| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Review
Volume 5, Number 1, March 2016, pages 1-16
Severe Infections in Obstetrics and Gynecology: How Early Surgical Intervention Saves Lives
Beryl Manning-Geista, Bassam H. Rimawia, b
aEmory University, School of Medicine, Department of Gynecology and Obstetrics, Division of Maternal Fetal Medicine, Division of Reproductive Infectious Diseases, 550 Peachtree Street, Atlanta, GA 30308, USA
bCorresponding Author: Bassam H. Rimawi, Emory University, School of Medicine, Department of Gynecology and Obstetrics, Division of Maternal Fetal Medicine, Division of Reproductive Infectious Diseases, 550 Peachtree Street, Atlanta, GA 30308, USA
Manuscript accepted for publication November 23, 2015
Short title: Infections in Obstetrics and Gynecology
doi: http://dx.doi.org/10.14740/jcgo368w
- Abstract
- Recognizing Severe Infections in Obstetrics and Gynecology
- Clostridial Species
- S. aureus
- S. pyogenes
- Clinical Approach/Management: Considerations for Hysterectomy
- Summary
- References
| Abstract | ▴Top |
Obstetricians and gynecologists commonly encounter many different infections. Early recognition and treatment of these infections is crucial. On many occasions, the use of antimicrobials alone is sufficient; however, in some instances, even with antimicrobials, early surgical intervention is necessary to treat the source of infection and prevent further dissemination. This review article focuses on the epidemiology, diagnosis and management of some of the aggressive microorganisms encountered in obstetrics and gynecology. A full literature review of why early surgical intervention is critical to saving lives was conducted. Clostridial species, Streptococcus pyogenes and Staphylococcus aureus bacteria are outlined in this review, given their aggressive ability to progress into disseminating sepsis, toxic shock syndrome and necrotizing soft tissue infections. This review serves as a guide to assist obstetricians and gynecologists with making life-saving decisions on early surgical intervention when encountering such critically ill patients. An algorithmic approach is also illustrated that an obstetrician gynecologist can follow when encountering such microorganisms.
Keywords: Staphylococcus aureus; Clostridial species; Streptococcus pyogenes; Sepsis; Hysterectomy; Toxic shock syndrome; Infection
| Recognizing Severe Infections in Obstetrics and Gynecology | ▴Top |
In the field of obstetrics and gynecology, the majority of pelvic infections are recognized early, either in an office setting or after inpatient admission. The classical presentation of most of these infections facilitates prompt initiation of appropriate antimicrobial agents and resolution of the disease. Of concern, a subset of pelvic infections have atypical presentations, or are not well controlled with antimicrobial agents alone; thereby, the severity of the process may go unrecognized, leading to endometritis, sepsis, septic shock, toxic shock syndrome (TSS), necrotizing soft tissue infections and even death. It is critical that this subset of infections be identified early so that appropriate, often surgical, interventions can be taken [1].
The typical scenarios that are associated with severe infections in obstetrics and gynecology include, but are not limited to, obstetrical procedures, such as abortions, vaginal deliveries with episiotomies or cesarean sections, as well as gynecological procedures, such as abdominal surgeries for pelvic masses or vulvar infections, particularly in diabetic or immunocompromised patients.
In obstetrics, genital tract infections are strongly associated with preterm birth, premature rupture of membranes, low birth weight, intrauterine fetal demise and intrauterine infection [2]. In general, these bacterial, viral and protozoal infections ascend from the lower genital tract; however, additional sources include intrauterine infections, systemic maternal infections, asymptomatic bacteruria, and maternal periodontitis [2]. Goldenberg and colleagues analyzed different infectious routes that lead to preterm birth [3]. They determined that infection can culminate within the uterus and amniotic fluid via hematogenous spread, incidental introduction via a diagnostic or therapeutic amniocentesis or retrograde spread from the abdominal cavity through the fallopian tubes. Ultimately, they found that infection ascending from the vagina had the highest risk factor for preterm birth.
In gynecology, genital tract infections are characterized by location, with lower genital tract infections involving the vagina, vulva and perineum and upper genital tract infections encompassing tissues above the level of the vagina, including the cervix, uterus, adnexa, ovaries and lower pelvis. In general, upper genital tract infections derive from ascending lower genital tract infections. Microorganisms from these infections can further disseminate, leading to bacteremia and subsequent systemic inflammation that forms sepsis. If left untreated, sepsis can evolve into septic shock, a life-threatening condition associated with hypotension (systolic blood pressure < 90 mm Hg or a reduction of 40 mm Hg from baseline) despite adequate fluid resuscitation, perfusion abnormalities and multi-organ dysfunction. These abnormalities can quickly lead to lactic acidosis, oliguria, obtundation and death [4]. This review article will focus on the most common microorganisms that cause life-threatening infections in obstetrics and gynecology (Table 1). This article also argues that source control is the most important factor in combatting infection. On some occasions, particularly with necrotizing soft tissue infection, antimicrobial therapy alone is not sufficient to treat these critically ill patients [5]. In these cases, early surgical intervention must be employed to remove the necrotic infected tissue. For example, in patients with Clostridium sordellii infection, or postpartum or postabortal streptococcal TSS, hysterectomy needs to be considered early in patient management [6].
 Click to view | Table 1. Microorganisms Commonly Associated With Serious Infections in Obstetrics and Gynecology |
We review the most important clinical factors an obstetrician gynecologist should consider when encountering a critically ill patient with a life-threatening infection. Most obstetrician gynecologists are reluctant to perform aggressive surgery, particularly a hysterectomy, in young nulliparous patients; however, these surgical interventions can be necessary to save patient lives. This article specifically focuses on clostridial species, Staphylococcus aureus and group A streptococcal bacteria, given that these particular microbes have been associated with necrotizing soft tissue infections, TSS, sepsis and death in cases pertaining to obstetrics and gynecology. We also review the obstetric and gynecological procedures that place these patients at risk for the life-threatening infections caused by these microbes. Figure 1 illustrates an algorithmic approach an obstetrician gynecologist can follow when encountering such microorganisms.
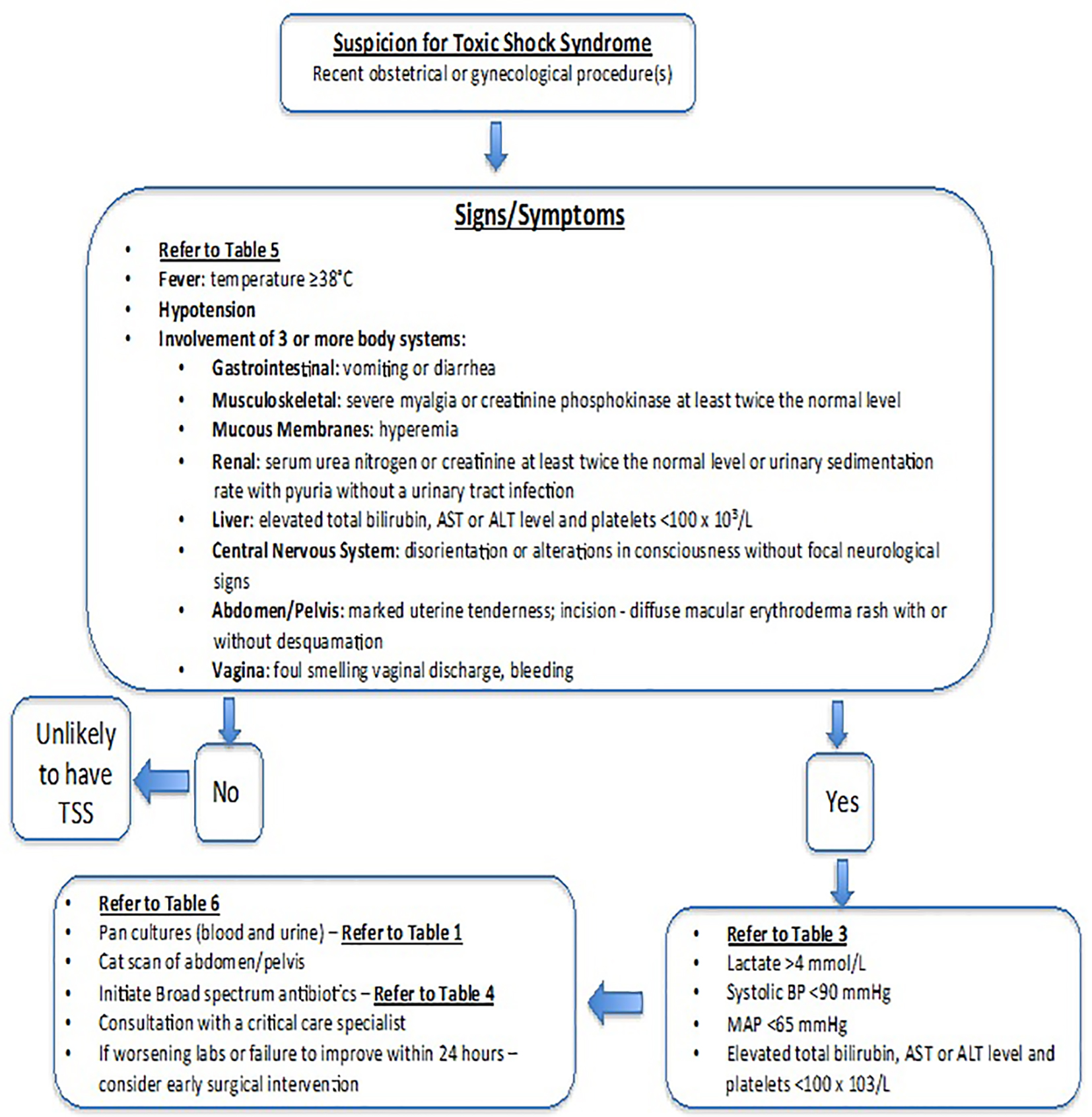 Click for large image | Figure 1. Algorithm for diagnosing and managing suspected toxic shock syndrome in obstetrics and gynecology. |
| Clostridial Species | ▴Top |
The bacterial genus clostridium is recognized for its highly fatal gynecologic manifestations including endometritis, surgical site infection and TSS. A family of large clostridial cytotoxins are responsible for many lethal effects of species such as C. sordellii, Clostridium septicum and Clostridium perfringens [7]. These cytotoxins exert their effects on varying GTPase subfamilies that control growth and cytoskeletal integrity. Although many clostridial soft tissue infections are traumatic in origin, spontaneous clostridial myonecrosis represents a common manifestation of gynecological clostridial infection [8].
C. sordellii
Microbiology
The Gram-positive, spore-forming, obligate-anaerobe C. sordellii is responsible for one of the most fatal TSSs known. The bacterium produces two major virulence factors called lethal toxin (TesL) and hemorrhagic toxin (TesH), which boast similar structures and mechanisms as toxins A and B of Clostridium difficile [9]. The two cytotoxins inactivate their protein substrates by adding UDP-glucose to threonine residues: TesL glycosylates Ras, Cdc42 and Rac subfamily GTP-binding proteins, while TesH glycosylates different substrates on the Rho, Cdc42 and Rac GTP-binding proteins. The inactivation of these important signaling molecules fundamentally changes cell activity and structure in mammalian hosts. For example, Ras subfamilies are responsible for growth control, and their inactivation can alter apoptosis and gene transcription, while Rho subfamily proteins maintain cytoskeletal integrity, and their inhibition compromises the microfilament cytoskeleton [7, 10].
The resulting cytoskeletal changes caused by TesL and TesH lead to altered growth and compromised endothelial integrity, which results in a myriad of symptoms, including massive third spacing of intravascular fluid. Table 2 [11-26] illustrates a summary of all the C. sordellii infection cases reported in the literature, pertaining to obstetrics and gynecology.
 Click to view | Table 2. Summary of Obstetric and Gynecologic C. sordelli Cases Reported in the Literature |
Pathophysiology of infection
At first, patients with C. sordellii infection develop non-specific symptoms including nausea, fatigue and lethargy; however, these symptoms quickly progress to a fulminant sepsis characterized by refractory hypotension, tachycardia, capillary leak with marked pleural edema, hemoconcentration, substantial leukocytosis with white blood cell counts > 50 × 103 cells/mm3 and an often-afebrile state [11-14]. The infectious source can be difficult to identify, as patients frequently lack abdominal pain [15]. This highly fatal clinical course is now referred to as C. sordellii-like TSS, with its ambiguous naming only due to the fact that it is difficult to distinguish C. sordellii from C. perfringens infections.
There are 27 reports of C. sordellii endometritis and TSS in the literature, including one spontaneous occurrence and several others following vaginal delivery, medical or spontaneous abortion, cesarean section or cervical procedures [11-23]. Only one of 27 postpartum or post-abortive patients reported in the literature has survived: the remaining infections proved fatal, despite efforts to medically resuscitate patients. This mortality rate stands in stark contrast to the 50% fatality rate of C. sordellii in injection drug users or the 53% fatality rate in post-traumatic or postoperative patients with culture-proven C. sordellii infection [11]. This discrepancy has been attributed to the delay in diagnosis resulting from the ambiguous presentation of gynecologic C. sordellii infection as well as the inability to visualize the infection, as is often possible in injection drug users or post-surgical patients.
The source of C. sordellii in gynecological infection remains unknown; however, reports have described rectal colonization in 2.8% of surveyed women, which could represent an origin for vaginal, and subsequent uterine, contamination [27]. It has been suggested that vaginal misoprostol may facilitate the ascension of C. sordellii into the uterus by introducing the bacterium, dilating the cervix or loosening the mucus plug; however, C. sordellii infection with only buccal misoprostol has also been reported [16, 28]. Isolated reports describe iatrogenic introduction of the bacterium, for example with retained vaginal sponges [15]. Once in the uterus, C. sordellii spores readily germinate: firstly, in vitro studies show that spores are more apt to germinate in response to progesterone, and secondly spores require a pH between 5.7 and 6.6 to germinate, which the amniotic fluid of the gravid uterus provides [29, 30]. Additional studies suggest that the development of septic shock may be aided in part by the inhibition of anti-inflammatory cytokine IL-10 in post-abortive patients due to mifepristone’s blockade of glucocorticoid receptors [28]. It should be noted, however, that other experimental applications of mifepristone in the treatment of chronic diseases have failed to result in a C. sordellii-like toxic shock like syndrome [31].
Diagnosis and treatment
The ambiguous presentation of C. sordellii infection, the delay and difficulty of culturing anaerobes in bloodstream infection and the relative rarity of the infection substantially delay diagnosis and treatment. Studies suggest that 0.54% of deaths in women aged 14 - 44 are caused by C. sordellii TSS; however, even these numbers may be underestimated [20]. More recent studies of C. sordellii infection indicate that the most effective diagnostic mechanisms include immunohistochemical staining of uterine tissue samples with anti-Clostridium spp. antibody or PCR gene analysis of 16SrRNA and phospholipase C genes [20]. More recently, a duplex microsphere assay has been developed that expedites the identification of C. sordellii and C. perfringens in formaldehyde fixed, paraffin-embedded tissues using smaller amounts of DNA [32]. This relative difficulty in diagnosing C. sordellii infection indicates that the infection may be more common than expected, as illustrated in a 2009 retrospective analysis of cases suspicious for C. sordellii-associated toxic shock death in a California patient population [20]. Table 3 illustrates the pertinent laboratory findings that can assist with diagnosis of invasive clostridial infections.
 Click to view | Table 3. Pertinent Laboratory Findings Associated With Infections Resulting in Invasive Streptococcus pyogenes (Group A Streptococcus), Clostridial Species and Staphylococcus aureus in Obstetrics and Gynecology |
The rapid progression of the infection from symptom onset to death also complicates the clinician’s ability to administer treatment in an impactful timeframe. Resistance studies suggest most C. sordellii strains are susceptible to several antibiotics including B-lactams, vancomycin, tetracycline, imipenem, linezolid, chloramphenicol, metronidazole, clindamycin and clindamycin-adjuvants [33]. Table 4 illustrates the recommended antibiotic treatment for invasive infections caused by clostridial species. Recent studies in murine models suggest that clindamycin may have unique efficacy in treating gas gangrene, as it can decrease toxin synthesis and the release of cytokines like TNF-alpha that lead to hypotension and other features of clinical toxic shock [34, 35]. At this time, C. sordellii antitoxins remain unavailable, although their development would likely prove to be a great advancement in the treatment of C. sordellii infection [11]. In addition to the available antibiotic regimens, debridement of necrotic tissue remains a cornerstone of treatment, as it represents the most efficient means of source control [36]. In all autopsy reports of patients with postpartum or post-abortus C. sordellii infection, bacteria were found in immunohistochemical staining of uterine samples, suggesting that the prompt removal of this infectious source is key.
 Click to view | Table 4. Antibiotic Treatment for Invasive Infections Caused by Invasive Streptococcus pyogenes (Group A Streptococcus), Clostridial Species and Staphylococcus aureus in Obstetrics and Gynecology |
C. septicum
Microbiology
C. septicum is a Gram-positive bacterium that distinguishes itself from its relatives by its relative aerotolerance, a quality that allows it to infect tissues independent of trauma. C. septicum produces four toxins, called alpha, beta, delta and gamma toxins [37]. Of these, alpha toxin is responsible for the cytotoxic effects of C. septicum. First released as an inactive prototoxin, alpha toxin is later converted to an active cytolysin that vacuolates and lyses cells. The toxin forms ion-permeable channels in membrane lipid bilayers that induce rapid outflow in potassium, ATP depletion, and cell necrosis [38]. This alpha toxin is ushered into tissues by additional enzymes including a hyaluronidase, fibrinolysin, deoxyribonuclease and hemolysins.
Pathophysiology of infection
The source of C. septicum remains unknown: previous studies have suggested that normal intestinal flora may include C. septicum, although several studies later contradicted these results. For example, one study failed to identify C. septicum among the 1,442 bacterial species found in the stool samples of three adult males over 5 months [39]. A different study, however, identified C. septicum in the stool of one of 33 patients fed both Western and Japanese diets, and an additional study isolated C. septicum in the stool of two of 125 patients [40, 41]. Like C. sordellii, uterine C. septicum infection is likely introduced from the vagina, even after a cesarean delivery [42, 43].
After introduction, the effects of the uterine environment on C. septicum infection are poorly defined. Previous reports suggest that necrotic environments may promote C. septicum spore germination, such as those observed in necrotic colon cancers or enterocolitis [44]. Other studies, however, have demonstrated that C. septicum is significantly more tolerant of aerobic tissues, indicating that it can infect viable tissues as well [45].
As it stands, C. septicum is a rare source of gynecological infection. Instead, 72% of C. septicum bacteremia is associated with colorectal or hematologic disorders and malignancies, with diabetic patients being particularly susceptible [46, 47]. There are, however, a few reports of infection in post-cesarean and post-abortive patients as well as isolated cases of C. septicum infection in ovarian cancer and choriocarcinoma [42, 48-50]. The clinical course of these patients mirrors other published reports: C. septicum infection is abrupt in onset, quick to progress, and its mortality rates fall between 67% and 100% depending on rapidity of treatment [47].
Patients typically present with acute onset of severe pain and edema followed by skin discoloration, hemorrhagic bullae, and crepitus [47]. Often, disseminated intravascular coagulation (DIC) and multiorgan failure follow. Accompanying laboratory signs can include leukocytosis > 25,000 cells/μL, anemia with hemoglobin < 11 mg/dL and bicarbonate levels below 22 mmol/L [42, 47, 50].
Diagnosis and treatment
The cornerstone of treatment in C. septicum necrotizing fasciitis is aggressive antibiotic treatment accompanied by debridement of the infectious site to remove necrotic tissue and drain wound abscesses. Tissue removal prevents extension of the infection, removes sources of bacteria and improves survival. In cases of C. septicum necrotizing fasciitis, including those involving the bowel, 57% of patients undergoing operations survive versus 26% of those treated with antibiotic regimens alone [46]. Hence, aggressive and prompt surgical intervention is crucial for patient survival. Without treatment, 100% of patients with C. septicum bacteremia die within 48 h [51]. Surgery can be complemented by an antibiotic regimen including high-dose penicillin and clindamycin as well as carbenicillin, cefazolin, cephalothin, chloramphenicol or metronidazole. Table 4 illustrates the antibiotic treatment of choice for invasive infections caused by clostridial species. Certain strains may also be sensitive to vancomycin, cefamandole and rifampin [52]. Resistance to some antibiotics, however, including clindamycin and carbenicillin, has been demonstrated, and thus sensitivity testing is imperative [53, 54]. As with other clostridial species, identification of the bacterium in the bloodstream is difficult, and accurate diagnosis often requires molecular microbiological analysis. Table 3 illustrates the pertinent laboratory findings associated with invasive clostridial infections.
The benefits of hyperbaric oxygen therapy are questionable in C. septicum infection, in light of its in vitro resistance to aerobic environments [45]. Certain cases, however, have reported improved clinical course related to decreased extension of infection with hyperbaric oxygen therapy [45].
C. perfringens
Microbiology
Formerly known as Clostridium welchii, C. perfringens is a Gram-positive, spore-forming, obligate anaerobe. Although there are multiple types of C. perfringens, type A is responsible for gas gangrene, food poisoning and necrotizing enterocolitis, which represent the most common infectious manifestations of the bacterium in humans. In all conditions except food poisoning, an exotoxin underlies the diseases’ pathogeneses. For gas gangrene, the condition most commonly implicated in gynecologic C. perfringens infections, a phospholipase C and a thiol-activated hemolysin cause the condition. Phospholipase C, or α-toxin, also displays both sphingomyelinase activity and platelet aggregation that contribute to the local tissue necrosis and cytotoxicity observed in C. perfringens infection [55, 56]. Additional animal studies have demonstrated that α-toxin also decreases myocardial function, perhaps through inhibition of the calcium-magnesium ATPase in the cardiac sarcoplasmic reticulum [57]. These findings may suggest an additional mechanism for the shock symptoms observed in patients with C. perfringens septicemia. The hemolysin, or θ-toxin, targets cholesterol receptors in the cell membrane to form pores and subsequently hemolysis [55]. This toxin also plays a role in advancing tissue necrosis by downregulating polymorphonuclear leukocyte adhesion to endothelial cells [58].
Pathophysiology of infection
Although endometrial C. perfringens infections are rare, especially after the legalization of therapeutic abortion, there are numerous accounts in the literature describing episodes occurring after cesarean section, amniocentesis, cordocentesis, endometrial ablation, abortion, molar pregnancy or vaginal delivery [59-70]. There is a particularly prominent role of C. perfringens in hospital-acquired gynecologic infection [70]. Furthermore, as with C. septicum, there are reported cases of C. perfringens infection in the setting of choriocarcinoma as well as endometrial or ovarian cancer [71-73]. Several reports have documented C. perfringens isolates from vaginal and cervical cultures, with some finding a 0.8-8% prevalence in normal vaginal flora, a 1-9% prevalence postnatally, and up to a 29% prevalence after abortion [63, 70, 74, 75]. These prevalence rates may even underestimate prevalence: culture-independent methods including high-throughput sequencing techniques of 16S rRNA genes suggest that the vaginal microbiome is even more diverse than originally appreciated [76]. Regardless of the accurate prevalence rates, colonization with exotoxin-producing C. perfringens strains occurs in a minority of cases.
Diagnosis and treatment
Infection often onsets rapidly, with signs of hemolysis, thrombocytopenia, leukocytosis, jaundice, renal failure, tissue necrosis and characteristic gas gangrene crepitis [60, 61, 63, 68, 69, 73, 74, 77-82]. Initial presentation of clostridial endometritis includes dizziness, abdominal or pelvic pain, vaginal bleeding and rapidly expanding uterine size due to air within the uterine wall and cavity [63, 66, 74, 83, 84]. Patients can present both with and without fever [61-63, 70, 74, 80]. Table 3 illustrates the pertinent laboratory findings associated with invasive clostridial infections. Although some cases have reported treatment success with local debridement and antibiotic regimens including high-dose intravenous (IV) penicillin with or without an additional macrolide or gentamycin, ceftriaxone and metronidazole, other reports have documented rapidly fatal sepsis in clostridial endometritis despite debridement, antibiotic therapy, and even hysterectomy [61, 74, 83, 84]. The most commonly reported resistance is to clindamycin, with resistance rates between 3.2% and 14%, depending on the tested bacterial strain [71, 85]. Although antibiotic regimens differ depending on treatment team for conservative management of clostridial endometritis, various susceptibility testing suggests prominent responses to metronidazole, ampicillin/sulbactam, piperacillin, penicillin, gentamicin or cefoxitin [85, 86]. Table 4 illustrates the optimal antibiotic treatment for invasive infections caused by clostridial species.
Despite extensive study, there is little demonstrable role for use of hyperbaric oxygen in treating C. perfringens, although one case reports pain relief and infectious control with hyperbaric oxygen, when C. perfringens complicated that case requiring chemotherapy for choriocarcinoma [87]. The same report also proposed that presurgical treatment with hyperbaric oxygen could help delineate areas of myonecrosis.
Because of the rapid expansion of clostridial infection, antibiotic administration or simple debridement is often insufficient to control disease progression, especially considering the mortality risk of clostridial sepsis. Recent analyses have found that the progression of endometrial infection to clostridial sepsis carries a 30-day mortality rate of 27-44% [85]. Researchers arguing for the operative control of infection with hysterectomy first gained credence in the 1960s when 10 of 11 patients were successfully treated for C. perfringens endometritis and sepsis with hysterectomy [88]. An additional report reviewing a diversity of surgical sources of C. perfringens infection demonstrated a relative risk of mortality associated with surgical intervention of 0.27 when compared to medical treatment (95% CI: 0.08 - 0.89) [89]. Nevertheless, some reports have advocated for more liberal treatment modalities [75, 86]. One report documented the successful treatment of five clinically stable women with rapidly administered, broad-spectrum antibiotics [86]. Although the extent of infection in these women included both intrauterine and blood stream infiltration by C. perfringens, prophylactic doxycycline prior to therapeutic abortion and prompt initiation of antibiotics within 1- 2 h of febrile presentation were adequate to control infection [86].
| S. aureus | ▴Top |
Although primarily associated with tampon use during menstruation, TSS is a condition broadly implicated across many disciplines. In fact, TSS was first described in a pediatric population presenting with a shock characterized by desquamating rash, hypotension and multisystem dysfunction [90]. By the early 1980s, however, epidemiological studies tied TSS to menstruation, with one study finding that women accounted for 37 of 38 surveyed cases, and that almost all women presented during their menstrual cycle [91]. This hallmark study also discovered the connection between TSS and tampon usage: 34 of 35 cases of TSS in menstruating women occurred in tampon users [91]. Several public health measures emerged from these findings, including the removal of highly absorbent tampons with cross-linked carboxymethylcellulose from the market. Since that time, the share of TSS associated with menstruation has declined. One study analyzing 61 TSS cases between 2000 and 2006 found that female patients accounted for 48 of the cases; however, only 33 of these patients were menstrual [92]. The same study found the incidence of menstrual TSS to be 0.69 per 100,000 women, with patients aged 13 - 24 years most affected at incidence rates of 1.41 per 100,000 [92].
Within obstetrics and gynecology, the greatest concern lies in staphylococcal infection as related to menstrual, postpartum and post-surgical TSS [93]. Under the most commonly accepted definition created by the Centers for Disease Control and Prevention (CDC), TSS must occur within 3 days of the beginning or end of the menstrual cycle in order to be classified as menstrual [94]. Most postpartum TSS presents shortly after delivery; however, cases have been described up to 2 months into the postpartum period [95]. There are a few additional cases of septic abortion reported, although it should be noted that S. aureus can also be asymptomatic: one study demonstrated presence of S. aureus on two of 53 pre-abortive cervices and three of 53 sampled laminaria tents [96, 97]. Other rare presentations of TSS include those presenting after intrauterine device insertion [98, 99].
Microbiology
A multitude of strains of S. aureus have been identified; however, unlike other bacteria, the majority of S. aureus’s TSS is due to an exotoxin encoded by the tst gene, called toxic shock syndrome toxin-1 (TSST-1). In fact, 95% of menstrual TSS patients are colonized with tst-carrying S. aureus strains [100]. To induce menstrual TSS, TSST-1 traverses the stratified squamous epithelium of the vaginal canal, simultaneously binding to CD40 on epithelial cells [101]. This action promotes the secretion of interleukin-6 (IL-6), IL-8 and MIP-3α from the vaginal epithelial cells as well as the production of norepinephrine. In turn, these factors recruit both T cells and macrophages to the vaginal submucosa and, in the case of norepinephrine, decrease the barrier capabilities of the vaginal epithelium to potentiate cytokine secretion [101]. Once in contact with T cells and macrophages, TSST-1 induces macrophage production of IL-1 and tumor necrosis factor, which promote the systemic inflammatory response. Additionally, TSST-1 also bypasses monocyte processing to directly bind the T-cell receptor and major histocompatibility complex II and produce additional effects including NF-κB-mediated neutrophil migration, tissue factor, prostaglandin, and nitric oxide secretion, as well as cytokine storm [102].
Pathophysiology of infection
Studies have demonstrated that although about 9% of the female population is vaginally colonized with S. aureus, only 1% of women carry the toxigenic strain [103]. In fact, the presence of the bacterium is transient, often depending on menstrual timing and changes in oxygenation, iron saturation and pH [103]. This fluctuation in bacterial colonization is also thought to be due to the lack of binding sites for S. aureus; unlike the nasal passages, vaginal mucosa lacks cytokeratin 10 that S. aureus’s clumping factor B can bind [103]. Original associations between TSS and tampon use were tied to the initiation of tampon use: typically, the symptom presents toward the middle or end of the menstrual cycle after a brief period of tampon use [91]. Although the subsequent mechanism of disease progression is poorly understood, it has been suggested that this bacterial colonization is exacerbated by the aerobic exposure promoted by tampon presence and the medium provided by persistence of endometrial blood in the vaginal canal [104]. Additional factors associated with menstruation, including the suppression of IL-1 by the low levels of estrogen and progesterone observed during menstruation, further the inflammatory response [105].
Outside of the menstrual environment, the postpartum state can also foster S. aureus infection. Numerous cases of postpartum TSS have been documented, with patients presenting up to 2 months after vaginal delivery [95, 106, 107]. Often, cases are distinguished as early onset when occurring within 3 days of delivery and as late onset when occurring 2 or more weeks after delivery [108]. Isolated cases of concomitant neonatal TSS have also been documented [109]. It has been postulated that the trauma associated with delivery may facilitate diffusion of the TSST-1 across the vaginal epithelium and into the bloodstream [95]. Additional reports, however, suggest the possibility of iatrogenic contamination, particularly in cases of methicillin-resistant Staphylococcus aureus (MRSA) infection [107]. Few studies of endometrial environments after vaginal delivery have been conducted; however, one study did demonstrate the presence of S. aureus in one of 14 women sampled [110].
The vaginal environment promotes production of TSST-1. At sufficient levels, TSST-1 produces the symptoms observed in TSS: it is responsible for 95% of cases [100]. Although most carriers develop high titers to TSST-1, up to 3% of those with vaginal colonization of toxigenic S. aureus lack titers above 1:4 [111]. In those populations lacking antibodies, the infection progresses rapidly to fulminant shock. Often, antibody titers will elevate independently of the colonization status: women who clear toxigenic S. aureus often experience persistent titer elevation [111]. For the 37% of women who fail to develop antibodies to TSST-1, recurrence of TSS is common [112].
Diagnosis and treatment
Both menstrual and non-menstrual TSSs present in similar manners, and for definite diagnosis patients must display the five CDC-designated criteria: fever > 38.9 °C, desquamation, rash, hypotension and multisystem dysfunction. For probable diagnosis, four of these criteria must be displayed. Additional symptoms often displayed include gastrointestinal abnormalities, headache and weakness [113]. For most accurate diagnosis, these symptomatic criteria should be complemented with laboratory findings, including culture for S. aureus. Further testing for susceptibility is nearly universal, as it allows for the classification of the bacterium’s methicillin susceptibility and for the optimization of antibiotic treatment. Table 3 illustrates the pertinent laboratory findings associated with invasive S. aureus infections.
To best treat TSS, it is of utmost importance that patients are clinically stabilized in an efficient manner. At the present time, mortality rates in S. aureus TSS can approach 10%, with mortality most directly correlated to the duration and severity of ARDS [95]. Appropriate treatment, particularly of menstrual TSS, can lower mortality rates to 0% [100]. Table 4 illustrates the antibiotic treatment for invasive infections caused by S. aureus.
In addition to initial treatment, antibiotic use, especially with B-lactamase resistant antibiotics, can sharply decrease recurrence rates [91]. Susceptibility of S. aureus strains depend on the community, and all regimens should cross cover for S. pyogenes due to difficulty of differentiating S. aureus from S. pyogenes TSS. Previous reports have described high percentages of susceptibility to vancomycin, gentamicin, bactrim, quinolones, oxacillin and, in some cases, clindamycin [92]. Preliminary in vitro studies of clindamycin suggest that it may inhibit toxin production [114]. Unlike clostridial infections or streptococcal infections, staphylococcal TSS can often be treated with antibiotics alone. This is largely due to the fact that the clinical presentation is driven by an exotoxin as opposed to the bacteria itself.
In many cases, the S. aureus exotoxin has already disseminated throughout the body, and the inflammatory response into the exotoxin drives the clinical manifestations of the syndrome. Manifestations including DIC may necessitate the transfusion of fresh frozen plasma, cryopreciptate, or platelets, especially in the setting of hemorrhage. These additional manifestations require hemodynamic monitoring, and it is thus important that the patient be treated in an intensive care setting. Aggressive pulmonary treatments, including oxygen supplementation and even intubation, should be quickly instituted. Additional supportive care should include fluid resuscitation with normal saline or lactated ringers as well as vasopressors, including dopamine or norepinephrine, in the case of prolonged hypotension, tachycardia or end-organ damage. Electrolytes must be monitored closely, as should organ function. Preliminary studies in animal and human models have found benefits of IV immunoglobulin as well as IL therapy in blocking T-cell activation by the TSST-1 superantigen [115]. Therapeutic control of fever or inflammatory response is controversial, and results are conflicting regarding the advantages of cyclooxygenase inhibitors, non-steroidal anti-inflammatories or glucocorticoids [95]. Other studies are beginning to probe the possible addition of antioxidants, including N-acetyl cysteine, to decrease NF-κB activation [116]. Within the first several hours, if the patient is clinically deteriorating on antibiotics, surgical intervention should be considered, as clostridial infection cannot be ruled out. There are numerous cases of infectious resolution with hysterectomy, particularly in the setting of recurrent MRSA-driven endometritis [107]. Any surgical-site infection, after cesarean section, episiotomy or other surgery should be immediately and aggressively debrided.
After treatment of the initial infection and eradication of the bacterium on culture, TSS can recur at rates between 25% and 50%, with lower recurrence rates demonstrated in patients treated with B-lactamase resistant antibiotics [95, 117]. Typically, recurrences present within 2 months; however, susceptibility is increased for up to a year after initial infection [95].
| S. pyogenes | ▴Top |
S. pyogenes, also known as group A streptococcus (GAS), is a potentially lethal microorganism that is rarely encountered in obstetrics and gynecology. GAS was first reported by Ignaz Semmelweis in 1847, an obstetrician practicing in Vienna, France [118]. He described how the failure of obstetricians to wash their hands prior to operating resulted in a surge of deaths secondary to GAS. By encouraging hand-washing techniques, the cases of fatal puerperal fever decreased from 12% to 2% [118]. When encountered, however, this microorganism can quickly progress to sepsis, necrotizing soft tissue infections, TSS and death. The overall case fatality rate is approximately 20%, with more than half of patients dying if septic shock develops [119]. The CDC estimates that approximately 11,500 cases (3.5 per 100,000 people) of invasive GAS disease occur annually in the United States. Bacteremia without a source (29%), pneumonia (15%), necrotizing fasciitis (7%) and streptococcal TSS (6%) represent the most common causes of invasive GAS infections [119].
Today, although invasive GAS infection is infrequent in developed countries, it still causes about 40% of septic deaths among patients with postpartum endometritis, necrotizing fasciitis and TSS infection [120]. GAS is rarely present in the normal vaginal flora, and is generally secondary to inoculation from a woman’s own pharynx or from a close contact source. The colonization rate of GAS in the vagina is approximately 0.03%, and colonization is highly transient [121]. Obstetricians should be aware that if GAS is noted on a recto-vaginal swab collection culture during pregnancy, a test routinely performed to check for group B streptococcus, it should be promptly treated given its very infectious nature. If left untreated, this microorganism can result in invasive GAS infection after delivery [122].
Microbiology
GAS is a Gram-positive coccus that can survive in both aerobic and anaerobic environments, hence its facultative nature. This microorganism has been associated with numerous infections in humans, particularly pharyngitis, tonsillitis, scarlet fever, erysipelas, cellulitis, lymphangitis, necrotizing fasciitis, myonecrosis and TSS. It also boasts post-infective complications including rheumatic fever and post-streptococcal glomerulonephritis. In obstetrics and gynecology, yet rare, this microorganism has been associated with severe postpartum endometritis and subsequent widespread invasive disease [5, 6, 123]. Similar to other bacteria, GAS ascends from the vagina into the uterus after delivery. Given its facultative nature, the large amount of blood and necrotic decidual tissue within the uterus acts as an excellent medium for GAS growth [122].
Table 5 illustrates the different infectious conditions that ultimately arise from untreated, invasive GAS. There are a wide variety of infectious conditions that can result from this microbe, which are largely due to the culmination of complex interactions between the human host defense mechanisms and specific virulence factors of the streptococcus. In humans, the only known reservoir for GAS is the skin and mucous membranes. It is very infectious and easily transmitted, either by respiratory droplets or by direct contact with the microorganism. After gaining access to the human body, GAS can seed the bloodstream, resulting in bacteremia and sepsis. Unfortunately, the pathogenic mechanisms underlying the more widespread GAS infections remain poorly understood.
 Click to view | Table 5. Infectious Sequelae From Untreated Streptococcus pyogenes (Group A Streptococcus) in Obstetrics and Gynecology |
Pathogenesis of infection
The pathogenesis resulting from invasive GAS infections harbors from virulence factors associated with this microorganism. These factors enable GAS to attach to the host tissue, evade the immune response and spread by penetrating host tissue layers. There are several virulence factors, all of which have unique pathological features that result in invasive disease [124]. However, of these factors, M protein is considered the major somatic virulence factor. This protein allows GAS to adhere to endothelium of blood vessels, causing vascular leakage and hypercoagulability. Even with treatment, this protein can cause DIC, multi-organ failure and death. Additionally, M protein resists the human immune response by preventing macrophage phagocytosis.
It is not clear why some patients with GAS progress into widespread disseminated invasive disease while others do not. With the right environment and specific trigger, such as surgery or a vaginal laceration after a delivery, GAS can release its virulence factors and quickly progress to an acute highly lethal state.
Diagnosis and treatment
As mentioned previously, the colonization rate of GAS in the vagina is approximately 0.03% [121]. Table 4 illustrates how aggressive GAS is; therefore, when found, this should be treated promptly. Although GAS can rapidly disseminate throughout the bloodstream, blood cultures are frequently negative. Obtaining tissue from the infectious source to isolate the bacterium or its virulence factors is often the only reliable laboratory test. Although laboratory values are often non-diagnostic, in the context of a strongly suspected severe soft tissue infection, they can support the diagnosis of necrotizing fasciitis [5]. Table 3 illustrates the pertinent laboratory findings associated with invasive GAS infections: particular concern for necrotizing fasciitis should be paid to patients with a white blood cell count > 25,000/mL, a hemoglobin level of < 11 mg/dL, a serum sodium of < 135 mEq/L, a creatinine level of > 1.6 mg/dL and a glucose level of > 180 mg/dL [5]. There may be marked hemoconcentration as fluid pours into the area of necrosis, as well as evidence of DIC and septic shock. Hypocalcemia is common as a consequence of the necrotic fat binding to calcium to form soap [5].
Once a presumptive diagnosis of invasive GAS is suspected, all patients should be hospitalized; blood, urine, and endometrial cultures should be obtained; and IV antibiotics should be immediately started. The cornerstone of GAS antibiotic therapy is penicillin, as GAS remains exquisitely sensitive. Carbapenems, such as meropenem, can be used as an alternative. In addition, toxin release is highly dependent on protein synthesis; therefore, antibiotics such as clindamycin that inhibit protein synthesis should be started. Furthermore, clindamycin performs better than penicillin when bacteria are in the stationary phase of their growth cycle [125, 126]. Table 4 illustrates the antibiotic treatment for invasive infections caused by GAS.
| Clinical Approach/Management: Considerations for Hysterectomy | ▴Top |
In most scenarios, women with serious gynecologic infections present to the emergency department and are shortly thereafter admitted to the intensive care unit by emergency practitioners, given the life-threatening severity of their condition. A multidisciplinary team involving an obstetrician gynecologist, intensivist and an infectious disease specialist should be the first consulted to these critically ill patients. In addition, a gynecological oncologist or a general surgeon should also be consulted early in the management of these patients, as aggressive surgical intervention is often crucial to preserve patient life. After collection of multiple cultures, including blood, cervical and vaginal swabs, and urine, broad-spectrum antibiotics should immediately be initiated. Postpartum or postabortal patients should also have a uterine aspirate sent for aerobic and anaerobic culture. Imaging studies may be ordered, depending on the clinical prognosis of the patient; however, surgical intervention should not be postponed until after imaging or cultures are made available, as these patients can rapidly deteriorate. Tissue should be collected and sent for polymerase chain reaction (PCR) testing to isolate these microorganisms when the conventional cultures are negative. Surgical intervention should be performed in any patient that is failing medical therapy, has worsening laboratory results, or has clinical signs of worsening sepsis despite appropriate antibiotic therapy.
In these critically ill patients, clostridial species, GAS or S. aureus should be considered early [1]. The treatment of these serious infections involves a combination of antimicrobial agents, supportive care, hemodynamic monitoring and most importantly, source control. Antimicrobial therapy should include coverage against Gram-positive, Gram-negative and anaerobic microorganisms. Most clinicians agree that a combination of vancomycin to cover MRSA, meropenem, to cover Gram-negative microbes, and clindamycin, to cover anaerobes and provide anti-toxin effects, is the most appropriate initial regimen. These antibiotics should be tailored once the susceptibility panel is made available. Table 4 illustrates the antibiotic treatment for these invasive infections.
Source control is the biggest factor that will set the fate of patient lives. In particular, antimicrobial therapy alone is not sufficient to treat necrotizing soft tissue infection [5]. In these cases, early surgical intervention is critical to remove the necrotic infected tissue. For example, in patients with postpartum or postabortal streptococcal TSS, hysterectomy needs to be considered early in patient management [6]. Table 6 illustrates the indications for surgical intervention with serious infections in obstetrics and gynecology. In patients with vulvar or incisional necrotizing soft tissue infections, early wide local debridement is critical. All necrotic tissue, including the overlying skin, should be resected until healthy bleeding tissue is encountered. On many occasions, these patients require serial surgical procedures to remove all the necrotic tissue [5].
 Click to view | Table 6. Indications for Hysterectomy in Women With Invasive Streptococcus pyogenes (Group A Streptococcus), Clostridial Species and Staphylococcus aureus in Obstetrics and Gynecology |
| Summary | ▴Top |
Invasive infections caused by S. pyogenes (GAS), clostridial species and S. aureus in obstetrics and gynecology are serious and potentially fatal in both pregnant and non-pregnant women. Increasing your index of suspicion will allow you to consider the diagnosis and order the appropriate laboratory tests that can lead you to early diagnosis and intervention.
Combination antibiotic therapy with penicillin is recommended to treat the described bacteria; however, if S. aureus is suspected, then vancomycin should be empirically started until a susceptibility panel is available. In addition, clindamycin should be added to the above regimen, to not only treat the underlying infection, but to also limit toxin production and the resultant inflammatory response. Carbapenems, such as meropenem, also provide excellent coverage and can be substituted for penicillin.
Surgical therapy to remove the source of infection and its toxin production is crucial to the effective therapy of necrotizing soft tissue infections and TSS. Surgical intervention should not be delayed in patients not improving to aggressive medical therapy within the first 24 h, as early surgical intervention, such as a hysterectomy, is necessary and is often lifesaving in women with either pregnancy-associated or gynecological-associated necrotizing soft tissue infections as well as those with TSS.
A multidisciplinary approach in an intensive care setting is recommended for women with the serious clinical manifestations of invasive infections caused by S. pyogenes (GAS), clostridial species and S. aureus in obstetrics and gynecology.
Synopsis
When antimicrobials alone are insufficient to control an infection, early surgical intervention is often needed to treat the source of infection and prevent further dissemination.
Conflict of Interest
None of the authors declare any conflicts of interest in the manuscript, including financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
| References | ▴Top |
- Soper DE. Early recognition of serious infections in obstetrics and gynecology. Clin Obstet Gynecol. 2012;55(4):858-863.
doi pubmed - Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17(7):357-365.
doi pubmed - Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84.
doi - Bone RC. The sepsis syndrome. Definition and general approach to management. Clin Chest Med. 1996;17(2):175-181.
doi - Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44(5):705-710.
doi pubmed - Rimawi BH, Soper DE, Eschenbach DA. Group A streptococcal infections in obstetrics and gynecology. Clin Obstet Gynecol. 2012;55(4):864-874.
doi pubmed - Busch C, Aktories K. Microbial toxins and the glycosylation of rho family GTPases. Curr Opin Struct Biol. 2000;10(5):528-535.
doi - Stevens DL, Bisno AL, Chambers HF, Everett ED, Dellinger P, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41(10):1373-1406.
doi pubmed - Martinez RD, Wilkins TD. Comparison of Clostridium sordellii toxins HT and LT with toxins A and B of C. difficile. J Med Microbiol. 1992;36(1):30-36.
doi pubmed - Popoff MR, Chaves-Olarte E, Lemichez E, von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, et al. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271(17):10217-10224.
doi pubmed - Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43(11):1436-1446.
doi pubmed - Sinave C, Le Templier G, Blouin D, Leveille F, Deland E. Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and postabortion disease. Clin Infect Dis. 2002;35(11):1441-1443.
doi pubmed - Sosolik RC, Savage BA, Vaccarello L. Clostridium sordellii toxic shock syndrome: a case report and review of the literature. Infect Dis Obstet Gynecol. 1996;4(1):31-35.
doi pubmed - Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, Poukens V, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353(22):2352-2360.
doi pubmed - McGregor JA, Soper DE, Lovell G, Todd JK. Maternal deaths associated with Clostridium sordellii infection. Am J Obstet Gynecol. 1989;161(4):987-995.
doi - Cohen AL, Bhatnagar J, Reagan S, Zane SB, D'Angeli MA, Fischer M, Killgore G, et al. Toxic shock associated with Clostridium sordellii and Clostridium perfringens after medical and spontaneous abortion. Obstet Gynecol. 2007;110(5):1027-1033.
doi pubmed - Meites E, Zane S, Gould C. Fatal Clostridium sordellii infections after medical abortions. N Engl J Med. 2010;363(14):1382-1383.
doi pubmed - Bitti A, Mastrantonio P, Spigaglia P, Urru G, Spano AI, Moretti G, Cherchi GB. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J Clin Pathol. 1997;50(3):259-260.
doi pubmed - Rorbye C, Petersen IS, Nilas L. Postpartum Clostridium sordellii infection associated with fatal toxic shock syndrome. Acta Obstet Gynecol Scand. 2000;79(12):1134-1135.
pubmed - Ho CS, Bhatnagar J, Cohen AL, Hacker JK, Zane SB, Reagan S, Fischer M, et al. Undiagnosed cases of fatal Clostridium-associated toxic shock in Californian women of childbearing age. Am J Obstet Gynecol. 2009;201(5):459 e451-457.
- Wiebe E, Guilbert E, Jacot F, Shannon C, Winikoff B. A fatal case of Clostridium sordellii septic shock syndrome associated with medical abortion. Obstet Gynecol. 2004;104(5 Pt 2):1142-1144.
doi pubmed - Soper DE. Clostridial myonecrosis arising from an episiotomy. Obstet Gynecol. 1986;68(3 Suppl):26S-28S.
pubmed - Hogan SF, Ireland K. Fatal acute spontaneous endometritis resulting from Clostridium sordelli. Am J Clin Pathol. 1989;91(1):104-106.
pubmed - Reis T, Chaves C, Soares A, Moreira M, Boaventura L, Ribeiro G. A Clostridium sordellii fatal toxic shock syndrome post-medical-abortion in Portugal, in 21st European Congress of Clinical Microbiology and Infectious Diseases. 2011: Milan, Italy.
- Harvey H, MF, A case of infection with Clostridium sordellii and gas gangrene treated by penicillin. Surgery. 1944;15(4):622-627.
- Golde S, Ledger WJ. Necrotizing fasciitis in postpartum patients. A report of four cases. Obstet Gynecol. 1977;50(6):670-673.
pubmed - Shannon C, VM, Chong E, Agnew K, Nucatola D, Newhall E, Sheehan K, Winkoff B, Vaginal and Rectal Clostridial Colonization among Women of Reproductive Age in the United States. Contraception. 2010;82:1.
doi - Miech RP. Pathophysiology of mifepristone-induced septic shock due to Clostridium sordellii. Ann Pharmacother. 2005;39(9):1483-1488.
doi pubmed - Ramirez N, Abel-Santos E. Requirements for germination of Clostridium sordellii spores in vitro. J Bacteriol. 2010;192(2):418-425.
doi pubmed - Liggins M, Ramirez N, Magnuson N, Abel-Santos E. Progesterone analogs influence germination of Clostridium sordellii and Clostridium difficile spores in vitro. J Bacteriol. 2011;193(11):2776-2783.
doi pubmed - Shannon C, Winikoff B. Comment on "Misoprostol impairs female reproductive tract innate immunity against Clostridium sordellii". J Immunol. 2008;181(4):2263; author reply 2263-2264.
doi pubmed - Bhatnagar J, Deleon-Carnes M, Kellar KL, Bandyopadhyay K, Antoniadou ZA, Shieh WJ, Paddock CD, et al. Rapid, simultaneous detection of Clostridium sordellii and Clostridium perfringens in archived tissues by a novel PCR-based microsphere assay: diagnostic implications for pregnancy-associated toxic shock syndrome cases. Infect Dis Obstet Gynecol. 2012;2012:972845.
doi pubmed - Nakamura S, Yamakawa K, Nishida S. Antibacterial susceptibility of Clostridium sordellii strains. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;261(3):345-349.
doi - Stevens DL, Bryant AE, Hackett SP. Antibiotic effects on bacterial viability, toxin production, and host response. Clin Infect Dis. 1995;20(Suppl 2):S154-157.
doi pubmed - Stevens DL, Maier KA, Mitten JE. Effect of antibiotics on toxin production and viability of Clostridium perfringens. Antimicrob Agents Chemother. 1987;31(2):213-218.
doi pubmed - Bryant AE, Stevens DL. Clostridial myonecrosis: new insights in pathogenesis and management. Curr Infect Dis Rep. 2010;12(5):383-391.
doi pubmed - Stevens DL, Aldape MJ, Bryant AE. Life-threatening clostridial infections. Anaerobe. 2012;18(2):254-259.
doi pubmed - Knapp O, Maier E, Mkaddem SB, Benz R, Bens M, Chenal A, Geny B, et al. Clostridium septicum alpha-toxin forms pores and induces rapid cell necrosis. Toxicon. 2010;55(1):61-72.
doi pubmed - Holdeman LV, Good IJ, Moore WE. Human fecal flora: variation in bacterial composition within individuals and a possible effect of emotional stress. Appl Environ Microbiol. 1976;31(3):359-375.
pubmed - Finegold SM, Attebery HR, Sutter VL. Effect of diet on human fecal flora: comparison of Japanese and American diets. Am J Clin Nutr. 1974;27(12):1456-1469.
pubmed - Finegold SM, Sutter VL, Sugihara PT, Elder HA, Lehmann SM, Phillips RL. Fecal microbial flora in Seventh Day Adventist populations and control subjects. Am J Clin Nutr. 1977;30(11):1781-1792.
pubmed - Rimawi BH, Graybill W, Pierce JY, Kohler M, Eriksson EA, Shary MT, Crookes B, et al. Necrotizing Fasciitis and Toxic Shock Syndrome from Clostridium septicum following a Term Cesarean Delivery. Case Rep Obstet Gynecol. 2014;2014:724302.
doi - Khoo CL, Meskhi A, Harris CP. Fatal Clostridium septicum following medical termination of pregnancy. J Obstet Gynaecol. 2013;33(5):530-531.
doi pubmed - Thiele EH, Arison RN, Boxer GE. Oncolysis by Clostridia. Iv. Effect of Nonpathogenic Clostridial Spores in Normal and Pathological Tissues. Cancer Res. 1964;24:234-238.
pubmed - Hill GB, Osterhout S. Experimental effects of hyperbaric oxgen on selected clostridial species. I. In-vitro studies. J Infect Dis. 1972;125(1):17-25.
doi pubmed - Hermsen JL, Schurr MJ, Kudsk KA, Faucher LD. Phenotyping Clostridium septicum infection: a surgeon's infectious disease. J Surg Res. 2008;148(1):67-76.
doi pubmed - Kornbluth AA, Danzig JB, Bernstein LH. Clostridium septicum infection and associated malignancy. Report of 2 cases and review of the literature. Medicine (Baltimore). 1989;68(1):30-37.
doi - Prinssen HM, Hoekman K, Burger CW. Clostridium septicum myonecrosis and ovarian cancer: a case report and review of literature. Gynecol Oncol. 1999;72(1):116-119.
doi pubmed - Lee CH, Hsieh SY. Case report: Clostridium septicum infection presenting as liver abscess in a case of choriocarcinoma with liver metastasis. J Gastroenterol Hepatol. 1999;14(12):1227-1229.
doi - Haas LE, Tjan DH, van Zanten AR. Fatal Clostridium septicum infection in a young pregnant woman. Neth J Med. 2006;64(7):254-255.
pubmed - Cline KA, Turnbull TL. Clostridial myonecrosis. Ann Emerg Med. 1985;14(5):459-466.
doi - Gabay EL, Rolfe RD, Finegold SM. Susceptibility of Clostridium septicum to 23 antimicrobial agents. Antimicrob Agents Chemother. 1981;20(6):852-853.
doi pubmed - Wilkins TD, Thiel T. Resistance of some species of Clostridium to clindamycin. Antimicrob Agents Chemother. 1973;3(1):136-137.
doi - Tally FP, Jacobus NV, Bartlett JG, Gorbach SL. In vitro activity of penicillins against anaerobes. Antimicrob Agents Chemother. 1975;7(4):413-414.
doi pubmed - Rood JI, Cole ST. Molecular genetics and pathogenesis of Clostridium perfringens. Microbiol Rev. 1991;55(4):621-648.
pubmed - Sugahara T, Takahashi T, Yamaya S, Ohsaka A. In vitro aggregation of platelets induced by alpha-toxin (phospholipase C) of Clostridium perfringens. Jpn J Med Sci Biol. 1976;29(5):255-263.
doi pubmed - Stevens DL, Troyer BE, Merrick DT, Mitten JE, Olson RD. Lethal effects and cardiovascular effects of purified alpha- and theta-toxins from Clostridium perfringens. J Infect Dis. 1988;157(2):272-279.
doi pubmed - Bryant AE, Bergstrom R, Zimmerman GA, Salyer JL, Hill HR, Tweten RK, Sato H, et al. Clostridium perfringens invasiveness is enhanced by effects of theta toxin upon PMNL structure and function: the roles of leukocytotoxicity and expression of CD11/CD18 adherence glycoprotein. FEMS Immunol Med Microbiol. 1993;7(4):321-336.
doi pubmed - Gold J, Cates W, Jr., Nelson M, Kimball AM, Rochat RW, Chester DA, Tyler CW, Jr. A cluster of septic complications associated with illegal induced abortions. Obstet Gynecol. 1980;56(3):311-315.
pubmed - Baltzer J, Geissler K, Gloning KP, Schramm T, Haider M. [Clostridium infection in the puerperium following cesarean section]. Geburtshilfe Frauenheilkd. 1989;49(11):1010-1013.
doi pubmed - Barrett JP, Whiteside JL, Boardman LA. Fatal clostridial sepsis after spontaneous abortion. Obstet Gynecol. 2002;99(5 Pt 2):899-901.
doi - Jasnosz KM, Shakir AM, Perper JA. Fatal Clostridium perfringens and Escherichia coli sepsis following urea-instillation abortion. Am J Forensic Med Pathol. 1993;14(2):151-154.
doi pubmed - Kirkpatrick CJ, Werdehausen K, Jaeger J, Breining H. Fatal Clostridium perfringens infection after normal term pregnancy. Arch Gynecol. 1982;231(2):167-170.
doi pubmed - Hendrix NW, Mackeen AD, Weiner S. Clostridium perfringens Sepsis and Fetal Demise after Genetic Amniocentesis. AJP Rep. 2011;1(1):25-28.
doi pubmed - Fray RE, Davis TP, Brown EA. Clostridium welchii infection after amniocentesis. Br Med J (Clin Res Ed). 1984;288(6421):901-902.
doi - Hovav Y, Hornstein E, Pollack RN, Yaffe C. Sepsis due to Clostridium perfringens after second-trimester amniocentesis. Clin Infect Dis. 1995;21(1):235-236.
doi pubmed - Hamoda H, Chamberlain PF. Clostridium welchii infection following amniocentesis: a case report and review of the literature. Prenat Diagn. 2002;22(9):783-785.
doi pubmed - Adams BN, Lekovic JP, Robinson S. Clostridium perfringens sepsis following a molar pregnancy. Am J Obstet Gynecol. 2014;210(1):e13-14.
doi pubmed - Plachouras N, Sotiriadis A, Dalkalitsis N, Kontostolis E, Xiropotamos N, Paraskevaidis E. Fulminant sepsis after invasive prenatal diagnosis. Obstet Gynecol. 2004;104(6):1244-1247.
doi pubmed - Ledger WJ, Hackett KA. Significance of clostridia in the female reproductive tract. Obstet Gynecol. 1973;41(4):525-530.
pubmed - Leal J, Gregson DB, Ross T, Church DL, Laupland KB. Epidemiology of Clostridium species bacteremia in Calgary, Canada, 2000-2006. J Infect. 2008;57(3):198-203.
doi pubmed - Braverman J, Adachi A, Lev-Gur M, Fallen S, Rosenzweig M, Greston WM, Kleiner GJ. Spontaneous clostridia gas gangrene of uterus associated with endometrial malignancy. Am J Obstet Gynecol. 1987;156(5):1205-1207.
doi - Kurashina R, Shimada H, Matsushima T, Doi D, Asakura H, Takeshita T. Spontaneous uterine perforation due to clostridial gas gangrene associated with endometrial carcinoma. J Nippon Med Sch. 2010;77(3):166-169.
doi pubmed - Dylewski J, Wiesenfeld H, Latour A. Postpartum uterine infection with Clostridium perfringens. Rev Infect Dis. 1989;11(3):470-473.
doi pubmed - O'Neill RT, Schwarz RH. Clostridial organisms in septic abortions. Report of 7 cases. Obstet Gynecol. 1970;35(3):458-461.
pubmed - Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. The vaginal microbiome: new information about genital tract flora using molecular based techniques. BJOG. 2011;118(5):533-549.
doi pubmed - Jemni L, Chatti N, Chakroun M, Allegue M, Chaieb L, Djaidane A. [Clostridium perfringens septicemia]. Rev Fr Gynecol Obstet. 1988;83(6):407-409.
pubmed - Montavon C, Krause E, Holzgreve W, Hosli I. [Uterine gas gangrene through clostridium perfringens sepsis after uterus rupture postpartum]. Z Geburtshilfe Neonatol. 2005;209(5):167-172.
doi pubmed - Singh S, Angra K, Davis B, Shokrani B. Complication of Invasive Molar Pregnancy with Clostridium perfringens Sepsis. Case Rep Obstet Gynecol. 2014;2014:282141.
- Halpin TF, Molinari JA. Diagnosis and management of clostridium perfringens sepsis and uterine gas gangrene. Obstet Gynecol Surv. 2002;57(1):53-57.
doi - Soper DE, Lee SI, Kim JY, McDonald AG. Case records of the Massachusetts General Hospital. Case 35-2011: A 33-year-old woman with postpartum leukocytosis and Gram-positive bacteremia. N Engl J Med. 2011;365(20):1916-1924.
doi pubmed - Nadisauskiene RJ, Kliucinskas M, Vitkauskiene A, Minkauskiene M, Vaitkiene D. Puerperal Clostridium perfringens sepsis in a patient with granulocytopenia. Gynecol Obstet Invest. 2008;65(1):32-34.
doi pubmed - Alsammani MA, Ahmed SR, Alsheeha MA, Saadia Z, Khairi SA. Co-infection with Toxoplasma gondii and Clostridium perfringens in a postpartum woman with uterine gas gangrene: a case report. J Obstet Gynaecol Res. 2012;38(7):1024-1027.
doi pubmed - Stroumsa D, Ben-David E, Hiller N, Hochner-Celnikier D. Severe Clostridial Pyomyoma following an Abortion Does Not Always Require Surgical Intervention. Case Rep Obstet Gynecol. 2011;2011:364641.
doi - Yang CC, Hsu PC, Chang HJ, Cheng CW, Lee MH. Clinical significance and outcomes of Clostridium perfringens bacteremia--a 10-year experience at a tertiary care hospital. Int J Infect Dis. 2013;17(11):e955-960.
doi pubmed - Lichtenberg ES, Henning C. Conservative management of clostridial endometritis. Am J Obstet Gynecol. 2004;191(1):266-270.
doi pubmed - Lacey CG, Futoran R, Morrow CP. Clostridium perfringens infection complicating chemotherapy for choriocarcinoma. Obstet Gynecol. 1976;47(3):337-341.
pubmed - Decker WH, Hall W. Treatment of abortions infected with Clostridium welchii. Am J Obstet Gynecol. 1966;95(3):394-399.
pubmed - van Bunderen CC, Bomers MK, Wesdorp E, Peerbooms P, Veenstra J. Clostridium perfringens septicaemia with massive intravascular haemolysis: a case report and review of the literature. Neth J Med. 2010;68(9):343-346.
pubmed - Todd J, Fishaut M, Kapral F, Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978;2(8100):1116-1118.
doi - Davis JP, Chesney PJ, Wand PJ, LaVenture M. Toxic-shock syndrome: epidemiologic features, recurrence, risk factors, and prevention. N Engl J Med. 1980;303(25):1429-1435.
doi pubmed - DeVries AS, Lesher L, Schlievert PM, Rogers T, Villaume LG, Danila R, Lynfield R. Staphylococcal toxic shock syndrome 2000-2006: epidemiology, clinical features, and molecular characteristics. PLoS One. 2011;6(8):e22997.
doi pubmed - Bartlett P, Reingold AL, Graham DR, Dan BB, Selinger DS, Tank GW, Wichterman KA. Toxic shock syndrome associated with surgical wound infections. JAMA. 1982;247(10):1448-1450.
doi pubmed - Hajjeh RA, Reingold A, Weil A, Shutt K, Schuchat A, Perkins BA. Toxic shock syndrome in the United States: surveillance update, 1979 1996. Emerg Infect Dis. 1999;5(6):807-810.
doi pubmed - Davis D, Gash-Kim TL, Heffernan EJ. Toxic shock syndrome: case report of a postpartum female and a literature review. J Emerg Med. 1998;16(4):607-614.
doi - Aslam AF, Aslam AK, Thakur AC, Vasavada BC, Khan IA. Staphylococcus aureus infective endocarditis and septic pulmonary embolism after septic abortion. Int J Cardiol. 2005;105(2):233-235.
doi pubmed - Evaldson GR, Fianu S, Jonasson A, Larsson B, Nord CE, Olund AR. Does the hygroscopic property of the laminaria tent imply a risk for ascending infection in legal abortions? A microbiological study. Acta Obstet Gynecol Scand. 1986;65(3):257-261.
doi pubmed - Klug CD, Keay CR, Ginde AA. Fatal toxic shock syndrome from an intrauterine device. Ann Emerg Med. 2009;54(5):701-703.
doi pubmed - Herzer CM. Toxic shock syndrome: broadening the differential diagnosis. J Am Board Fam Pract. 2001;14(2):131-136.
pubmed - Descloux E, Perpoint T, Ferry T, Lina G, Bes M, Vandenesch F, Mohammedi I, et al. One in five mortality in non-menstrual toxic shock syndrome versus no mortality in menstrual cases in a balanced French series of 55 cases. Eur J Clin Microbiol Infect Dis. 2008;27(1):37-43.
doi pubmed - Brosnahan AJ, Vulchanova L, Witta SR, Dai Y, Jones BJ, Brown DR. Norepinephrine potentiates proinflammatory responses of human vaginal epithelial cells. J Neuroimmunol. 2013;259(1-2):8-16.
doi pubmed - Brosnahan AJ, Schlievert PM. Gram-positive bacterial superantigen outside-in signaling causes toxic shock syndrome. FEBS J. 2011;278(23):4649-4667.
doi pubmed - Parsonnet J, Hansmann MA, Seymour JL, Delaney ML, Dubois AM, Modern PA, Jones MB, et al. Persistence survey of toxic shock syndrome toxin-1 producing Staphylococcus aureus and serum antibodies to this superantigen in five groups of menstruating women. BMC Infect Dis. 2010;10:249.
doi pubmed - Tang YW, Himmelfarb E, Wills M, Stratton CW. Characterization of three Staphylococcus aureus isolates from a 17-year-old female who died of tampon-related toxic shock syndrome. J Clin Microbiol. 2010;48(5):1974-1977.
doi pubmed - Polan ML, Loukides J, Nelson P, Carding S, Diamond M, Walsh A, Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989;69(6):1200-1206.
doi pubmed - Gibney RT, Moore A, Muldowney FP. Toxic-shock syndrome associated with post-partum staphylococcal endometritis. Ir Med J. 1983;76(2):90-91.
pubmed - Collet C, Petsaris O, Lafforgue N, Poulain P, Gautier P, Michelet C, Donnio PY. Postpartum toxic shock syndrome due to methicillin-resistant Staphylococcus aureus epidemic in community. Eur J Obstet Gynecol Reprod Biol. 2009;144(2):184-185.
doi pubmed - Katoh H, Ogihara T, Iyori S. Postpartum toxic shock syndrome: a report of a case. Jpn J Med. 1988;27(1):71-73.
doi pubmed - Green SL, LaPeter KS. Evidence for postpartum toxic-shock syndrome in a mother-infant pair. Am J Med. 1982;72(1):169-172.
doi - Eschenbach DA, Rosene K, Tompkins LS, Watkins H, Gravett MG. Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. J Infect Dis. 1986;153(6):1038-1045.
doi pubmed - Parsonnet J, Hansmann MA, Delaney ML, Modern PA, Dubois AM, Wieland-Alter W, Wissemann KW, et al. Prevalence of toxic shock syndrome toxin 1-producing Staphylococcus aureus and the presence of antibodies to this superantigen in menstruating women. J Clin Microbiol. 2005;43(9):4628-4634.
doi pubmed - Stolz SJ, Davis JP, Vergeront JM, Crass BA, Chesney PJ, Wand PJ, Bergdoll MS. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J Infect Dis. 1985;151(5):883-889.
doi pubmed - LeRiche T, Black AY, Fleming NA. Toxic shock syndrome of a probable gynecologic source in an adolescent: a case report and review of the literature. J Pediatr Adolesc Gynecol. 2012;25(6):e133-137.
doi pubmed - Stevens DL, Gibbons AE, Bergstrom R, Winn V. The Eagle effect revisited: efficacy of clindamycin, erythromycin, and penicillin in the treatment of streptococcal myositis. J Infect Dis. 1988;158(1):23-28.
doi pubmed - Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267(24):3315-3316.
doi pubmed - Krakauer T, Buckley M. The potency of anti-oxidants in attenuating superantigen-induced proinflammatory cytokines correlates with inactivation of NF-kappaB. Immunopharmacol Immunotoxicol. 2008;30(1):163-179.
doi pubmed - Dixit S, Fischer G, Wittekind C. Recurrent menstrual toxic shock syndrome despite discontinuation of tampon use: is menstrual toxic shock syndrome really caused by tampons? Australas J Dermatol. 2013;54(4):283-286.
doi pubmed - Wyklicky H, Skopec M. Ignaz Philipp Semmelweis, the prophet of bacteriology. Infect Control. 1983;4(5):367-370.
doi pubmed - O'Loughlin RE, Roberson A, Cieslak PR, Lynfield R, Gershman K, Craig A, Albanese BA, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis. 2007;45(7):853-862.
doi pubmed - Kramer HM, Schutte JM, Zwart JJ, Schuitemaker NW, Steegers EA, van Roosmalen J. Maternal mortality and severe morbidity from sepsis in the Netherlands. Acta Obstet Gynecol Scand. 2009;88(6):647-653.
doi pubmed - Mead PB, Winn WC. Vaginal-rectal colonization with group A streptococci in late pregnancy. Infect Dis Obstet Gynecol. 2000;8(5-6):217-219.
doi pubmed - Stefonek KR, Maerz LL, Nielsen MP, Besser RE, Cieslak PR. Group A streptococcal puerperal sepsis preceded by positive surveillance cultures. Obstet Gynecol. 2001;98(5 Pt 1):846-848.
doi - Deutscher M, Lewis M, Zell ER, Taylor TH, Jr., Van Beneden C, Schrag S. Incidence and severity of invasive Streptococcus pneumoniae, group A Streptococcus, and group B Streptococcus infections among pregnant and postpartum women. Clin Infect Dis. 2011;53(2):114-123.
doi pubmed - Brown EJ. The molecular basis of streptococcal toxic shock syndrome. N Engl J Med. 2004;350(20):2093-2094.
doi pubmed - Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18(12):1096-1100.
doi pubmed - Schlievert PM, Kelly JA. Clindamycin-induced suppression of toxic-shock syndrome--associated exotoxin production. J Infect Dis. 1984;149(3):471.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
