| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 7, Number 2, June 2018, pages 31-36
Ovarian Cancer: Post-Relapse Survival and Prognostic Factors
Ai Miyoshia, b, Serika Kanaoa, Hirokazu Naoia, Hirofumi Otsukaa, Takeshi Yokoia
aDepartment of Obstetrics and Gynecology, Kaizuka City Hospital, Kaizuka, Osaka, Japan
bCorresponding Author: Ai Miyoshi, Department of Obstetrics and Gynecology, Kaizuka City Hospital, 3-10-20 Hori Kaizuka, Osaka 597-0015, Japan
Manuscript submitted April 4, 2018, accepted April 20, 2018
Short title: Post-Relapse Survival for Ovarian Cancer
doi: https://doi.org/10.14740/jcgo488w
| Abstract | ▴Top |
Background: Patients with relapsing ovarian cancer have a particularly poor prognosis, it is thus important for oncology consultants to anticipate the patient’s adverse prognosis and to select an optimum treatment plan. We report here our retrospective review of the treatment outcomes of the post-relapse survival (PRS) for ovarian cancer and the different prognostic factors for relapsing patients.
Methods: Totally 242 patients with ovarian cancer were admitted to our institution. All underwent surgery, and all achieved complete remission of their primary disease. Of the 242 patients, 48 were subsequently diagnosed with a recurrence. We retrospectively reviewed their initial FIGO staging, the histology of their tumors, the treatment-free interval (TFI), the number of recurrent lesions, the treatment for the recurrence (whether treatment included surgery, radiotherapy or chemotherapy), and the number of chemotherapy regimens applied for treatment of the recurrence.
Results: The median age of the 48 relapse patients was 59 years (range 34 - 83); the median follow-up period was 40 months (range 4 - 103). The multivariate Cox proportional hazards model demonstrated that having a mucinous histology (P = 0.029), having a TFI of less than 6 months (P = 0.0002), having a solitary recurrent lesion (P = 0.011), and no chemotherapy (P = 0.007) were independent risk factors associated with PRS. The number of recurrent lesions, multimodal treatment for recurrence, the number of chemotherapy regimens used for treatment, and use of bevacizumab were not independent factors for PRS.
Conclusions: In regards to recurrent ovarian cancer, after achieving complete surgical remission of the primary disease, having a mucinous adenocarcinoma histology and/or a TFI of less than 6 months worsened the prognosis for the patient. Having a solitary recurrent lesion was a better prognostic factor regardless of whether or not they received surgical treatment. Chemotherapy could improve PRS, if the performance status (PS) of the patient allows her to receive chemotherapy, and if she is desirous of the attempt to extend her life.
Keywords: Post-relapse survival; Ovarian cancer; Prognostic factor
| Introduction | ▴Top |
Patients with ovarian cancer who have had a complete remission after their initial treatment still have a particularly poor prognosis [1]; it is thus important for us as oncology consultants to anticipate the patient’s adverse prognosis and to select an optimized treatment plan. It is just as important for the patient and her family to have a clear picture of this prognosis with which to consider their way of life going forward.
To give physicians the best current information to share with their patients, we report here our retrospective review of the treatment outcomes in our institution of the post-relapse survival (PRS) for ovarian cancer and the different prognostic factors for relapsing patients.
| Patients and Methods | ▴Top |
Between January of 2008 and December of 2016, 242 patients with ovarian cancer were admitted to our institution at the Department of Obstetrics and Gynecology of Kaizuka City Hospital in Osaka, Japan. All underwent surgery and all achieved complete remission of their primary disease. Of these 242 patients, 48 were subsequently diagnosed with a recurrence of their ovarian cancer; they became the subjects of our study of relapse outcomes.
In all cases, as their primary therapy, patients with ovarian cancer underwent a total abdominal hysterectomy, bilateral salpingo-oophorectomy, partial omentectomy and pelvic and para-aortic lymph node dissection. Cytological assessment of the peritoneal fluid was performed. Adjuvant postoperative chemotherapy with platinum-based drugs was administered to all patients with a tumor of grade 2 or higher, and/or a stage of Ic or higher.
Following the completion of primary treatment, all 242 cases were confirmed, with a thoracic/abdominal CT scan, to have achieved complete remission of their ovarian cancer. Patients were subsequently followed, at intervals of 3 - 6 months, with abdominal ultra-sonograms, serum CA125 analyses and thoracic/abdominal CT scans. The recurrence of disease was diagnosed and confirmed with emergence of a measurable lesion on CT scan, as previously described [2, 3].
The treatment for recurrent disease was basically with chemotherapy, the regimen of which depended on whether chemoresistance was encountered, and as judged by the treatment-free interval (TFI) [4]. The regimen used after progression following the first chemotherapy regimen was decided by individual doctors, based on any adverse effects and on the performance status (PS) of the patient. If there were no contraindications, a combination therapy with bevacizumab and maintenance was considered, regardless of former bevacizumab usage [5-7]. If the recurrent disease was resectable, a complete surgical resection was also attempted [8]. Radiotherapy for recurrent disease was applied only for the palliation of disease symptoms.
From medical records, we retrospectively reviewed the initial FIGO staging, the histology of the tumors, the interval between the end of the initial treatment and the recurrence, the number of recurrent lesions, the treatment for recurrence (whether that included operation or radiotherapy, or only chemotherapy) and the number of chemotherapy regimens applied for treatment of the recurrence. We obtained the patient’s status for recurrent disease and survival, as of December 31, 2016, from which we calculated PRS.
Statistical analysis
MedCalc (MedCalc Software, Ostend, Belgium) was used for statistical analysis. PRS curves were constructed using the Kaplan-Meier method and were evaluated for statistical significance by the log-rank test. For comparison among multiple groups, a Bonferroni correction was applied for the interpretation of significance. Multivariate Cox proportional hazards model (step-wise method) was calculated to select the independent risk factors for survival probabilities. Statistical results were recognized as significant when the P-value was less than 0.05.
Ethical issues
This investigation was a retrospective observational study. All patients had given their comprehensive consent for the investigational use of their clinical data.
| Results | ▴Top |
Medical records from 48 patients with a relevant relapse of their ovarian cancer were available for this study. Their median age was 59 years (range 34 - 83) and the median follow-up period was 40 months (range 4 - 103).
Initial FIGO stage
Eleven patients (23%) were FIGO stage I, two (4%) were stage II, 32 (67%) were stage III, and three (6%) were stage IV.
Histology
Microscopic pathology examination revealed that 29 (60%) of the 48 cases were serous adenocarcinoma, nine (19%) were clear cell adenocarcinoma, three (6%) were mucinous adenocarcinoma, two (4%) were endometrioid adenocarcinoma and five (11%) were of individual other types (carcinosarcoma, granulosa cell tumor, leiomyosarcoma, mixed carcinoma and undifferentiated carcinoma, respectively).
The interval between the end of initial treatment and the beginning of treatment for recurrence
Of 48 cases, the median of the interval between the end of initial treatment and the beginning of treatment for recurrence, namely, the TFI, was 9.2 months (range 0.1 - 60.4). Eighteen (38%) of the recurrent cases had a TFI of less than 6 months (range 0.1 - 5.2). These 18 had all received chemotherapy and, because of the shortened period to recurrence, were considered to have had a platinum-resistant recurrence. The remaining 30 (62%) cases had a TFI of more than the 6 month cut-off (range 6.6 - 60.6), thus were assumed to have had a recurrence of a more platinum-sensitive tumor.
The number of recurrence sites
Nine (19%) cases had a single recurrence site, 39 (81%) cases had multiple sites of recurrence.
The treatment for recurrence
Six (13%) cases had salvage surgical reduction performed, 42 (87%) cases received only chemotherapy. All six salvage surgeries succeeded in complete cytoreduction.
The chemotherapy for recurrent disease
Chemotherapy was conducted for 42 (89%) of the 48 recurrent cases. Twenty-two (46%) cases received one regimen of chemotherapy, 10 (21%) cases got two regimens, seven (15%) received three regimens, and three (6%) received four regimens. The remaining six (12%) cases turned down chemotherapy and instead were given best supportive care (BSC).
Survival analysis
Of the 48 cases analyzed, the post-relapse median survival was 32.3 months (Fig. 1). The log-rank test for the Kaplan-Meier method showed that the initial FIGO staging was not related to PRS (P = 0.824, data not shown); however, the histology of the tumor was (P < 0.001) (Fig. 2). Using the Bonferroni correction, we found significant differences between cases with serous adenocarcinoma and those with mucinous adenocarcinoma (P < 0.05) (Fig. 2).Those patients who were diagnosed as having a relapsing TFI of less than 6 months had a significantly worse PRS than patients with a TFI of more than 6 months (P < 0.001) (Fig. 3). Patients with multiple recurrent lesions also had a worse PRS than patients with a solitary lesion (P = 0.029) (Fig. 4). Patients who received only chemotherapy for recurrence treatment had a significantly worse prognosis than patients who received multimodality therapy, including surgery (P = 0.005). Patients treated with chemotherapy showed significantly better prognosis than patients without chemotherapy (P < 0.001) (Fig. 5). However, no trend was recognized between the number of chemotherapy regimens delivered and PRS (data not shown). Combining bevacizumab immunotherapy with chemotherapy did not improve PRS over chemotherapy alone (P = 0.951) (Fig. 6).
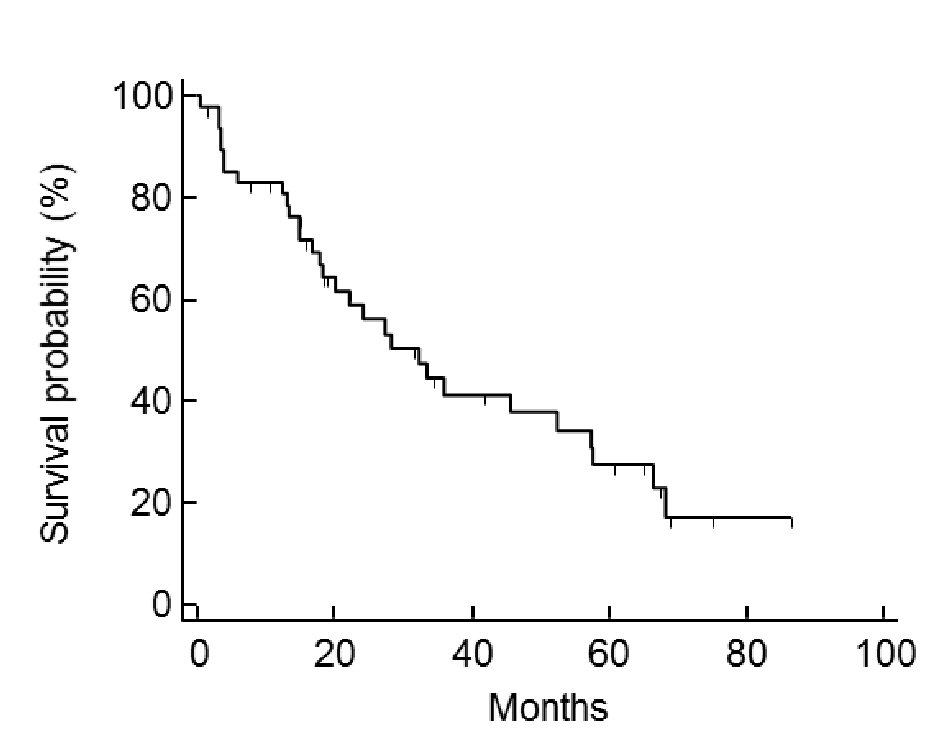 Click for large image | Figure 1. Case post-relapse survival. The median of post-relapse survival (PRS) was 32.3 months. |
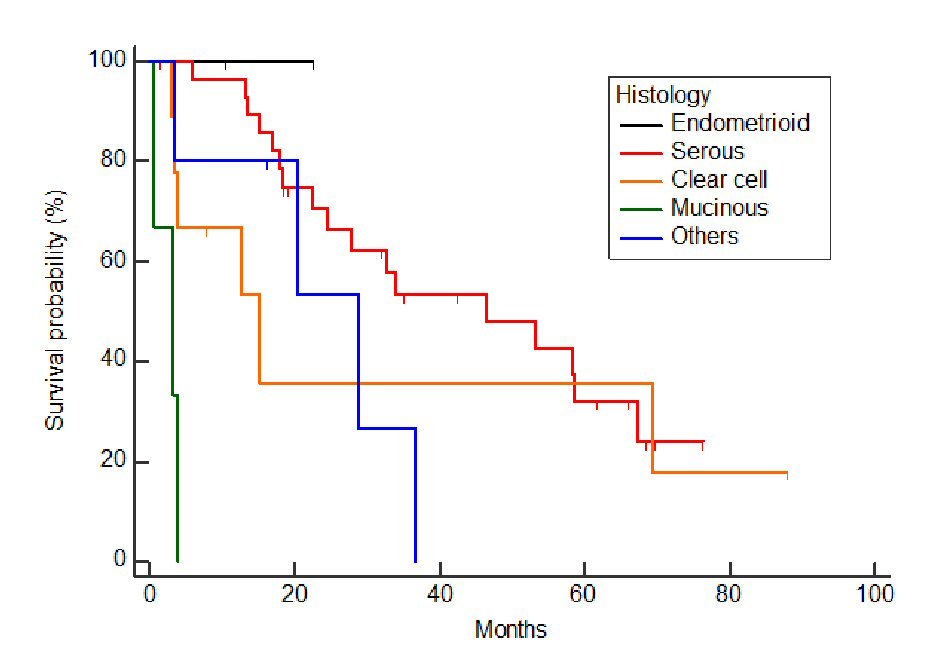 Click for large image | Figure 2. Relationship between histology and post-relapse survival. Tumor histology was significantly associated with PRS (P < 0.001). With the Bonferroni correction, there were significant differences in PRS recognized between serous adenocarcinoma and mucinous adenocarcinoma (P < 0.05). |
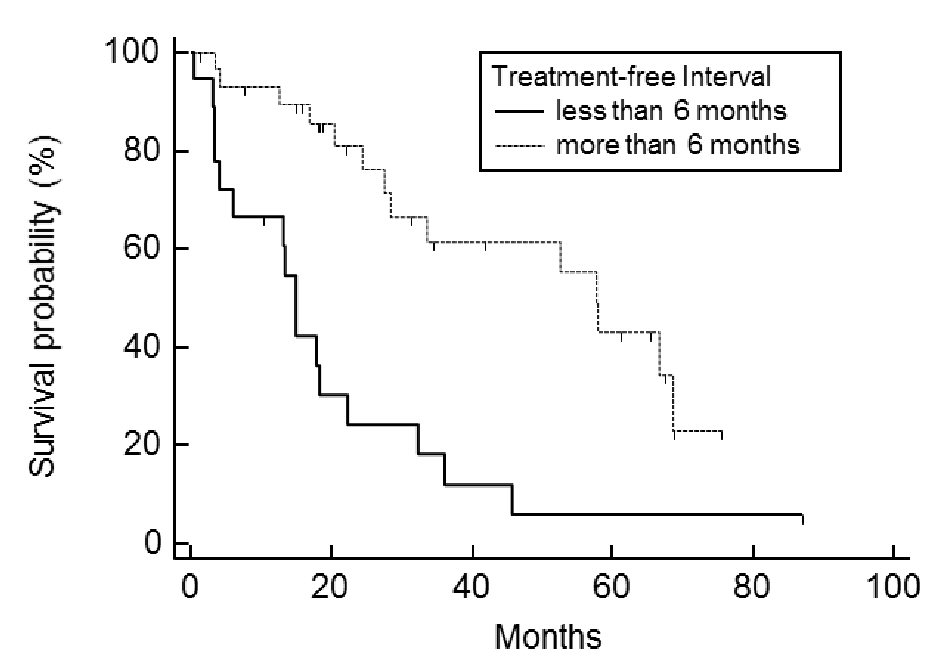 Click for large image | Figure 3. Post-relapse survival: comparison between treatment-free interval (TFI) of less or more than 6 months. Patients with a TFI of less than 6 months had a significantly worse prognosis than those with a TFI of more than 6 months (P < 0.001). |
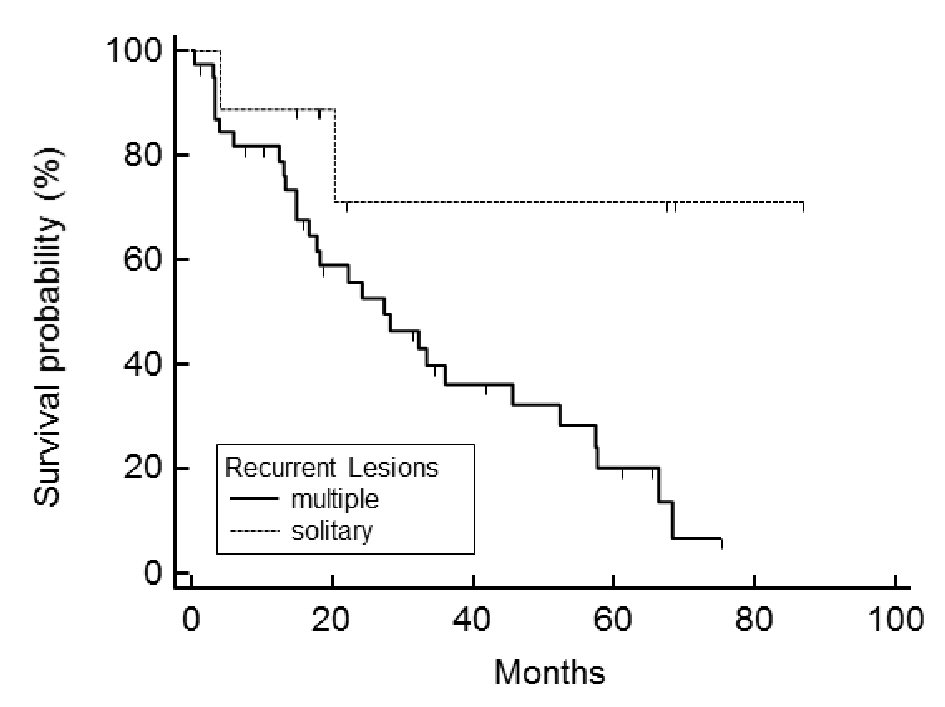 Click for large image | Figure 4. Post-relapse survival is related to the number of recurrent lesions. Patients with multiple recurrent lesions had a significantly worse prognosis than those with a solitary lesion (P = 0.029). |
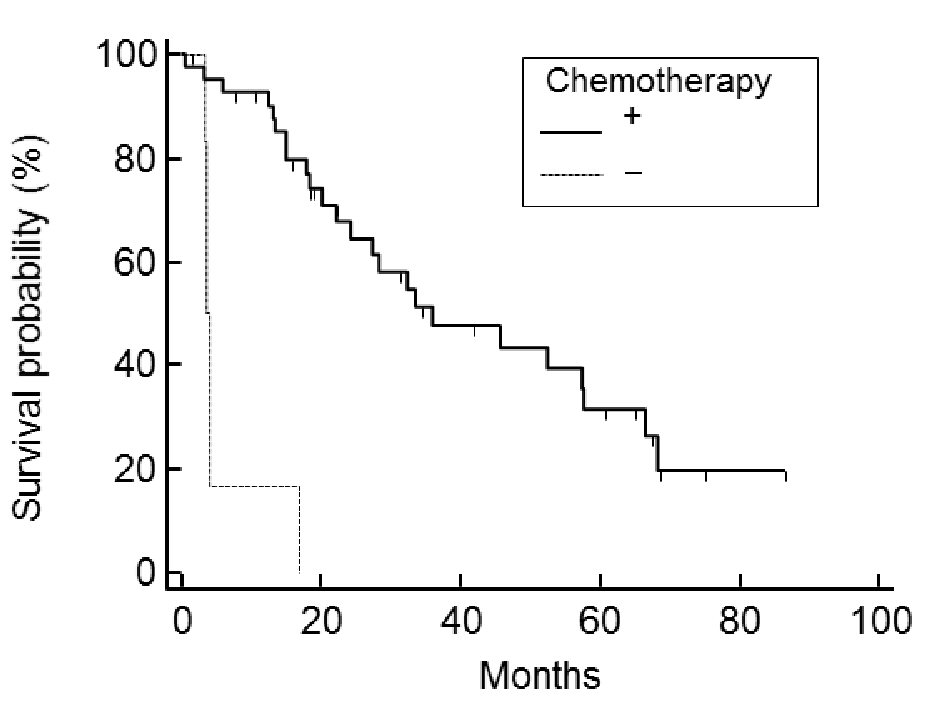 Click for large image | Figure 5. Post-relapse survival: comparison between the use and non-use of chemotherapy. Patients treated with chemotherapy showed significantly better prognosis than patients not treated with chemotherapy (P < 0.001). No trend was recognized between the number of chemotherapy regimens used and PRS. |
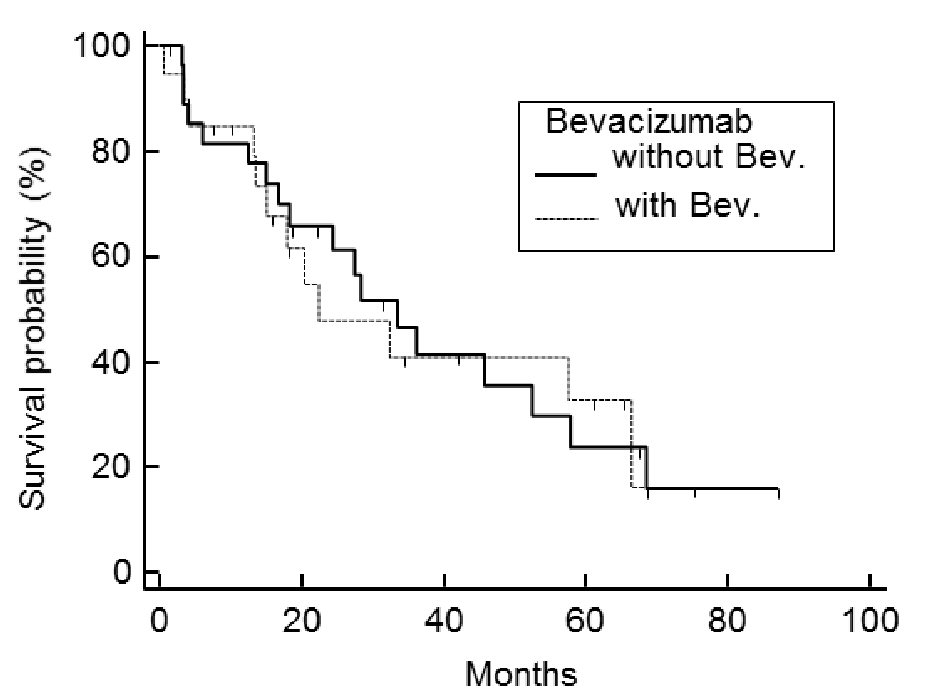 Click for large image | Figure 6. Post-relapse survival: comparison between chemotherapy with or without bevacizumab. Combining chemotherapy with bevacizumab does not improve PRS over chemotherapy-alone (P = 0.951). |
The multivariate Cox proportional hazards model demonstrated that mucinous histology (P = 0.029), TFI within 6 months (P = 0.0002), solitary recurrent lesion (P = 0.011), and no chemotherapy (P = 0.007) were independent risk factors associated with PRS (Table 1). The following were not independent factors for PRS: the number of recurrent lesions, multimodal treatment for recurrence, the number of chemotherapy regimens used, and use of bevacizumab.
 Click to view | Table 1. Post-Relapse Survival: Multivariate Analysis With Cox Proportional Hazards Regression |
Among 18 patients who relapsed within 6 months after initial therapy, 16 died within 46 months of the diagnosis of recurrence and two patients were still alive at last check, 10 and 88 months, respectively, after recurrence.
| Discussion | ▴Top |
Analysis of the data using the multivariate Cox proportional hazards model demonstrated that having a TFI of less than 6 months (P = 0.0002) and a mucinous ovarian adenocarcinoma (P = 0.029) were significantly associated with unfavorable PRS (Table 1). This means that the primary ovarian tumor’s initial sensitivity to chemotherapy, in particular to platinum-based chemotherapy, is a primary prognostic factor for the treatment of recurrent ovarian cancer.
For primary ovarian cancer treatment, we routinely administered paclitaxel and carboplatin to patients as an adjuvant postoperative chemotherapy, thus cases with recurrence within 6 months after initial therapy were considered to have had platinum-resistance recurrence [4]. The platinum-resistant ovarian cancers included tumors which were refractory to the primary platinum-based chemotherapy and had progression within 6 months.
Gore et al reported that the progression-free interval, i.e., the period between the end of the initial treatment and the diagnosis of a relapse, was a significant prognostic factor for both recurrence response to treatment and PRS; in their study only 17% of patients who relapsed before 18 months responded to treatments, as compared to 53% who responded when relapsing after 18 months [9].
Patients may be distinguished as platinum-refractory (progression occurs under the initial platinum-based therapy), platinum-resistant (a relapse occurs within 6 months), or as platinum-sensitive (relapsed after 6 months) [10]. Ideally, patients with platinum-resistance should receive non-platinum therapy for recurrence [10]. Paclitaxel, topotecan, pegylated liposomal doxorubicin (PLD), and gemcitabine, which have all demonstrated some activity in platinum-resistant patient populations, are reasonable treatment options [11-15]. Although there are reports that combination therapy with non-platinum agents demonstrates an improvement for progression-free survival (PFS) compared with single agent chemotherapy [12, 14, 16], a monotherapy should be considered first, given that no advantage appears to accrue from the use of non-platinum-containing combination chemotherapy in patients with platinum-resistant disease [17, 18].
In the present investigation, all 48 cases of ovarian cancer achieved complete remission after the initial surgery and chemotherapy. That suggests that the TFI in this study was equivalent to the disease-free interval used in many other studies. We indicate that, in regards to cases with complete remission and then delayed recurrence, i.e., platinum-sensitive ovarian cancers, the length of the delay more directly affects survival probability after the recurrence than in cases directly refractory to the initial platinum treatments.
For the platinum-sensitive recurrence of ovarian cancer, the OCEANS study indicated that platinum-based chemotherapy with bevacizumab, followed by bevacizumab maintenance, significantly improved PFS after recurrence [5]. Pujade-Lauraine et al, in the AURELIA Phase III trial, found that bevacizumab combined with chemotherapy also statistically improved PFS for platinum-resistant recurrent ovarian cancer [6]. However, the OCEANS and AURELIA studies have yet to prove that bevacizumab use contributes to the elongation of overall survival [6, 19]. In our institution, we add bevacizumab to non-platinum single-agent chemotherapy (weekly paclitaxel, topotecan, PLD, or gemcitabine) for any platinum-resistant recurrent patients who match the eligibility criteria of the AURELIA trial. For cases of platinum-sensitive recurrence, we use platinum-based chemotherapy (paclitaxel/carboplatin or gemcitabine/carboplatin) accompanied with bevacizumab, in accordance with the OCEANS study. In this study we combined the platinum-sensitive with the platinum-resistant cases; however, the platinum-refractory cases were excluded because they never achieved complete remission of the primary disease. In addition, bevacizumab was not administered throughout the PRS term in all cases. Under the conditions of the present report, the log-rank test for the Kaplan-Meier procedure showed that adding bevacizumab was not responsible for determining PRS (P = 0.952) (Fig. 6). For treatment of recurrence of ovarian cancer after a complete remission, we have not found any advantage to bevacizumab use, which is in agreement with the results of the OCEANS and AURELIA studies.
Not surprisingly, we found that the histology of the tumor was significantly associated with PRS. With the Bonferroni correction, there were significant differences in PRS recognized between serous adenocarcinoma and mucinous adenocarcinoma (P < 0.05). There have been several recent reports showing that ovarian serous adenocarcinomas have a worse prognosis and greater likelihood of recurrence. The higher-grade serous ovarian carcinomas are characterized by frequent p53 mutations, which are related to why they are more aggressive than other histological subtypes [20, 21]. On the other hand, clear cell and mucinous adenocarcinomas are known to be more likely to incur chemoresistance, and that their survival rates are significantly poorer than that of serous adenocarcinoma, known as a much more chemo-sensitive cancer [22]. It is also well known that mucinous adenocarcinomas of the ovary show poorer response/reactivity to chemotherapy and a poorer prognosis than tumors of other histology [23-26].
In addition to tumor aggressiveness, which is known to be defined by tumor type and genetic profile, we think that chemo-sensitivity varies greatly among the histological subtypes of ovarian cancer; and that this greatly affects PRS. Patients treated with chemotherapy showed significantly better prognosis than patients not treated with chemotherapy (P < 0.001) (Fig. 6). Six patients with recurrence did not receive chemotherapy. Three (50%) of the six patients wanted to receive only best supportive care, the other three (50%) couldn’t receive chemotherapy because of their poor PS (≥ 2) at the time the recurrence was detected. Low PS at diagnosis meant that these patients were almost automatically included in the no-chemotherapy group, perhaps skewing these results. Our present results indicate that use of chemotherapy after recurrence should independently give survival advantages; however, further consideration of the usefulness of chemotherapy for recurrent ovarian cancer, including randomized studies, will be necessary to prove this point.
We have found that the finding of a solitary recurrent lesion was significantly associated with improved PRS. We found the presence of a solitary recurrent lesion in nine patients, six (67%) of whom received salvage surgical reduction and three (33%) who received only chemotherapy. We offered salvage surgical reduction to patients who had recurrent lesions that we expected to be able to remove completely. Cases of solitary recurrent lesion were more likely to receive surgical treatment, but not all received it. The multivariate Cox proportional hazards model demonstrated that use of multimodality treatment for recurrence didn’t significantly associate with PRS. We conclude that a solitary recurrent lesion is a good prognostic factor.
However, due to the small number of cases analyzed, there is limitation to adapt the statistical results directly to general population.
Conclusions
In ovarian cancer patients, post-relapse median survival is still a dismal 32.3 months. Having the histology of mucinous adenocarcinoma and a TFI less than 6 months worsens the prognosis for recurrent ovarian cancer patients. The solitary recurrent lesion is a good prognostic factor regardless of whether they received surgical treatment or not. Chemotherapy could improve PRS, if the PS of the patient allows her to receive chemotherapy and she desires to.
Acknowledgments
We would like to gratefully thank Dr. Takashi Miyatake for his advice regarding this manuscript, and Dr. GS Buzard for his editing.
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Funding
It is funded for editing by Kaizuka City Hospital.
| References | ▴Top |
- Ozols RF. Systemic therapy for ovarian cancer: current status and new treatments. Semin Oncol. 2006;33(2 Suppl 6):S3-11.
doi pubmed - Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247.
doi pubmed - Rustin GJ, van der Burg ME, Griffin CL, Guthrie D, Lamont A, Jayson GC, Kristensen G, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376(9747):1155-1163.
doi - Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, Wheeler S, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361(9375):2099-2106.
doi - Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A, Sovak MA, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17):2039-2045.
doi pubmed - Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, Sorio R, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302-1308.
doi pubmed - Bennouna J, Sastre J, Arnold D, Osterlund P, Greil R, Van Cutsem E, von Moos R, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14(1):29-37.
doi - Harter P, du Bois A, Hahmann M, Hasenburg A, Burges A, Loibl S, Gropp M, et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol. 2006;13(12):1702-1710.
doi pubmed - Gore ME, Fryatt I, Wiltshaw E, Dawson T. Treatment of relapsed carcinoma of the ovary with cisplatin or carboplatin following initial treatment with these compounds. Gynecol Oncol. 1990;36(2):207-211.
doi - Gadducci A, Conte P, Cianci C, Negri S, Genazzani AR. Treatment options in patients with recurrent ovarian cancer. Anticancer Res. 2001;21(5):3557-3564.
pubmed - Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka-Gorska I, Bidzinski M, Scambia G, et al. Randomized, open-label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J Clin Oncol. 2012;30(31):3841-3847.
doi pubmed - Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghamande SA, Gabrail NY, et al. PRECEDENT: a randomized phase II trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD) in combination versus PLD alone in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2013;31(35):4400-4406.
doi pubmed - Lortholary A, Largillier R, Weber B, Gladieff L, Alexandre J, Durando X, Slama B, et al. Weekly paclitaxel as a single agent or in combination with carboplatin or weekly topotecan in patients with resistant ovarian cancer: the CARTAXHY randomized phase II trial from Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens (GINECO). Ann Oncol. 2012;23(2):346-352.
doi pubmed - Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S, Mosconi AM, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16(5):561-568.
doi - Takei Y, Takahashi Y, Machida S, Taneichi A, Takahashi S, Nagashima T, Morisawa H, et al. Response to and toxicity of gemcitabine for recurrent ovarian cancer according to number of previous chemotherapy regimens. J Obstet Gynaecol Res. 2017;43(2):358-364.
doi pubmed - Le TN, Harvey RE, Kim CK, Brown J, Coleman RL, Smith JA. A retrospective evaluation of activity of gemcitabine/platinum regimens in the treatment of recurrent ovarian cancer. Gynecol Oncol Res Pract. 2017;4:16.
doi pubmed - Buda A, Floriani I, Rossi R, Colombo N, Torri V, Conte PF, Fossati R, et al. Randomised controlled trial comparing single agent paclitaxel vs epidoxorubicin plus paclitaxel in patients with advanced ovarian cancer in early progression after platinum-based chemotherapy: an Italian Collaborative Study from the Mario Negri Institute, Milan, G.O.N.O. (Gruppo Oncologico Nord Ovest) group and I.O.R. (Istituto Oncologico Romagnolo) group. Br J Cancer. 2004;90(11):2112-2117.
doi pubmed - Sehouli J, Stengel D, Oskay-Oezcelik G, Zeimet AG, Sommer H, Klare P, Stauch M, et al. Nonplatinum topotecan combinations versus topotecan alone for recurrent ovarian cancer: results of a phase III study of the North-Eastern German Society of Gynecological Oncology Ovarian Cancer Study Group. J Clin Oncol. 2008;26(19):3176-3182.
doi pubmed - Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015;139(1):10-16.
doi pubmed - Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, Setiawan VW, et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888-2898.
doi pubmed - Vang R, Shih Ie M, Kurman RJ. Ovarian low-grade and high-grade serous carcinoma: pathogenesis, clinicopathologic and molecular biologic features, and diagnostic problems. Adv Anat Pathol. 2009;16(5):267-282.
doi pubmed - Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(24):3621-3627.
doi pubmed - Shimada M, Kigawa J, Ohishi Y, Yasuda M, Suzuki M, Hiura M, Nishimura R, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113(3):331-334.
doi pubmed - Perren TJ. Mucinous epithelial ovarian carcinoma. Ann Oncol. 2016;27(Suppl 1):i53-i57.
doi pubmed - Ledermann JA, Luvero D, Shafer A, O'Connor D, Mangili G, Friedlander M, Pfisterer J, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for mucinous ovarian carcinoma. Int J Gynecol Cancer. 2014;24(9 Suppl 3):S14-19.
doi pubmed - Naik JD, Seligmann J, Perren TJ. Mucinous tumours of the ovary. J Clin Pathol. 2012;65(7):580-584.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
