| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 6, Number 1, March 2017, pages 23-27
Primary Neuroendocrine Carcinoma of the Uterine Cervix Treated With Complete Surgical Resection and Adjuvant Combination Chemotherapy
Yukihiro Nishioa, Takashi Miyatakea, c, Hironao Yasuokab, Hiromi Tsujib, Mai Temukaia, Takeshi Hisamatsua, Koji Hisamotoa, Masahiko Tsujimotob
aDepartment of Obstetrics and Gynecology, Osaka Police Hospital, Osaka, Japan
bDepartment of Diagnostic Pathology, Osaka Police Hospital, Osaka, Japan
cCorresponding Author: Takashi Miyatake, Department of Obstetrics and Gynecology, Osaka Police Hospital, 10-31, Kitayama, Tennouji, Osaka 5430035, Japan
Manuscript accepted for publication February 15, 2017
Short title: Large Cell Neuroendocrine Carcinoma of Cervix
doi: https://doi.org/10.14740/jcgo432w
| Abstract | ▴Top |
We describe our experience with one case of cervical large cell neuroendocrine carcinoma (LCNC), with an attempt of an adjuvant chemotherapy after complete surgery. The patient was a 66-year-old female (gravida 3, para 2) presenting with genital bleeding. A cervical mass was diagnosed as high-grade neuroendocrine carcinoma. Radical hysterectomy, pelvic lymph node dissection and bilateral salpingo-oophorectomy were performed as primary treatment for the cervical cancer. The surgical specimen of the uterus had an enlarged cervix of 4 cm in diameter with parametrial invasion. Microscopically, the surgical specimen exhibited invasive proliferation of relatively large tumor cells. Peripheral nuclear palisading and central necrosis were also histologically observed. Tumor cells had abundant cytoplasm with vesicular nuclei. The final pathological conclusion was high-grade neuroendocrine carcinoma (LCNC). The postoperative diagnosis was cervical cancer, high-grade neuroendocrine carcinoma (LCNC), pT2bN0M0, FIGO stage IIB, ly (+), v (+). Adjuvant treatment with cisplatin and irinotecan was planned at 4-week intervals. With the completion of three cycles of adjuvant chemotherapy, there was no evidence of recurrent disease. We determined the adjuvant therapy to be effective and well tolerated, and the therapy is now planned to be continued. In conclusion, we experienced a rare case of uterine cervical LCNC. It was surgically resected completely, and adjuvant chemotherapy has been continued. Adjuvant combination chemotherapy with cisplatin and irinotecan is expected to improve the prognosis of cervical LCNC.
Keywords: Large cell; Neuroendocrine carcinoma; Uterus
| Introduction | ▴Top |
In uterine cervical cancer, neuroendocrine tumors are relatively rare, accounting for 5% of all cervical carcinomas [1]. Among them, large cell neuroendocrine carcinoma (LCNC), one of the types of high-grade neuroendocrine carcinoma, is considerably rarer, with only dozens of cases reported [2-8]. LCNC is known to have highly aggressive clinical features with poor prognosis [1]. Surgical resection, chemotherapy and radiotherapy are multimodally attempted for LCNC [9]. However, an established therapeutic procedure has not yet been described [9]. The efficacy of combination chemotherapy with irinotecan hydrochloride and cisplatin against advanced stage small cell neuroendocrine carcinoma (SCNC) of the lung was reported [10]. This therapy was also reported to be effective for a surgically residual case of cervical LCNC [11].
This report describes the clinical features of a rare case of uterine cervical LCNC, which was successfully treated with complete surgery followed by adjuvant chemotherapy with irinotecan and cisplatin.
| Case Report | ▴Top |
The patient was a 66-year-old female (gravida 3, para 2). Menopause began at the age of 53. There was no particular familial medical history. At 53 years old, right hip replacement was performed due to hip osteoarthritis. At 61 years old, she was diagnosed with primary hypertension and hyperlipidemia. Medications had been prescribed for these comorbidities.
She presented with complaints of postmenopausal genital bleeding for a month when she visited our hospital. Internal examination and transvaginal ultra-sonogram revealed a cervical mass of 3 cm in diameter. Findings on colposcopy were atypical vessels and invasive cancer (IC). Cervical cytology was positive for malignant cells, and cervical biopsy of the overt carcinoma resulted in diagnosis of high-grade neuroendocrine carcinoma. Immunohistochemical studies on the cervical biopsy were positive for synaptophysin, NCAM and chromogranin-A.
Her serum levels of CA125, squamous cell carcinoma (SCC) and carcinoembryonic antigen (CEA) were 19 U/mL, 0.8 ng/mL and 59.9 ng/mL, respectively, with elevation of CEA. Serum neuron-specific enolase [12] was 12.5 ng/mL, within the normal range.
Pelvic magnetic resonance imaging [13] demonstrated a cervical mass of 3 cm in diameter with right parametrial space invasion (Fig. 1). Thoracic and abdominal computed tomography (CT) showed no metastatic lesions.
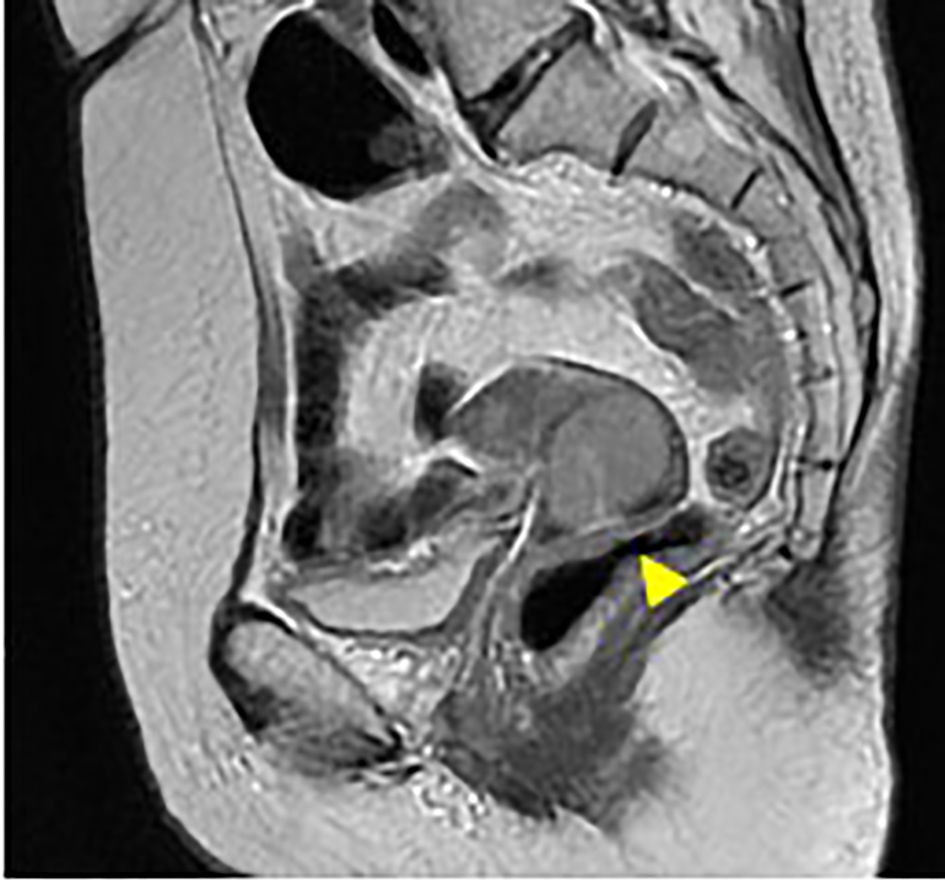 Click for large image | Figure 1. Pelvic MRI images of T2 intensified (1-1: sagittal section; 1-2: transverse section). Uterine cervical tumor of 3 cm in diameter was visualized on MRI (arrow). The tumor invaded to the right parametrial space with interruption of the stromal ring (arrow in 2-2). The MRI indicates stage IIB cervical cancer. |
Preoperatively, we diagnosed her with cervical cancer of FIGO stage IIb. Radical hysterectomy, pelvic lymph node dissection and bilateral salpingo-oophorectomy were performed as primary treatment for the cervical cancer.
The surgical specimen of the uterus exhibited an enlarged cervix of 4 cm in diameter with parametrial invasion. The microscopic findings of the surgical specimen were relatively large tumor cells invasively proliferating in an organoid nesting pattern (Fig. 2). Peripheral nuclear palisading and central necrosis were also histologically observed (Fig. 3). Tumor cells showed abundant cytoplasm with vesicular nuclei and with prominent nucleoli (Fig. 4). Mitotic activity of the tumor cells was high (Fig. 4). Immunohistochemical staining of the tumor was strongly positive for synaptophysin, weakly positive for chromogranin-A and partially positive for NCAM (Fig. 5). The Ki-67 index of the tumor cells was almost 100% (Fig. 5). Together with histology and immunohistochemistry, the final pathological conclusion was high-grade neuroendocrine carcinoma (LCNC). The tumor exhibited lymphovascular space invasion and right parametrial invasion. The entire surgical stump was negative for malignant tissue. The postoperative diagnosis was cervical cancer, high-grade neuroendocrine carcinoma, pT2bN0M0, FIGO stage IIB, ly (+), v (+). We performed complete surgical resection and there was no residual disease.
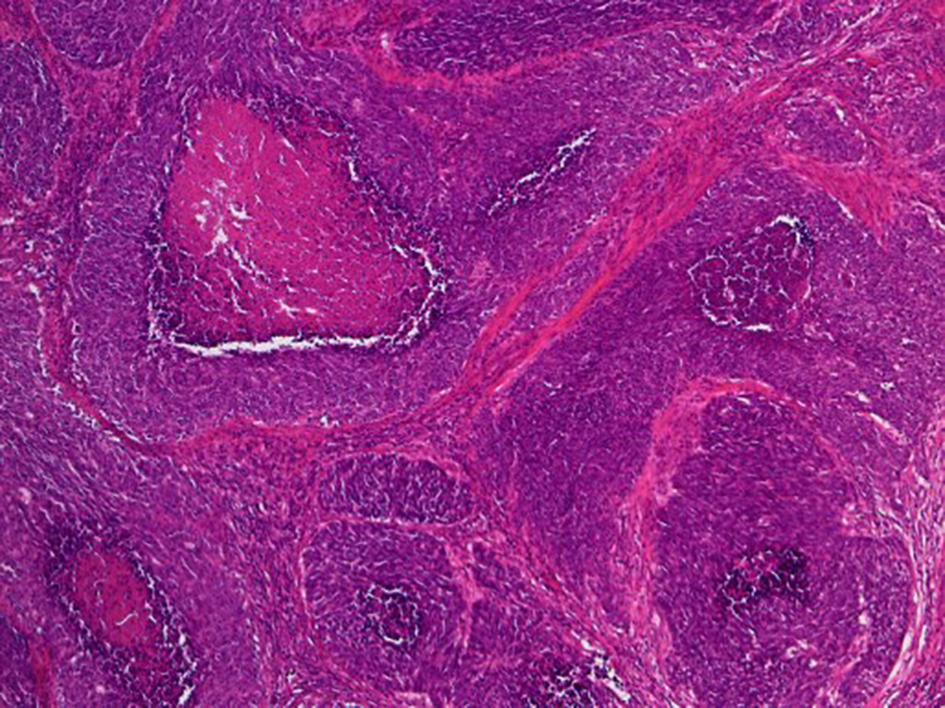 Click for large image | Figure 2. Histological findings of surgical specimen of cervical tumor (H&E stain, original magnification × 40). Relatively large tumor cells invasively proliferate with organoid nesting pattern and central necrosis. |
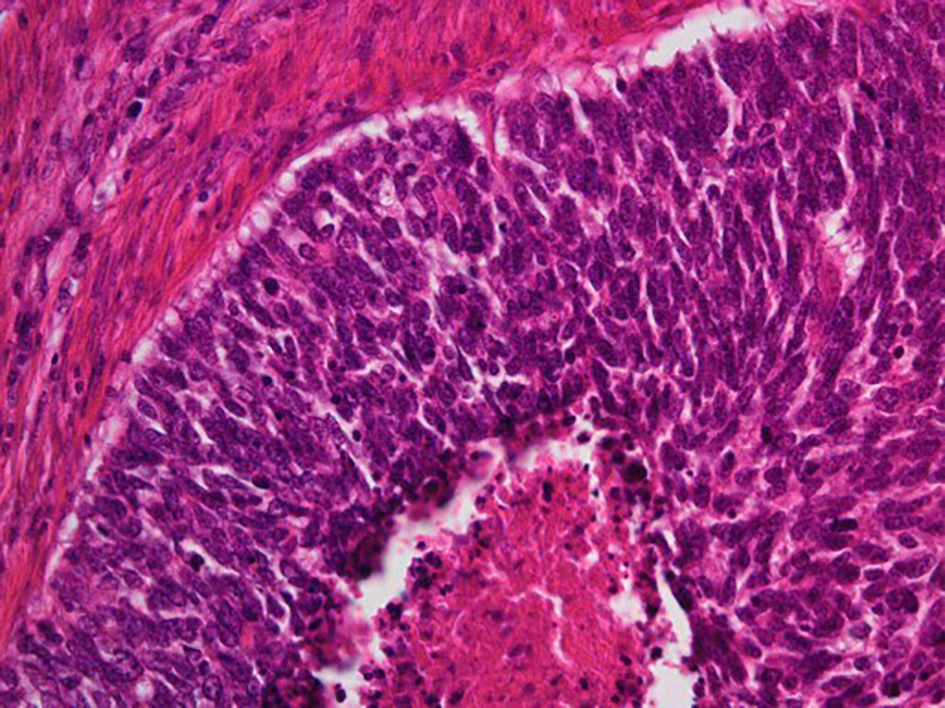 Click for large image | Figure 3. Histological findings of surgical specimen of cervical tumor (H&E stain, original magnification × 200). Peripheral nuclear palisading and central necrosis are histologically observed. |
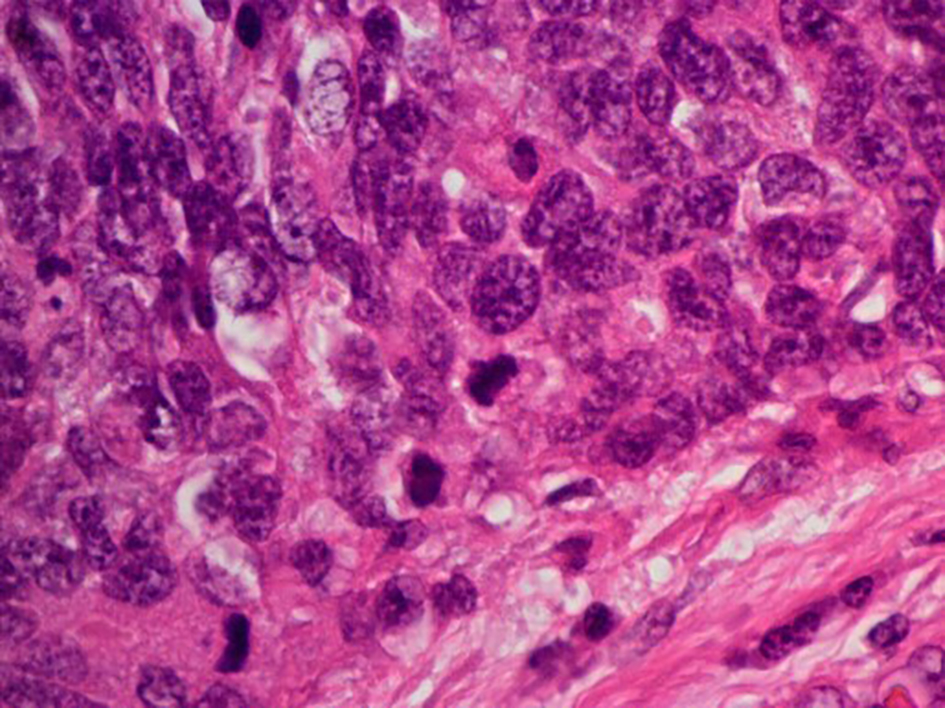 Click for large image | Figure 4. Histological findings of surgical specimen of cervical tumor ( H&E stain, original magnification × 400). Tumor cells exhibit abundant cytoplasm with vesicular nuclei. Mitotic activity of the tumor cell was high. |
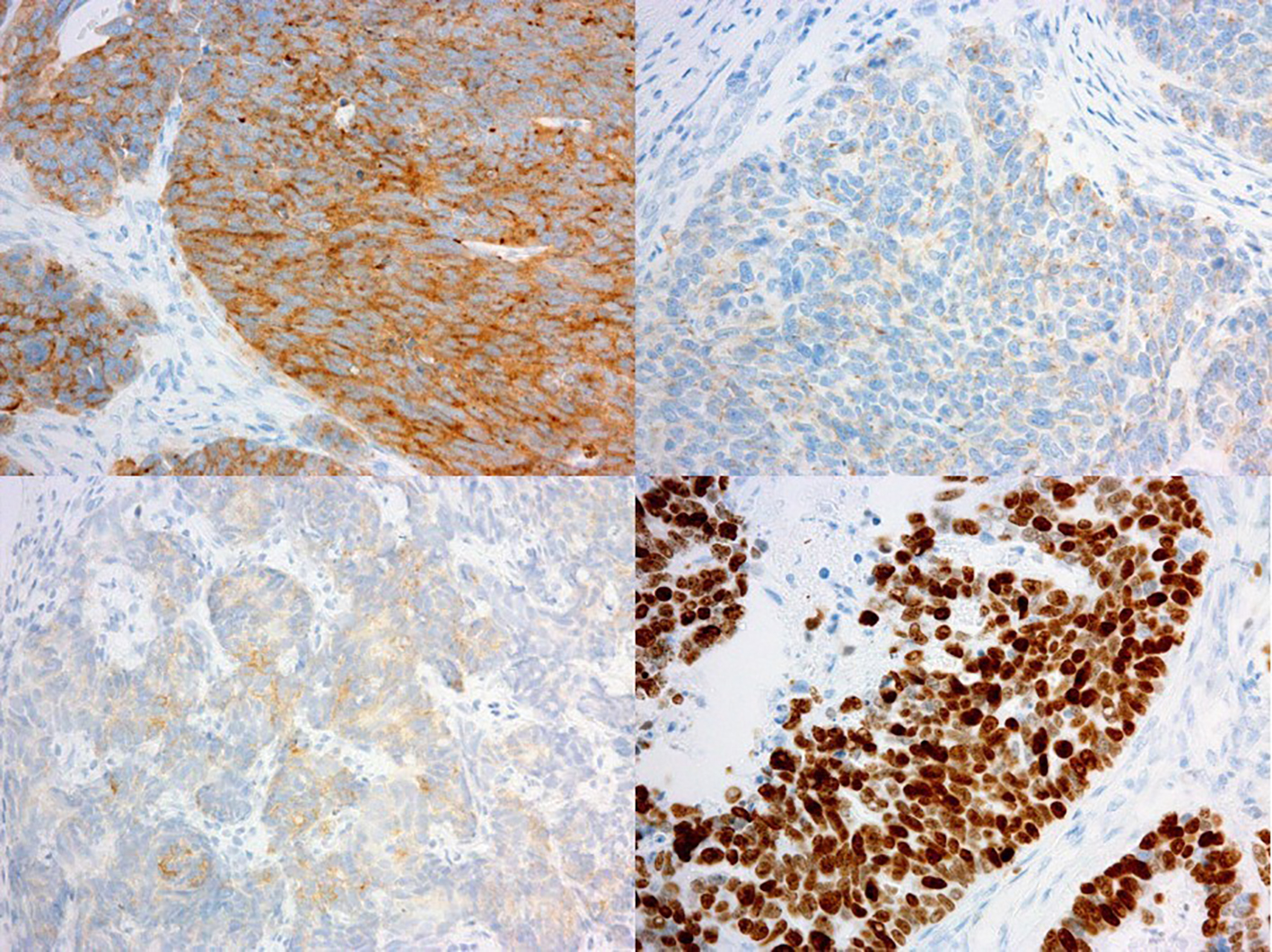 Click for large image | Figure 5. Immunohistochemical findings of surgical specimen of cervical tumor (immunohistochemistry, original magnification × 100). Immunohistochemical staining of the tumor was strongly positive for synaptophysin (upper left), weakly positive for chromogranin-A (upper right) and partially positive for NCAM (bottom left). The Ki-67 index of the tumor cells was almost 100% (bottom right). |
LCNC is known to have aggressive features with a poor prognosis; however, there is no established treatment modality for LCNC of the cervix. We adopted an adjuvant chemotherapy regimen which is reported as effective on remission induction for cervical LCNC [9]. Treatment with cisplatin (60 mg/m2 at day 1) and irinotecan (60 mg/m2 at day 1, 8 and 15) was planned, and given intravenously at 4-week intervals. With the completion of three cycles of adjuvant chemotherapy, there was no evidence of recurrent disease, but an adverse effect of grade 2 diarrhea. We determined the adjuvant therapy to be effective and tolerated. As reported in remission induction, the adjuvant combination chemotherapy is now planned to be continued for up to six cycles.
| Discussion | ▴Top |
Neuroendocrine tumors are rare in the uterine cervix, accounting for 5% of uterine cervical carcinomas [1, 7, 14]. According to the World Health Organization (WHO), neuroendocrine tumors of the uterine cervix are classified into two grades, namely, low-grade neuroendocrine tumors and high-grade neuroendocrine carcinomas. Low-grade neuroendocrine tumors include grade 1 carcinoid tumors and grade 2 atypical carcinoid tumors. High-grade neuroendocrine carcinomas are composed of high-grade malignant cells of either small cell (SCNC) or large cell type (LCNC). Both types of high-grade neuroendocrine carcinomas are classified as grade 3 [8, 15-17]. SCNC is most common among cervical neuroendocrine tumors. LCNC usually develops in the lungs. In the gynecological organs, LCNC generally affects in the uterine cervix and ovary, but is a rare entity [10-12]. There are less than 60 cases of cervical LCNCs reported to date [2-7].
The features of neuroendocrine tumors include the inconspicuous presence of neurosecretory granules in the cytoplasmic component. Tumors are immunohistochemically positive for synaptophysin, chromogranin, CD56 and NSE as neuroendocrine markers [18]. The carcinoids are characterized with having abundant cytoplasm, granular chromatin and visible to prominent nucleoli, and carcinoids have no cytological atypia, no necrosis and mitotic figures are rare. On the other hand, atypical carcinoids possess nuclear atypia and greater mitotic activity with rare focal necrosis. SCNC presents as a monotonous population of small cells with ovoid hyper-chromatic nuclei, with scant cytoplasm, high mitotic activity and extensive necrosis. Histological criteria of cervical LCNC include the presence of large cells with abundant cytoplasm, vesicular nuclei with prominent nucleoli and a high mitotic rate. The growth pattern of LCNC is an insular, organoid, trabecular or solid pattern, often with peripheral palisading or rosette formation, and focal tumor necrosis is observed. The immunohistochemically positive rates in LCNC are 87% for chromogranin, 56% for synaptophysin, and 88% for at least one of chromogranin, synaptophysin or NSE [19].
LCNCs have occasionally been misdiagnosed due to the rarity of disease as well as to the coexistence of other histological types [3]. Pathologically, LCNC should be distinguished from atypical carcinoids, SCNC, SCC and adenocarcinoma. It was reported that 8/23 of uterine cervix LCNCs were histologically diagnosed as mixed type, coexistent with SCNC, adenocarcinoma or SCC [19]. Thus, adequately sized biopsy is necessary for accurate diagnosis. For the differentiation of histology, an atypical carcinoid exhibits mitoses of less than 10/10 HPFs. SCNC consists of small cells with scant cytoplasm. SCC and adenocarcinoma lack cytoplasmic neurosecretory granules and conspicuous nucleoli.
Clinically, cervical LCNCs are diagnosed at a younger age than other cervical cancers, with a median age of 37 - 42 years [9, 18]. On diagnosis, LCNCs are 58% stage I disease, 16% stage II, 2% stage III and 8% stage IV disease [7]. The median survival rates of patients with cervical LCNC are 19 months for stage I, 17 months for stage II, 3 months for stage III and 1.5 months for stage IV [7], respectively. In another report, it was reported that 65% of LCNC patients had died of the disease within 3 years. Even in stage I cervical LCNC, 12 of 21 patients died of the disease with a median survival of 16 months [20]. Liver and/or pulmonary metastases are most commonly observed at the relapse [9]. Embry et al reported that younger age, earlier stage, any surgery, radical hysterectomy, chemotherapy during primary treatment and platinum-based chemotherapy were associated with improved survival [9].
Accurate diagnosis of cervical LCNC is of prognostic importance. However, an established treatment procedure for cervical LCNC remains unknown due to the rarity of the disease. In the treatment guidelines of uterine cervical cancer, neuroendocrine carcinomas, including small cell carcinomas, are declared to be out of the scope of the guidelines due to lack of clinical therapeutic evidence [20]. Aggressive primary multimodal treatment with radical hysterectomy, adjuvant chemotherapy or chemoradiotherapy is usually considered [3, 9, 17] for cervical LCNC. SCNC of the cervix is a relatively common type of cervical neuroendocrine carcinomas. Thus, the investigation on the treatment of cervical neuroendocrine carcinoma is usually considered based on the clinical data of cervical SCNC, or more generally, of lung SCNC. For cervical SCNC, Chang et al reported superior survival with the adjuvant combination chemotherapy of vincristine, doxorubicin and cyclophosphamide (VAC), or of cisplatin and etoposide (PE) [21]. Lee et al concluded that there was no difference in 5-year survival of cervical SCNC between the adjuvant chemotherapy and the adjuvant chemoradiotherapy [22]. Cohen et al also recommended adjuvant chemotherapy with PE or adjuvant chemoradiotherapy for the improvement of cervical SCNC [23]. From the analogy of cervical SCNC, in cervical LCNC, Embry et al reviewed 67 cases of cervical LCNC and concluded that perioperative chemotherapy with platinum, with or without etoposide, improves survival with LCNC [7]. In the present reports on cervical neuroendocrine carcinomas, perioperative platinum-based chemotherapy, including PE, is the most attempted and reported treatment for the improvement of prognosis.
For the treatment of metastatic SCNC of the lung, Noda et al have demonstrated that the combination therapy of irinotecan (60 mg/m2, days 1, 8, and 15) and cisplatin (60 mg/m2, day 1) with a 4-week interval was more effective than the combination of PE [10]. The results were introduced into the treatment guidelines of lung SCNC as the recommendation for advanced stage disease [24]. Tanimoto et al reported the efficacy of postoperative irinotecan plus cisplatin with six cycles for a case of cervical LCNC with positive surgical margin [11].
Although there is a lack of evidence on the efficacy of adjuvant settings for the prognosis of cervical LCNC, the combination therapy of irinotecan and cisplatin can be considered as an expected perioperative therapy for cervical LCNC, which may be superior to PE therapy. We selected adjuvant irinotecan and cisplatin for the present case. The adverse effects are tolerated after the completion of three cycles. As in the treatment guidelines of lung SCNC [24], we intend to continue the therapy for four to six cycles for the completion of primary therapy.
Conclusion
We experienced a rare case of uterine cervical LCNC. It was surgically resected completely and adjuvant chemotherapy has been continued. Adjuvant combination chemotherapy with cisplatin and irinotecan is expected to improve the prognosis of cervical LCNC, as it was proven to be effective for pulmonary SCNC.
Conflicts of Interest
The authors report no conflicts of interest.
Financial Support
There was no funding source for this report.
| References | ▴Top |
- Wang KL, Wang TY, Huang YC, Lai JC, Chang TC, Yen MS. Human papillomavirus type and clinical manifestation in seven cases of large-cell neuroendocrine cervical carcinoma. J Formos Med Assoc. 2009;108(5):428-432.
doi - Walker AN, Mills SE, Taylor PT. Cervical neuroendocrine carcinoma: a clinical and light microscopic study of 14 cases. Int J Gynecol Pathol. 1988;7(1):64-74.
doi pubmed - Gilks CB, Young RH, Gersell DJ, Clement PB. Large cell neuroendocrine [corrected] carcinoma of the uterine cervix: a clinicopathologic study of 12 cases. Am J Surg Pathol. 1997;21(8):905-914.
doi pubmed - Mannion C, Park WS, Man YG, Zhuang Z, Albores-Saavedra J, Tavassoli FA. Endocrine tumors of the cervix: morphologic assessment, expression of human papillomavirus, and evaluation for loss of heterozygosity on 1p,3p, 11q, and 17p. Cancer. 1998;83(7):1391-1400.
doi - Wistuba, II, Thomas B, Behrens C, Onuki N, Lindberg G, Albores-Saavedra J, Gazdar AF. Molecular abnormalities associated with endocrine tumors of the uterine cervix. Gynecol Oncol. 1999;72(1):3-9.
doi pubmed - Yun K, Cho NP, Glassford GN. Large cell neuroendocrine carcinoma of the uterine cervix: a report of a case with coexisting cervical intraepithelial neoplasia and human papillomavirus 16. Pathology. 1999;31(2):158-161.
doi pubmed - Embry JR, Kelly MG, Post MD, Spillman MA. Large cell neuroendocrine carcinoma of the cervix: prognostic factors and survival advantage with platinum chemotherapy. Gynecol Oncol. 2011;120(3):444-448.
doi pubmed - Kurman RJ. International Agency for Research on Cancer. World Health Organization. WHO classification of tumours of female reproductive organs, 4th ed. Lyon: International Agency for Research on Cancer; 2014. p. 307.
- Strosberg JR, Coppola D, Klimstra DS, Phan AT, Kulke MH, Wiseman GA, Kvols LK. The NANETS consensus guidelines for the diagnosis and management of poorly differentiated (high-grade) extrapulmonary neuroendocrine carcinomas. Pancreas. 2010;39(6):799-800.
doi pubmed - Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, et al. Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med. 2002;346(2):85-91.
doi pubmed - Tanimoto H, Hamasaki A, Akimoto Y, Honda H, Takao Y, Okamoto K, Teramoto M, et al. [A case of large cell neuroendocrine carcinoma (LCNEC) of the uterine cervix successfully treated by postoperative CPT-11+CDDP chemotherapy after non-curative surgery]. Gan To Kagaku Ryoho. 2012;39(9):1439-1441.
pubmed - Grande E, Santamaria Sandi J, Capdevila J, Navarro Gonzalez E, Zafon Llopis C, Ramon YCAT, Gomez Saez JM, et al. Consensus on management of advanced medullary thyroid carcinoma on behalf of the Working Group of Thyroid Cancer of the Spanish Society of Endocrinology (SEEN) and the Spanish Task Force Group for Orphan and Infrequent Tumors (GETHI). Clin Transl Oncol. 2016;18(8):769-775.
doi pubmed - Dieler R, Dammrich J. Immunohistochemical and fine structural characterization of primary carcinoid tumors of the larynx. Eur Arch Otorhinolaryngol. 1995;252(4):229-235.
doi pubmed - Rouzbahman M, Clarke B. Neuroendocrine tumors of the gynecologic tract: select topics. Semin Diagn Pathol. 2013;30(3):224-233.
doi pubmed - Lax SF. [New features in the 2014 WHO classification of uterine neoplasms]. Pathologe. 2016;37(6):500-511.
doi pubmed - Bocker W. [WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics]. Verh Dtsch Ges Pathol. 2002;86:116-119.
pubmed - Albores-Saavedra J, Gersell D, Gilks CB, Henson DE, Lindberg G, Santiago H, Scully RE, et al. Terminology of endocrine tumors of the uterine cervix: results of a workshop sponsored by the College of American Pathologists and the National Cancer Institute. Arch Pathol Lab Med. 1997;121(1):34-39.
pubmed - Dikmen Y, Kazandi M, Zekioglu O, Ozsaran A, Terek MC, Erhan Y. Large cell neuroendocrine carcinoma of the uterine cervix: a report of a case and review of the literature. Arch Gynecol Obstet. 2004;270(3):185-188.
doi pubmed - Rekhi B, Patil B, Deodhar KK, Maheshwari A, R AK, Gupta S, Tongaonkar HB, et al. Spectrum of neuroendocrine carcinomas of the uterine cervix, including histopathologic features, terminology, immunohistochemical profile, and clinical outcomes in a series of 50 cases from a single institution in India. Ann Diagn Pathol. 2013;17(1):1-9.
doi pubmed - Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Cho KR, Chu C, et al. Cervical Cancer, Version 2.2015. J Natl Compr Canc Netw. 2015;13(4):395-404; quiz 404.
pubmed - Chang TC, Lai CH, Tseng CJ, Hsueh S, Huang KG, Chou HH. Prognostic factors in surgically treated small cell cervical carcinoma followed by adjuvant chemotherapy. Cancer. 1998;83(4):712-718.
doi - Lee JM, Lee KB, Nam JH, Ryu SY, Bae DS, Park JT, Kim SC, et al. Prognostic factors in FIGO stage IB-IIA small cell neuroendocrine carcinoma of the uterine cervix treated surgically: results of a multi-center retrospective Korean study. Ann Oncol. 2008;19(2):321-326.
doi pubmed - Cohen JG, Kapp DS, Shin JY, Urban R, Sherman AE, Chen LM, Osann K, et al. Small cell carcinoma of the cervix: treatment and survival outcomes of 188 patients. Am J Obstet Gynecol. 2010;203(4):347 e341-346.
- Kalemkerian GP, Akerley W, Bogner P, Borghaei H, Chow LQ, Downey RJ, Gandhi L, et al. Small cell lung cancer. J Natl Compr Canc Netw. 2013;11(1):78-98.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
