medical Center, New York, NY, USA
cCorresponding Author: Miranda A. Farage, The Procter and Gamble Company, Winton Hill Business Center, 6110, Center Hill Avenue, Box 136, Cincinnati, OH 45224, USA
Manuscript accepted for publication July 09, 2015
Short title: Urogenital Biomarkers in Post-Menopausal Women
doi: http://dx.doi.org/10.14740/jcgo347e
| Abstract | ▴Top |
Background: A clinical study was conducted to evaluate biomolecules and physical measures in the urogenital skin of pre-menopausal and post-menopausal women.
Methods: A total of 45 female subjects (aged 21 - 70 years) were enrolled in the study and assigned to one of three groups: 15 pre-menopausal (pre-M), 15 post-menopausal receiving no form of hormone replacement therapy (post-M non-HRT), and 15 post-menopausal receiving HRT (post-M HRT). Self-assessed symptoms were recorded from each group (i.e. dryness, itch and difficulty with intercourse). Skin temperature and pH, and quantification of biomolecules (via tape strip samples) were obtained from the labia majora, labia minora and introitus. Vaginal pH was also collected.
Results: More post-menopausal subjects (HRT and non-HRT) reported genital symptoms of external and vaginal dryness, and difficulties with intercourse. External genital skin itch was reported more frequently in the post-M non-HRT group. Skin temperature was lower in both post-M groups indicating reduced blood perfusion. The pH was significantly higher for the post-M non-HRT group at the vagina, introitus and labia minora compared to the pre-M group. Histamine was significantly reduced in both post-M groups; however, histamine levels did not correlate with complaints of itching. The concentration of natural moisturizing factors (NMFs) did not differ consistently between groups. Concentrations of IL-1α tended to be lower in the pre-M group at all three body sites.
Conclusions: This is the first report of non-invasive measures of temperature, pH, biomolecules of vulvar tissue in post- and pre-menopausal women. Based on results of histamine analyses, we propose that reduced histamine levels in post-menopausal women are related to sexual and lubrication difficulties, and reduced blood perfusion.
Keywords: Post-menopausal; Histamine; Skin temperature; Skin pH; Histidine; NMF; IL-1α; IL-1ra; Urogenital biomarkers; Urogenital tissue; Cytokines
| Introduction | ▴Top |
The skin, a full one-sixth of body weight, is a sophisticated and dynamic organ, which protects the sensitive internal tissues of the body from the external environment. However, skin is not a mere barrier. It is essential to the maintenance of body temperature and internal hydration, sensory functions, and immunological surveillance [1]. Skin is a highly active metabolic tissue, and there is growing interest in the relationship between the presence and concentrations of certain biomolecules and the existence of certain dermatologic conditions. Lee and colleagues [2] examined and compared the potential roles of IL-1α and TNF-α in the activation and release of secondary cytokines/chemokines in irritant contact dermatitis. Gerber and colleagues [3] reviewed the role of cytokines and chemokines in rosacea. Distinct patterns of cytokine secretion from the skin surface have been demonstrated in patients with psoriasis compared to patients with atopic dermatitis [4]. Psoriasis is associated with the differential expression of a wide variety of inflammatory and immune-related mediators. Those markers most easily accessible in the skin are those associated with abnormal keratinocyte differentiation and proliferation [5]. In addition to dermatologic conditions, a variety of systemic and internal pathological conditions may be reflected in the skin [4], including diabetes mellitus, atherosclerosis, inflammatory bowel diseases, AIDS, mental stress, and aging.
A wide variety of compounds can be extracted from the skin using minimally-invasive or non-invasive methods such as scraping, tape stripping or skin surface washing [4]. With this capability, there is growing interest in quantifying biomolecules in the skin as a means of monitoring skin disorders or other clinical conditions. As a manufacturer of feminine protection products, we were interested in evaluating cytokines and other biomarkers from genital tissue to better understand and distinguish between the urogenital skin environment of pre-menopausal and post-menopausal women (estrogenized and non-estrogenized). In addition, these measures have the potential to provide additional information for traditional safety and efficacy testing, thereby increasing the ability of these tests to discriminate between very similar product or material options. This is the first report of non-invasive measures of temperature, pH, cytokines and other biomarker measures of vulvar tissue in post- and pre-menopausal women.
| Materials and Methods | ▴Top |
Test subjects
Testing was conducted in compliance with the Good Clinical Practice Regulations (21 Code of Federal Regulations (CFR) 50), and in accordance with the Declaration of Helsinki [6]. Test protocols were approved by the test facility’s Institutional Review Board. Subject recruitment and sample collection were conducted at an independent test facility (Radiant Research, Cincinnati, OH). Subjects were healthy, adult female volunteers, 21 - 70 years of age, who had signed an informed consent. Participation was completely voluntary. Subjects were excluded from participation if they had had a partial or full hysterectomy or irregular menstrual cycle, they had skin abnormalities in the vulvar area, they had diabetes, kidney, heart or circulatory disease, or were currently pregnant or breast-feeding, they took certain immunosuppressive or anti-inflammatory medications that may interfere with test results, or they were participating in another clinical study.
Forty-five female subjects, aged 21 - 70 years, who met all entrance criteria, were enrolled. A summary of the test group demographics is presented in Table 1. The groups consisted of 15 pre-menopausal females (pre-M, vaginal atrophy score ≤ 2 and pH ≤ 5), 15 post-menopausal females who were not receiving any type of hormone replacement therapy (HRT) and who showed signs of urogenital atrophy based on a urogenital examination (post-M non-HRT, vaginal atrophy score ≥ 6 and pH ≥ 5), and 15 post-menopausal females receiving HRT for at least 12 consecutive months (via oral, vaginal or transdermal patch) and showing no signs of urogenital atrophy (post-M HRT, vaginal atrophy score ≤ 2 and pH ≤ 5). A urogenital examination was performed and the degree of urogenital atrophy was graded. The mean score for each group is given in Table 1. The average time on HRT was 5 years. Approximately half the subjects (seven out of 15) were taking oral HRT.
 Click to view | Table 1. Summary of Demographics and Baseline Vaginal Atrophy Scores |
Test protocols
Subjective assessment
Self-assessed subject-reported symptoms were recorded. Panelists were asked to rate specific urogenital symptoms, including genital skin dryness and itch, and vaginal dryness and itch on a scale of “0” for no symptoms, “1” for slight symptoms, “2” for moderate symptoms, and “3” for considerable symptoms. Perceived difficulty having intercourse was rated on a scale of “0” to “4”, with “0” to “3” as described above, and “4” indicating unbearable symptoms.
Physical measurements
Subjects were asked to shave their labial hair 7 days before the scheduled test visit. Skin temperature and pH were obtained from three different body sites: labia majora, labia minora and introitus. The upper thigh was used as a control site for the skin temperature measurement. A hand-held infrared thermographic scanner (Exergen DermaTemp, Exergen Corporation, Watertown, MA) was used to record skin temperature, and pH measurements were done using a portable meter (SkinCheck HI-98109, Hanna Instruments, Woonsocket, RI) fitted with a specialized electrode designed to measure skin pH. In addition, vaginal pH was measured using a 2-inch strip of pH paper (3.0 - 7.0 range) grasped by a ring forceps and inserted and placed against the middle to upper third of the lateral wall of the vagina.
Tape strip collection
Ten sequential tape strips (22 mm D-Squame discs, CuDerm Corporation, Catalog #D100) were collected from each of the three sites (labia majora, labia minora (outer surface) and introitus (6 o’clock position)). Tape strips were placed on the site and rubbed, then tapped repeatedly (20 times) by pressing down on the tape strip with the blunt end of the forceps so that good contact was achieved. Once removed, the next tape in the sequence was applied immediately using the same procedure. Each tape strip sample was placed in 12-well polystyrene plates and stored at -70 °C or below until analysis. For each panelist, if the tape stripping procedure caused any discomfort, it was discontinued immediately.
Cytokine analysis
The first and second D-Squame® tape strips were used for cytokine analysis. Tape strips were transferred to 2 mL polypropylene vials (BioPlas, Inc.) and extracted with phosphate-buffered saline (PBS) containing an additional 0.25 M NaCl and a commercially available protease inhibitor cocktail (Roche Applied Science, Inc., Indianapolis, IN, USA) for 30 min with sonication on ice. The extracts were then centrifuged for 5 min at 2,100 g, and supernatants were divided into two aliquots for cytokine and protein analysis. One aliquot was analyzed for soluble protein content using the BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) using bovine serum albumin (BSA) as a reference standard. The second aliquot of each supernatant was supplemented with 2% BSA frozen at -70 °C or below until cytokine analysis. Human cytokines (IL-1α, IL-1ra, IL-6 and IL-10) were simultaneously quantitated using a Human Cytokine Multiplex Immunoassay Kit (Bio-Plex Pro™; Bio-Rad Laboratories, Hercules, CA, USA).
Natural moisturizing factors (NMFs) and histamine analysis
The third tape strip was used for NMF analysis, which included measurement of histamine, histidine, 2-pyrrolidone-5-carboxylic acid, proline, trans-urocanic acid, cis-urocanic acid and protein. For histamine analysis, tape strips were placed into individual polypropylene vials, each vial was spiked with stable isotope-labeled histamine (D4-histamine) internal standard (ISTD) and extracted with acidified for water (0.1% formic acid in distilled-deionized water) using sonication for 10 min. Each extract was isolated from the tape strip and an aliquot of each sample was placed into a specified position of a 96-well polypropylene plate. A set of histamine standards were prepared in the 96-well plate over an appropriate calibration range in acidified water. For analysis of histidine, 2-pyrrolidone-5-carboxylic acid, proline, trans-urocanic acid, cis-urocanic acid, tape strips were spiked with stable isotope internal standards (D3-proline, D5-pyrrolidone-5-carboxylic acid, 13C3-cis-urocanic acid), extracted and placed into a 96-well polypropylene plate as described for histamine. A set of combined standards (histidine, 2-pyrrolidone-5-carboxylic acid, proline, cis-urocanic acid and trans-urocanic acid) were prepared in the 96-well plate over an appropriate calibration range in acidified water. The standards and extracts of the tape strips were analyzed using gradient reversed-phase high performance liquid chromatography/tandem mass spectrometry (LC/MS/MS). Histamine and D4-histamine were monitored by positive ion electrospray using multiple reaction monitoring (MRM) with precursor ions of 112 m/z (histamine) and 116 m/z (D4-histamine) and product ions of 95 m/z (histamine) and 99 m/z (D4-histamine). Standard curves were constructed by plotting the signal, defined here as the peak area ratio (peak area of histamine/peak of ISTD) vs. the mass of the histamine. The mass of histamine in the calibration standards and the tape strip extracts were then back-calculated using the generated regression equation. The results were reported as the mass of histamine per tape strip (ng/strip). These values were normalized for the amount of tissue on the tape strip by dividing the ng histamine/strip by the amount of protein found in the extract, expressed as μg protein/tape strip. The net results are ultimately expressed as ng histamine/μg protein. The protein levels found in the tape strip extract were determined using the BCA Protein Assay Kit (Pierce Biotechnology/Thermo Scientific, Rockford, IL, USA). The detection limits for histamine and histidine were 100 pg and 10 ng, respectively.
Skin multiple analyte profile (MAP) analysis
D-Squame® tape strips number 4 and 5 were used for skin MAP analysis. Tape strips were transferred to 2 mL polypropylene vials (BioPlas, Inc.) and extracted with PBS containing 0.2% sodium dodecyl sulfate (SDS), 0.5% propylene glycol and protease inhibitor cocktail for 30 min with sonication on ice. The extracts were then centrifuged for 5 min at 2,100 g, and supernatants were divided into two aliquots for skin MAP and protein analysis, and frozen at -70 °C. One aliquot was analyzed for soluble protein content using the BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL, USA) using BSA as a reference standard. The second aliquot was analyzed for multiple human skin analytes (human serum albumin (or HSA), keratin-1, 10 and involucrin) using an MILLIPLEX™ MAP Human Skin Magnetic Bead Panel (EMD Millipore).
Infrared densitometry
Stratum corneum protein content was estimated using a bench-top infrared densitometer (Squame Scan™ 850A, Heiland electronic GmbH, Wetzlar, Germany) to measure the optical absorption of D-Squame® skin sampling discs at a wavelength of 850 nm. Squame Scan™ 850A readings are linearly proportional to stratum corneum protein content, and were used to indirectly measure amount of protein present on each D-Squame® tape strip sample [7, 8].
Analyses of data
A separate linear mixed model was used to analyze each measurement at three different sites (introitus, labia minora and labia majora) with BMI and age used as covariates. Data were transformed to the natural log scale before analysis and then back-transformed to the original scale for the adjusted means. All statistical analyses were conducted using SAS 9.3.
| Results | ▴Top |
There were differences between groups when subjects were asked about self-assessed subject-reported symptoms (Fig. 1). Compared to the pre-menopausal group, a higher proportion of post-menopausal women in both the non-HRT and the HRT groups reported external dryness, although the differences did not reach significance (i.e., P > 0.05). When asked about vaginal dryness, only 7% of the pre-M group reported this symptom. A higher incidence was reported by the post-menopausal groups, with 33% of the post-M non-HRT subjects and 47% of the post-M HRT subjects responding in the affirmative. The difference was significant when the pre-M group was compared to the post-M HRT group (P = 0.035). External itch was experienced by a significantly higher proportion of the post-M non-HRT, i.e., 47%, compared to the other two groups (each with 7% responding in the affirmative, P = 0.035). Difficulties with intercourse were reported by a higher proportion of post-menopausal women in both groups (non-HRT and HRT) compared to pre-menopausal women. The difference was significant when the pre-M group was compared to the post-M HRT group (P = 0.006). In the post-M HRT group, no significant differences were found between reported subjective symptoms and the type of HRT, i.e., oral, injection or transdermal patch (data not shown).
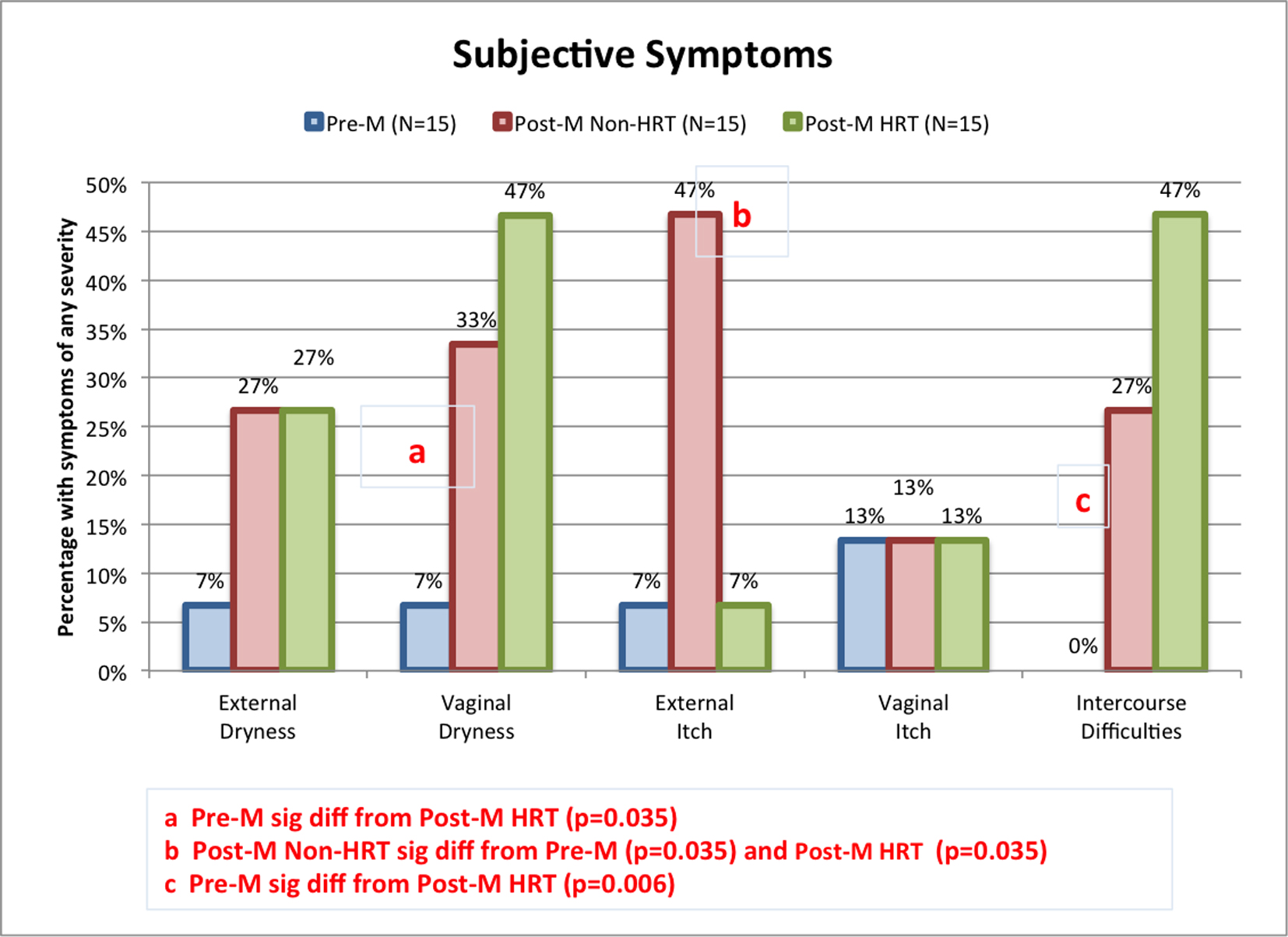 Click for large image | Figure 1. Incidence of subjective urogenital symptoms among test groups. Panelists were asked to rate specific urogenital symptoms, including genital skin dryness and itch, and vaginal dryness and itch on a four-point scale (none, slight, moderate, or considerable). Perceived difficulty having intercourse was rated on the five-point scale (none, slight, moderate, considerable, or unbearable). The proportion of individuals in each test group claiming some degree of symptoms is plotted. Pairwise comparisons were conducted using Fisher’s exact test. Significant differences between groups are indicated on the graph. |
Skin temperature and pH are shown in Figure 2. Differences in skin temperature at all the anatomic sites were small, but were significant at the labia minora and the labia majora (Fig. 2a). At the labia minora, surface skin temperature was lower in the post-M non-HRT subjects compared to pre-M and post-M HRT subjects (P = 0.0087 and 0.0388, respectively). At the labia majora, surface skin temperature was significantly higher in the pre-M group when compared to either post-menopausal group (P = 0.0025 for non-HRT and 0.0035 for HRT).
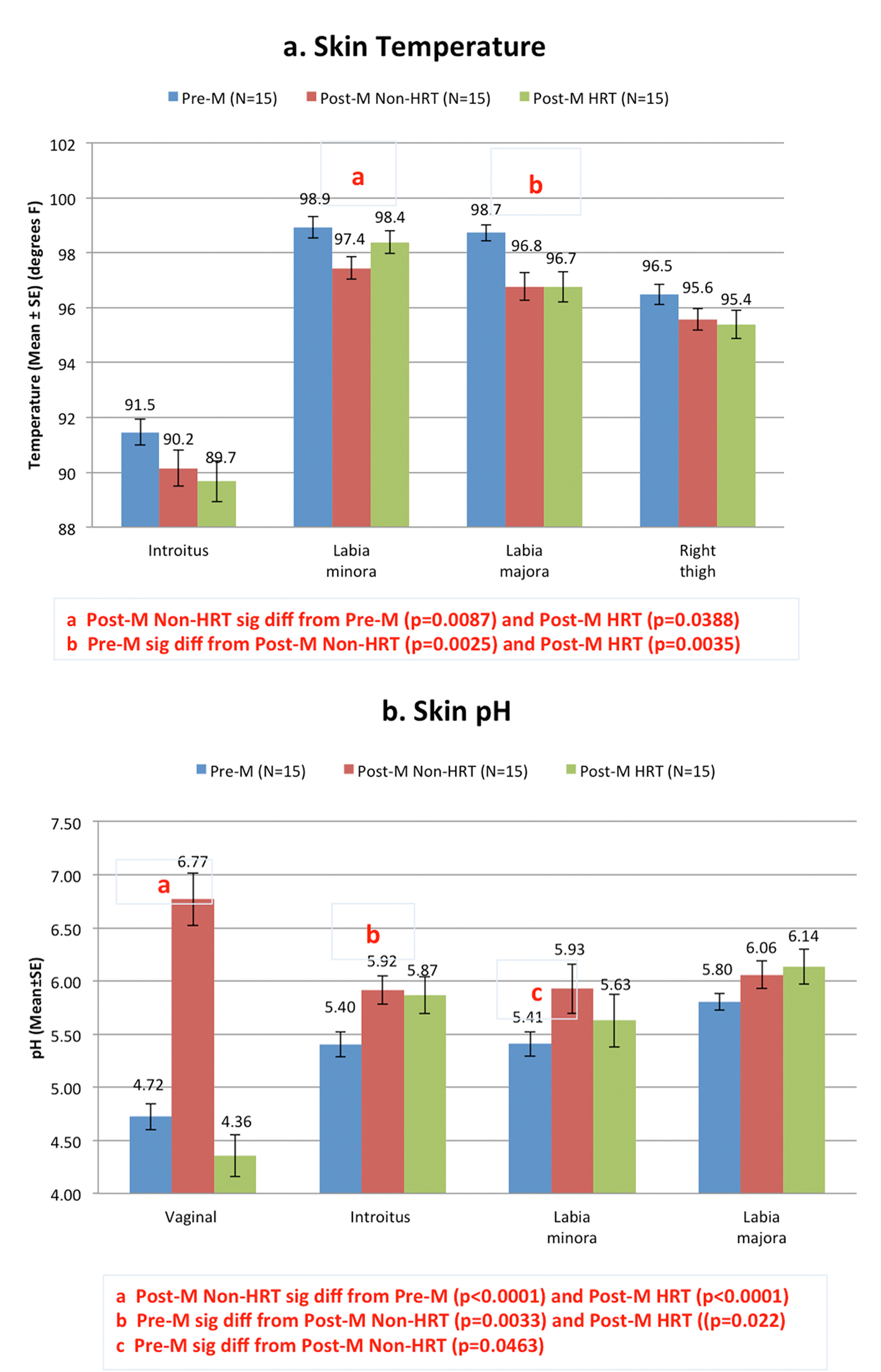 Click for large image | Figure 2. Skin temperature and pH among test groups. Skin temperature (a) and pH (b) were obtained from three different body sites; labia majora, labia minora and introitus. The upper thigh was uses as a control site for the skin temperature measurement. In addition, vaginal pH was measured using litmus paper against the middle to upper third of the lateral wall of the vagina. Pairwise comparisons were conducted using a mixed linear model. Significant differences between groups are indicated on the graph. |
Measures of skin pH (Fig. 2b) show that the pre-menopausal women had a significantly lower surface pH at the introitus (P = 0.0033) and at the labia minora (P = 0.0463) when compared to the post-M non-HRT group. The pH measurements in the post-M HRT group showed that the vaginal pH and the pH of the labia minora were similar to those of the pre-M group. The pH of the introitus for the post-M HRT group was similar to the post-M non-HRT.
As shown in Table 2, the levels of both histamine and histidine were higher at all three anatomic sites for the pre-menopausal group compared to both post-menopausal groups (non-HRT and HRT) (Table 2). For histamine, the differences were significant at all three sites when the pre-M group was compared to the post-M non-HRT group (introitus, P = 0.0003; labia minora, P = 0.0001; labia majora P = 0.006) and to the post-M HRT group (introitus, P = 0.041; labia minora, P = 0.003; labia majora, P = 0.003). Levels of histidine were significantly different between the pre-M and the post-M non-HRT groups at the labia minora (P = 0.045) and labia majora (P = 0.006) (Table 2). Histidine levels were significantly different at the labia minora when the pre-M group was compared to the post-M HRT group (P = 0.010). Due to the higher levels of histamine, the histidine/histamine ratio was much lower for the pre-menopausal group at all three sites (Table 2). The differences were significant between the pre-M and the post-M non-HRT groups at the introitus (P = 0.030) and the labia minora (P = 0.017).
 Click to view | Table 2. Measurements of Histamine and Histidine |
To further explore the potential relationship between histamine levels and the presence of self-assessed subject-reported symptoms, levels of histamine for those individuals who reported symptoms of skin and vaginal dryness, external and vaginal itch, and difficulties with intercourse were compared to the individuals who reported an absence of symptoms. As shown in Figure 3, women from the entire test population who reported having symptoms (i.e., regardless of group assignment) showed consistently lower levels of histamine compared to women who did not have those symptoms.
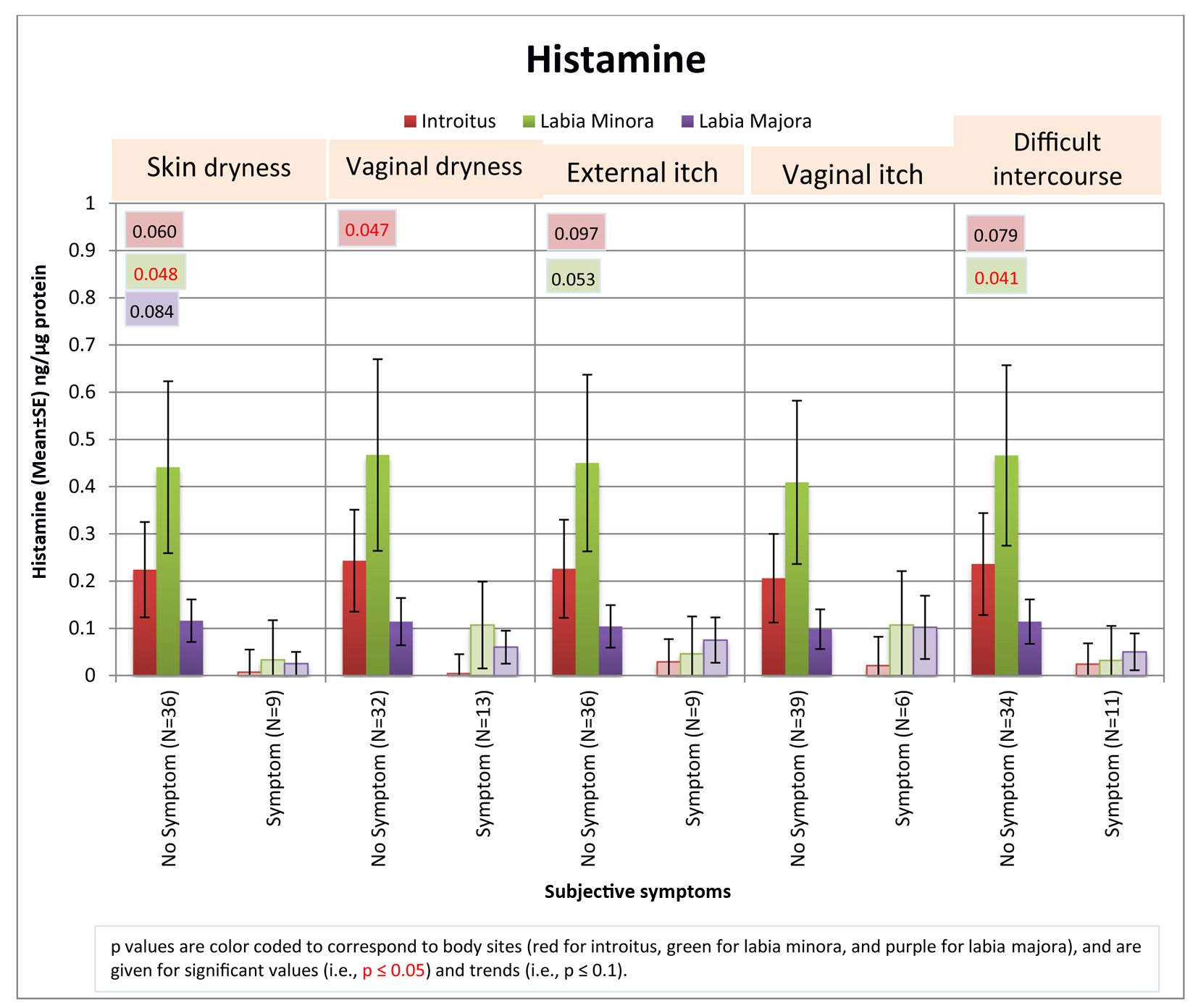 Click for large image | Figure 3. Relationship between histamine levels and subjective symptoms. The levels of histamine detected from individuals who claimed the presence of subjective symptoms was compared to that from individuals who did not claim to experience the symptoms. The entire test population was considered as a whole, regardless of group assignment. Pairwise comparisons were conducted using Fisher’s exact test. Significant differences (P ≤ 0.05) and trends (P ≤ 0.1) between groups are indicated on the graph. |
Measures of NMF were not significantly different when groups were compared with the exception of NMF at the labia minora, where the pre-M group measurement was significantly higher than the post-M HRT group (P = 0.016) (Table 3). The levels of NMF tended to increase as the tissue type changed to a more keratinized type, i.e., introitus < labia minora < labia majora. When amino acids components of NMF were measured, there were no significant differences between the pre-M and the post-M non-HRT groups except in the proline content, which was higher at the labia majora for the pre-M group (P = 0.033). Comparisons of the pre-M and the post-M HRT groups indicate 2-pyrrolidone-5-acid was higher for the pre-M group at the labia minora (P = 0.024), and proline was higher at the labia minora and labia majora (P = 0.0023 and 0.0040, respectively). One significant differences were also noted when amino acid levels were compared for the two post-menopausal groups; proline was higher in the post-M non-HRT group at the labia minora (P = 0.0045). Cis-urocanic acid was evaluated but not detected in these samples.
 Click to view | Table 3. Measurements of NMF, Cytokines and Other Biomarkers |
Cytokine measures showed that IL-1α tended to be higher among the post-menopausal groups compared to the pre-menopausal group. This difference was significant at the labia minora and labia majora for the comparison between the pre-M and post-M non-HRT groups (P = 0.0091 and 0.045, respectively), and at the labia majora for the comparison between the pre-M and post-M HRT groups (P = 0.014). Levels of IL-1ra did not show a consistent trend. The ratio of IL-1ra/IL-1α tended to be higher in the pre-M group compared to the post-M non-HRT group, but the differences were not significant. Levels of IL-6 and IL-10 were evaluated but not detected in these samples.
Analysis of Squame is a means of quantifying dry skin [9]. Squame was lower for post-M non-HRT at the introitus compared to both the pre-M (P < 0.0001) and post-M HRT groups (P = 0.0021). This material was lower for post-M HRT at the labia minora and labia majora when compared to the pre-M group (P < 0.0001 and P = 0.0080, respectively) and post-M non-HRT group (P < 0.0001 and P = 0.0002, respectively).
HSA was significantly higher in the post-M non-HRT group at the labia minora when compared to the pre-M group (P = 0.044) and the post-M HRT group (P = 0.0083). The level of keratin 110 was significantly higher in the pre-M group compared to the post-M non-HRT group (P = 0.041). The protein skin MAP was significantly lower in the post-M HRT group compared to pre-M group (P = 0.0041). Our measurements indicated no differences between groups in content of involucrin or in the more general measure of total protein cytokine.
| Discussion | ▴Top |
Menopause is accompanied by a number of physical and psychological changes. These include vasomotor symptoms (hot flushes), sleep disorders, decreased sexual response, genitourinary factors, and mood. HRT is known to relieve many of these symptoms [10], and to have a positive impact on overall quality of life [10]. One objective of this study was to determine if there was an indication that HRT relieved specific, genital sensations that may also be associated with menopause.
A recent review reported that up to 55% of women with vaginal atrophy reported vaginal dryness, and 44% report pain during intercourse [11]. In our study, reports of these particular subjective symptoms were lower among the post-M non-HRT group showing significant vaginal atrophy, with 33% of women in this group reporting vaginal dryness and 27% reporting difficulties with intercourse (Fig. 1). In this study, surprisingly, there was no evidence that HRT improved these subjective symptoms. In fact, the post-M HRT group reported a directionally higher incidence of vaginal dryness and difficulties with intercourse than the post-M non-HRT group, although differences were not significant. From this study, it appears that HRT may be associated with relief from external itch (Fig. 1). The percentage of women reporting this symptom was equivalent in the pre-M and post-M HRT groups (7% in each). In the post-M non-HRT group, the percentage was significantly higher at 47%.
An interesting observation in the course of this study was the number of tape strips tolerated by panelists in each test group. Although the genital skin of post-menopausal women is generally considered quite fragile, the post-M non-HRT group tolerated a greater number of tape strips at each anatomic site compared to the other two groups (Table 4). The difference was significant at the introitus compared to the pre-M group, and at all sites compared to the post-M HRT group. This is consistent with previous observations that vulvar sensitivity to mechanical stimuli declines after menopause, but is restored by estrogen supplementation [12].
 Click to view | Table 4. Number of Tape Strips Tolerated by Subjects |
Surface skin temperature is the result of the equilibrium between the body’s internal sources of heat supplied to the skin by vascular perfusion and heat loss to the external environment. We found the temperature of the skin at the labia minora and labia majora was lower in post-menopausal women reflecting the underlying decrease in blood perfusion (Fig. 2a). To the authors’ knowledge, this is the first time skin surface temperature of anatomic sites on the genitalia has been reported for pre- and post-menopausal women.
The vaginal pH of premenopausal women without vulvovaginal atrophy is typically reported as 4.5 or less [13, 14]. Prior to menopause, the glycogen released from the epithelial cells that are exfoliated from the vaginal wall is converted to glucose, which is acted upon by lactobacilli to produce lactic acid. This lactic acid maintains the acidic vaginal pH. With menopause, estrogen production is reduced resulting in a thinning of the vaginal epithelial cell layer, with a subsequent reduction in the number of exfoliated cells, ultimately leading to less production of lactic acid. This allows the postmenopausal pH to increase to the range of 5.0 - 7.5 [13, 14]. In our study, the vaginal pH of the pre-M group was similar to that previously reported for pre-menopausal women with a mean of 4.72 (Fig. 2b). The post-M HRT group showed a vaginal pH similar to the pre-M group. The vaginal pH of the post-M non-HRT group exhibited a mean of 6.77, within the previously cited range of 5.0 - 7.5. The pH values of the introitus and the labia minora were significantly higher in the post-M non-HRT compared to the pre-M group. To our knowledge, this is the first report of the pH on external genitalia for post-menopausal women compared to pre-menopausal women.
Histamine is derived from the decarboxylation of the amino acid histidine [15] and is most often associated with itching. A dose-dependent cause and effect between histamine and itching has been demonstrated many times using a variety of test methods. However, the sensation of itch can be caused by several other biochemical mediators [16], and possibly other stimuli, such as dryness. Claims of external itching in the genital area were significantly higher in the post-M non-HRT group (Fig. 1). However, histamine levels were significantly lower in this group compared to pre-menopausal women (Table 2). Figure 4a illustrates the striking change in histamine levels at the different life stages. Histamine levels at the introitus showed a 32-fold decrease when pre-M women were compared to post-M non-HRT women, and a nine-fold decrease when compared to post-M HRT women. A similar pattern was observed: the labia minora with a 25-fold and 13-fold decrease, and at the labia majora with a seven-fold and 10-fold decrease, respectively.
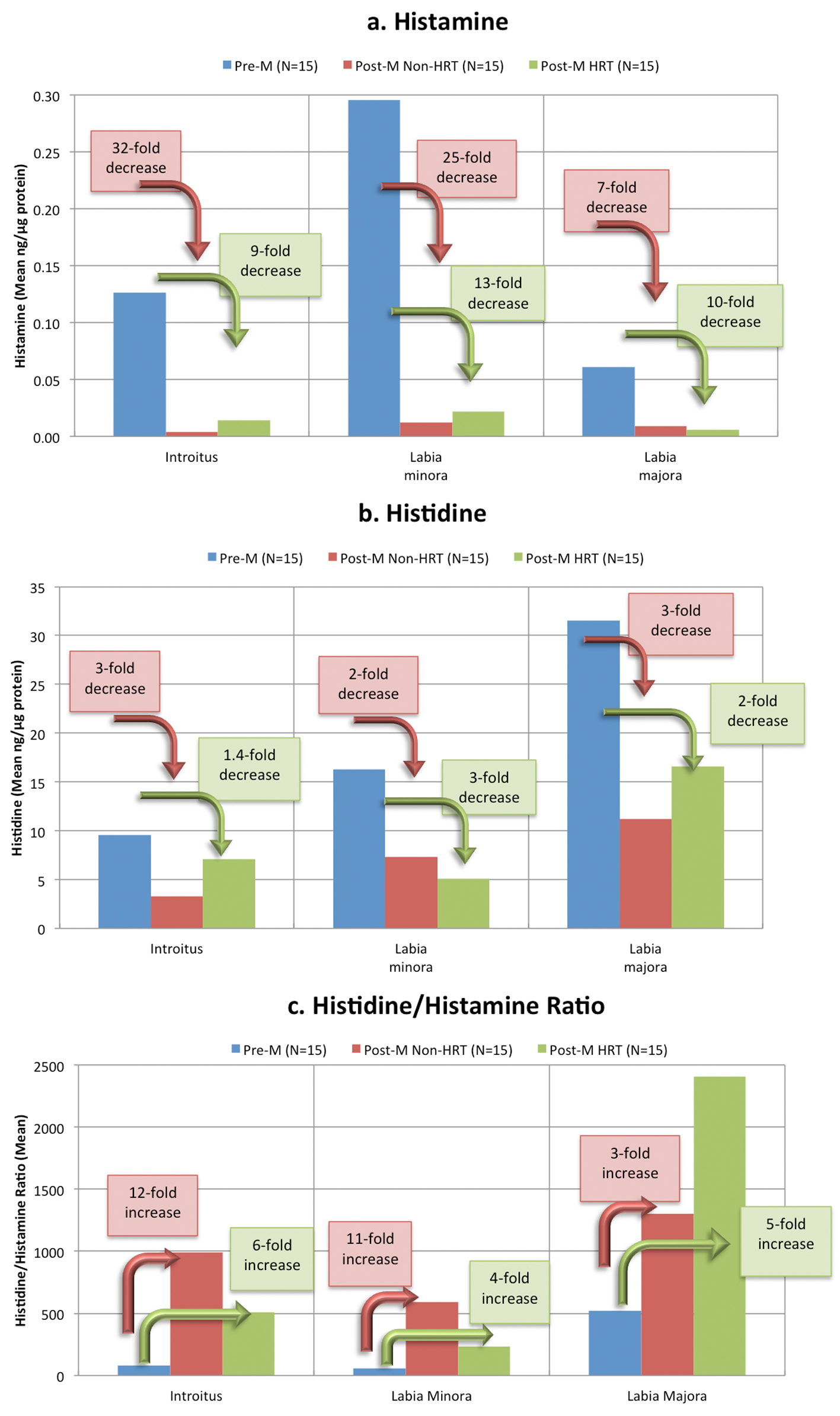 Click for large image | Figure 4. Changes in histamine and histidine at different life stages. The histamine (a), histidine (b) and histidine/histamine ratios (c) at three anatomic sites (given in Table 2) are plotted for each group to illustrate the change from the premenstrual group to the post-menstrual groups. Decrease from pre-M levels for post-M non-HRT group are illustrated by the pink arrows and text boxes, and for Post-M HRT group by the green arrows and text boxes. |
Interestingly, histidine levels were also lower in both post-M groups compared to the pre-M group (Fig. 4b) However, the ratio of histidine to histamine showed an increase (Fig. 4c) that reached significance when the pre-M group was compared to the post-M non-HRT group at the introitus and the labia minora (Table 2). Since histidine is a percursor of histamine, lower histidine levels in post-menopausal women will result in a decrease in histamine. Further, an altered ratio of histamine to histidine may indicate a change in the induction of histidine decarboxylase or a shift in the equilibrium between these two materials.
Histamine has a variety of functions in the body other than inducing itch, including some important ones related to sexual function [15]. At a central level, histamine receptors are important in brain areas involved in sexual arousal [17]. As a neurotransmitter, histamine levels are related to sexual desire [18]; a decrease in histamine causes a decrease in sexual desire, and an increase causes the reverse. At a local level, histamine has effects on smooth muscle and blood vessels critical to physiological sexual arousal [19]. In women, this involves an increase in clitoral cavernosal artery inflow and an increase in clitoral intracavernous pressure that leads to tumescence and extrusion of the clitoris [18]. Engorgement of the genital vascular network increases pressure inside the vaginal capillaries and results in lubrication of the epithelial surface of the vaginal wall [18]. Histamine also causes the sexual flush that occurs during arousal. Orgasm is triggered when histamine is released from the mast cells in the genitals, and sufficient histamine (and its precursor histidine) must be present in order to trigger an orgasm. For some women who fail to achieve sexual pleasure and orgasm, the problem may be a result of a biochemical imbalance. We propose that the reduced level of histamine in the genital area may be related to sexual and lubrication difficulties in post-menopausal women, and not related to a subjective perception of itch. Further, histamine may be an important biomarker for genital tissue health with regard to blood perfusion and sexual function.
NMF represents about 20-30% of the dry weight of the stratum corneum and is composed of a number of water-soluble compounds including 2-pyrrolidone-5-acid and urocanic acid, proline, lactic acid, urea, citrate, and sugars [20]. There are intrinsically lower levels of NMF present in aged skin compared to younger skin [21]. However, we did not find a consistent reduction in the components of NMF in the genital epithelium. In those instances where significant differences were found, the post-menopausal groups with and without HRT exhibited lower amounts compared to the pre-M group.
The cytokine IL-1α is produced by epithelial cells, and the normal human epidermis acts as a major reservoir of this material. Regulated cytokine expression is essential to the quality and function of the epidermal barrier, and deregulation of this complex signaling mechanism can result in multiple consequences in skin barrier function [22]. The cytokine IL-1ra functions as a competitive inhibitor to block the response to IL-1α [23]. Hirao and colleagues [24] reported that content of IL-1α and IL-1ra in the stratum corneum varied between body sites. The stratum corneum of an area of skin unexposed to sunlight, i.e., the inner side of the upper arm, contained more IL-1α than a sun-exposed area, i.e., the face. In contrast, the IL-1ra content was reversed, with the unexposed area containing lower amounts than the sun-exposed area. The ratio of IL-1ra to IL-1α was 8 in the unexposed area, and over 100 in the sun-exposed area [24]. These same authors reported that the IL-1α content in the unexposed site increased with age, while the content of IL-1ra decreased, resulting in an age-dependent decrease in the IL-1ra/IL-1α ratio. In infants suffering from diaper rash, Perkins and colleagues [25] reported a positive correlation between IL-1ra levels recovered from the buttocks and diaper rash severity. These authors also demonstrated significant increases in IL-8 levels in samples recovered from diaper rash versus control skin sites.
Our results are consistent with these observations in that the IL-1α content measured in the pre-M group was the lowest at all three sites (Table 3). The differences reached significance at the introitus and labia majora (pre-M group compared to post-M HRT group) and the labia minora (pre-M group compared to post-M non-HRT group).
Stute and colleagues [26] investigated levels of vaginal and serum cytokines (IL-1β, IL-6, IL-8, and tumor necrosis factor-α) in post-menopausal women not receiving hormone therapy. These authors found no differences in concentrations of any of these inflammatory markers when women with symptoms of vulvovaginal irritation (i.e., itching, burning, or pain) were compared to those without symptoms. An interesting area of future investigation would be a similar comparison of cytokine levels at other genital sites (i.e., introitus, labia minora and labia majora) in post-menopausal women with and without symptoms of vulvovaginal irritation.
The level of HSA measured at the labia minora was increased significantly in the post-M non-HRT group. Albumin is the most abundant plasma protein accounting for 55-60% [27]. Each day, 120 - 145 g of albumin is lost into the extravascular space: 41% of the extravascular HSA is in the skin [27]. Albumin does not diffuse freely through intact vascular endothelium, thus the loss of albumin into interstitial spaces may be an indication of increased vascular permeability [28].
Involucrin is a soluble protein precursor of the cross-linked envelope in human stratified squamous epithelium [29]. Keratins are major components of the epithelial cytoskeleton and important in mechanical integrity at the cellular and tissue level [30]. Our measurements indicated no consistent differences between groups in content of these materials.
In this study, no attempt was made to recruit equal numbers of women on different types of therapy, i.e., oral, genital or dermal/patch. Half of our panelists were on oral therapy, and those on local vaginal therapy had been using this form for a relatively short time. Differences have been reported in the effectiveness of different therapeutic approaches. Long and colleagues [31] conducted a comparison of oral and vaginal estrogen therapy in postmenopausal women and found that the vaginal therapy had greater impact on sexual function compared to the oral preparation despite a lower serum estradiol concentration in the vaginal group. Vaginal estrogen therapy was reported by the North American Menopause Society [32] as effective in 80-90% women in relieving symptoms of VVA compared to 75% of women on oral estrogen therapy. Studies of cytokines and physical markers in larger numbers of women using different HRT approaches (i.e., oral, vaginal and dermal) may provide insights into benefits of the different types of therapies and thus will need further investigation.
A limitation of the study is the use of sequential tape strips to measure different biomarkers collected from genital tissue. The large numbers of different analyses in this initial investigation necessitated this approach to the test protocol; however, the impact on the quantitation of the various biomarkers is unknown. Future investigations should focus on specific cytokines of interest, eliminating the need to collect multiple, sequential samples.
Testing for potential skin effects is a key part of the overall safety assessment for any consumer product, including feminine protection products. The current industry approach employs a multi-step process, with the final step involving exposure to the products or materials and evaluation for subtle signs of skin effects. For decades, this approach has served the consumer products industry well. However, modern feminine protection products are inherently very mild to skin, and produce few or no discernible skin effects when tested even under exaggerated exposure conditions. This does not negate the utility of current testing in evaluating product safety; however, it limits attempts in the development of further product improvements. We have been exploring various approaches to increase our abilities to discriminate between very similar products or materials with regard to potential skin effects [33]. Quantifying various biomarkers and physical measurements has the potential to further increase the discriminating power of the testing approaches typically used for consumer products.
There is growing interest in quantitating biomolecules in the skin as a means of monitoring skin disorders or other clinical conditions. Using minimally-invasive methods, we evaluated physical measurements such as temperature and pH, and quantified cytokines and other biomarkers from genital tissue in order to establish a baseline for pre-menopausal and post-menopausal women (with and without HRT). To our knowledge, this is the first published report of cytokine measures for the genital area. Parameters such as skin surface temperature, pH and histamine levels obtained from anatomic sites on the external genitalia, i.e., labia minora and labia majora, could be indicators of vaginal atrophy. Further, biophysical changes in external tissue can be monitored in a non-invasive manner to evaluate the potential benefits of treatments or products intended for post-menopausal women.
Acknowledgement
The authors thank Michael Noss, MD and his staff (Radiant Research, Cincinnati, OH) for assistance in the conduct of the clinical study, and Terresa L. Nusair, PhD, (Health and Environmental Safety Alliance, Cincinnati, OH) for technical input. This work was conducted for and funded by The Procter and Gamble Company, Cincinnati, OH.
Conflict of Interest
The author(s) confirm that this article content has no conflicts of interest.
| References | ▴Top |
- Farage MA, Miller KW, Elsner P, Maibach HI. Characteristics of the Aging Skin. Adv Wound Care (New Rochelle). 2013;2(1):5-10.
doi pubmed - Lee HY, Stieger M, Yawalkar N, Kakeda M. Cytokines and chemokines in irritant contact dermatitis. Mediators Inflamm. 2013;2013:916497.
doi pubmed - Gerber PA, Buhren BA, Steinhoff M, Homey B. Rosacea: The cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15(1):40-47.
doi pubmed - Portugal-Cohen M, Kohen R. Non-invasive evaluation of skin cytokines secretion: an innovative complementary method for monitoring skin disorders. Methods. 2013;61(1):63-68.
doi pubmed - Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii104-110.
doi pubmed - World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194.
doi pubmed - Voegeli R, Heiland J, Doppler S, Rawlings AV, Schreier T. Efficient and simple quantification of stratum corneum proteins on tape strippings by infrared densitometry. Skin Res Technol. 2007;13(3):242-251.
doi pubmed - Hahn T, Hansen S, Neumann D, Kostka KH, Lehr CM, Muys L, Schaefer UF. Infrared densitometry: a fast and non-destructive method for exact stratum corneum depth calculation for in vitro tape-stripping. Skin Pharmacol Physiol. 2010;23(4):183-192.
doi pubmed - Schatz H, Kligman AM, Manning S, Stoudemayer T. Quantification of dry (xerotic) skin by image analysis of scales removed by adhesive discs (D-Squames). J Soc Cosmet Chem. 1993;4453-4463.
- Freedman MA. Quality of life and menopause: the role of estrogen. J Womens Health (Larchmt). 2002;11(8):703-718.
doi - Wysocki S, Kingsberg S, Krychman M. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clin Med Insights Reprod Health. 2014;8:23-30.
doi pubmed - Farage MA, Miller KW, Zolnoun D, Ledger WJ. Assessing sensory perception on the vulva and on extragenital sites. The Open Women's Health Journal. 2012;6:6-18.
doi - Lindahl SH. Reviewing the options for local estrogen treatment of vaginal atrophy. Int J Womens Health. 2014;6307-6312.
doi - Mac Bride MB, Rhodes DJ, Shuster LT. Vulvovaginal atrophy. Mayo Clin Proc. 2010;85(1):87-94.
doi pubmed - Lieberman P. The basics of histamine biology. Ann Allergy Asthma Immunol. 2011;106(2 Suppl):S2-5.
doi pubmed - Stander S, Steinhoff M, Schmelz M, Weisshaar E, Metze D, Luger T. Neurophysiology of pruritus: cutaneous elicitation of itch. Arch Dermatol. 2003;139(11):1463-1470.
doi pubmed - Devidze N, Lee AW, Zhou J, Pfaff DW. CNS arousal mechanisms bearing on sex and other biologically regulated behaviors. Physiol Behav. 2006;88(3):283-293.
doi pubmed - Meston CM, Frohlich PF. The neurobiology of sexual function. Arch Gen Psychiatry. 2000;57(11):1012-1030.
doi - Adaikan PG, Karim SM. Male sexual dysfunction during treatment with cimetidine. Br Med J. 1979;1(6173):1282-1283.
doi - Rawlings AV, Harding CR. Moisturization and skin barrier function. Dermatol Ther. 2004;17(Suppl 1):43-48.
doi - Farage MA, Miller KW, Maibach HI. Degenerative Changes in Aging Skin. In: Farage MA, Miller KW, Maibach HI, eds. Textbook of Aging Skin. Berlin Heidelberg: Springer-Verlag, 2010:25-35.
doi - Hanel KH, Cornelissen C, Luscher B, Baron JM. Cytokines and the skin barrier. Int J Mol Sci. 2013;14(4):6720-6745.
doi pubmed - Borg M, Calleja-Agius J. The effect of cytokines on skin during menopause. In: Farage MA, Miller KW, Fugate-Woods, Maibach MI, eds. Skin, Mucosa and Menopause; Management of Clinical Issues. Berlin: Springer-Verlag, 2015:53-70.
doi - Hirao T, Aoki H, Yoshida T, Sato Y, Kamoda H. Elevation of interleukin 1 receptor antagonist in the stratum corneum of sun-exposed and ultraviolet B-irradiated human skin. J Invest Dermatol. 1996;106(5):1102-1107.
doi pubmed - Perkins MA, Osterhues MA, Farage MA, Robinson MK. A noninvasive method to assess skin irritation and compromised skin conditions using simple tape adsorption of molecular markers of inflammation. Skin Res Technol. 2001;7(4):227-237.
doi pubmed - Stute P, Kollmann Z, Bersinger N, von Wolff M, Thurman AR, Archer DF. Vaginal cytokines do not differ between postmenopausal women with and without symptoms of vulvovaginal irritation. Menopause. 2014;21(8):840-845.
doi pubmed - Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599-610.
doi pubmed - Hankins J. The role of albumin in fluid and electrolyte balance. J Infus Nurs. 2006;29(5):260-265.
doi pubmed - Watt FM. Involucrin and other markers of keratinocyte terminal differentiation. J Invest Dermatol. 1983;81(1 Suppl):100s-103s.
doi pubmed - Ramms L, Fabris G, Windoffer R, Schwarz N, Springer R, Zhou C, Lazar J, et al. Keratins as the main component for the mechanical integrity of keratinocytes. Proc Natl Acad Sci U S A. 2013;110(46):18513-18518.
doi pubmed - Long CY, Liu CM, Hsu SC, Wu CH, Wang CL, Tsai EM. A randomized comparative study of the effects of oral and topical estrogen therapy on the vaginal vascularization and sexual function in hysterectomized postmenopausal women. Menopause. 2006;13(5):737-743.
doi pubmed - Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888-902; quiz 903-884.
- Farage MA. Are we reaching the limits or our ability to detect skin effects with our current testing and measuring methods for consumer products? Contact Dermatitis. 2005;52(6):297-303.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
