| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Original Article
Volume 8, Number 2, June 2019, pages 39-43
Tendency Prediction for Atonic Bleeding Following a Problem-Free Pregnancy
Atsushi Yanaiharaa, b, Shouta Hatakeyamaa, Shirei Ougia, Aguri Hiranoa, Takumi Yanaiharaa
aYanaihara Women’s Clinic, Kanagawa, Japan
bCorresponding Author: Atsushi Yanaihara, Yanaihara Women’s Clinic, 1-26-29 Ofuna Kamakura, Kanagawa, Japan
Manuscript submitted March 3, 2019, accepted April 9, 2019
Short title: Prediction for Atonic Bleeding Following Pregnancy
doi: https://doi.org/10.14740/jcgo540
| Abstract | ▴Top |
Background: The incidence of postpartum hemorrhage (PPH) has increased globally; however, the reasons for this are largely unknown. PPH is potentially fatal and atonic PPH can occur even in low-risk pregnancies. In this study, we aimed to identify the causes of atonic bleeding following a problem-free pregnancy.
Methods: One thousand, four hundred sixty-six patients with problem-free pregnancies who experienced total bleeding 2 h after vaginal delivery were divided into two groups based on the amount of blood loss: control group (n = 1,325), with a blood loss of < 800 mL and study group (n = 141) with a blood loss of ≥ 800 mL. Several factors that may correlate with atonic bleeding were divided into three groups: maternal demographic (MD) factors, intrapartum factors, and fatal factors. Comparisons were made between the control group and study group regarding these factors. A multivariate analysis and receiver operating characteristic (ROC) analysis were performed to identify the independent risk factors for atonic bleeding. The continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were also calculated.
Results: The independent factors being statistically significant that predicted over 800 mL of atonic bleeding were in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) pregnancies (adjusted odd ratio (OR): 3.63; 95% confidence interval (CI): 2.46 - 5.36; P < 0.001), new-born weight (adjusted OR: 1.0016; 95% CI: 1.0011 - 1.0022; P < 0.001), and the cases of instrumental labor (IL) (adjusted OR: 2.48; 95% CI: 1.64 - 3.77; P < 0.001). ROCs for the final model (area under the curve (AUC): 0.765; 95% CI: 0.724 - 0.806), fatal model (AUC: 0.675; 95% CI: 0.627 - 0.723), intrapartum model (AUC: 0.654; 95% CI: 0.612 - 0.696) and MD model (AUC: 0.615; 95% CI: 0.575 - 0.656) were constructed. The NRI and IDI were 0.733 (95% CI: 0.565 - 0.901; P < 0.0001) and 0.073 (95% CI: 0.051 - 0.90; P < 0.0001) in the fatal model and final model, 0.057 (95% CI: -0.117 - 0.230; P = 0.288) and 0.015 (95% CI: -0.004 - 0.034; P = 0.521) in the MD model, and -0.146 (95% CI: -0.319 - 0.027; P = 0.098) and -0.003 (95% CI: -0.021 - 0.014; P = 0.700) in the intrapartum model.
Conclusions: We concluded that IVF/ICSI pregnancies, new-born weight, and IL were independent factors contributing to atonic bleeding. The coincidence of these three factors significantly predicts the likelihood of atonic bleeding.
Keywords: Postpartum hemorrhage; Atonic bleeding; Delivery; Instrumental labor; IVF
| Introduction | ▴Top |
Postpartum hemorrhage (PPH) or sudden bleeding after delivery may lead to maternal death from hemorrhagic shock, especially if the bleeding is heavy and difficult to control. It accounts for 20% of all maternal deaths in Japan. According to the Guidelines of the Perinatology Committee, Japan Society of Obstetrics and Gynecology 2014, the probability of PPH with over 1,000 mL, 1,500 mL, 2,000 mL, and 3,000 mL of blood loss is 17.7%, 7%, 3%, and 0.7%, respectively [1]. Globally, PPH accounts for 25% of maternal deaths, and ranks as the number one cause of death.
Over 500 mL of bleeding within 24 h has been defined as abnormal PPH [2] and over 1,000 mL of bleeding as severe PPH [3]. However, it has been recently reported by the society that the 90th percentile of blood loss after delivery is 800 mL (Guideline for PPH, Perinatology Committee, Japan, 2016). Thus, the cut-off value of blood loss after delivery in this study was set at 800 mL.
In this country, 60% of deliveries are carried out in small private clinics. When a problem during pregnancy is detected, patients are transferred to a large hospital before delivery. Thus, small clinics only take low-risk pregnancy cases.
Atonic bleeding is one of the most common causes of PPH and is a diagnosis made after delivery based on the total amount of blood loss. Thus, it is necessary for the delivering physician to be familiar with prophylaxis and the actions needed if bleeding occurs after delivery. Ideally, potential atonic bleeding cases would be recognized beforehand and actions taken to prevent atonic bleeding.
In this study, we focused on atonic bleeding in patients with low-risk pregnancies. To identify the causes of atonic bleeding and thus try to predict its occurrence, a retrospective study was performed using multivariate analysis and receiver operating characteristic (ROC) analysis.
| Materials and Methods | ▴Top |
One thousand, four hundred sixty-six patients who had a vaginal delivery of a singleton at ≥ 37 weeks of gestation between January 2014 and January 2017 with problem-free pregnancies were included in this study. The patients were divided into two groups, depending on the total amount of bleeding 2 h after vaginal delivery: a < 800 mL group (n = 1,325) and a ≥ 800 mL group (n = 141).
Patients with bleeding due to birth canal damage, uterus varus, hysterorrhexis, retained placenta, adhesive placentas, blood clotting abnormalities, and myoma were excluded.
To reduce the influence of individual doctor’s practices on bleeding after delivery, deliveries were performed by the same two board-certified doctors at the clinic. The medical treatments during and after delivery were based on the Japan Society of Obstetrics and Gynecology criteria. To avoid blood loss, hemostatic treatment was performed as soon as possible after delivery when it was judged that bleeding had increased or poor uterine contraction was detected. Uterine massage, oxytocin, ergometrine, balloon tamponade, and so on were used at the time if needed. Selective arterial embolisation was performed in four cases and a hysterectomy was performed in one case. The total amount of blood loss was calculated by weight of blood collection and soaked swabs.
The factors were divided three categories: maternal demographic (MD) factors (maternal age, parity (para 0 or para 1), gestational weeks, weight in first trimester of pregnancy, weight at the last examination, difference in weight, height, and body mass index (BMI) in the first trimester of pregnancy, BMI at the last examination, and method of conception (in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) pregnancy)), intrapartum factors (instrumental labor (IL) and duration of labor, and length of second and third stage of labor) and fatal factors (FF) (Apgar score, sex, weight of new-born, and head circumference of new-born).
A multivariate logistic regression (stepwise selection method) was used to establish the parsimonious model for bleeding risk prediction, which was adjusted for background information. For the final model, multicollinearity was assessed using the variance inflation factor. In order to evaluate predictive ability of the final model, a logistic regression model was constructed using the method of conception (MD model), IL (intrapartum model) and fatal weight (FF model) as the independent variables. Predictive abilities were assessed using ROC and area under the curve (AUC). The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) between the final model and other models were also calculated. We conducted all analyses using R version 3.3.2.1. The standard P < 0.05 was considered to be statistically significant in all tests.
This study was approved by the Institutional Review Board of the Yanaihara Women’s Clinic, and was conducted with the approval of the Ethics Committee of Yanaihara Women’s Clinic and with patient consent (ERBY/1, 2014).
| Results | ▴Top |
Stepwise selection method was used to establish the parsimonious model for bleeding risk prediction (Table 1). The multivariate logistic regression coefficients and adjusted odd ratio (OR) for the final model are presented in Table 2.
 Click to view | Table 1. Comparison of Clinical Features Predictive of Blood Loss Over 800 mL During Delivery |
 Click to view | Table 2. The Multivariate Logistic Regression Coefficients and Adjusted OR for the Final Model |
Variables such as weight of new-born (adjusted OR: 1.0016; 95% CI: 1.0011 - 1.0022; P < 0.001), IL (adjusted OR: 2.48; 95% CI: 1.64 - 3.77; P < 0.001) and method of conception (IVF/ICSI) (adjusted OR: 3.63; 95% CI: 2.46 - 5.36; P < 0.001) had a statistically significant relationship with bleeding events.
The ROC for the final model (AUC: 0.765; 95% CI: 0.724 - 0.806), FF model (weight of new-born) (AUC: 0.675; 95% CI: 0.627 - 0.723), MD model (IL) (AUC: 0.654; 95% CI: 0.612 - 0.696) and intrapartum model (method of conception) (AUC: 0.615; 95% CI: 0.575 - 0.656) are shown in Figure 1.
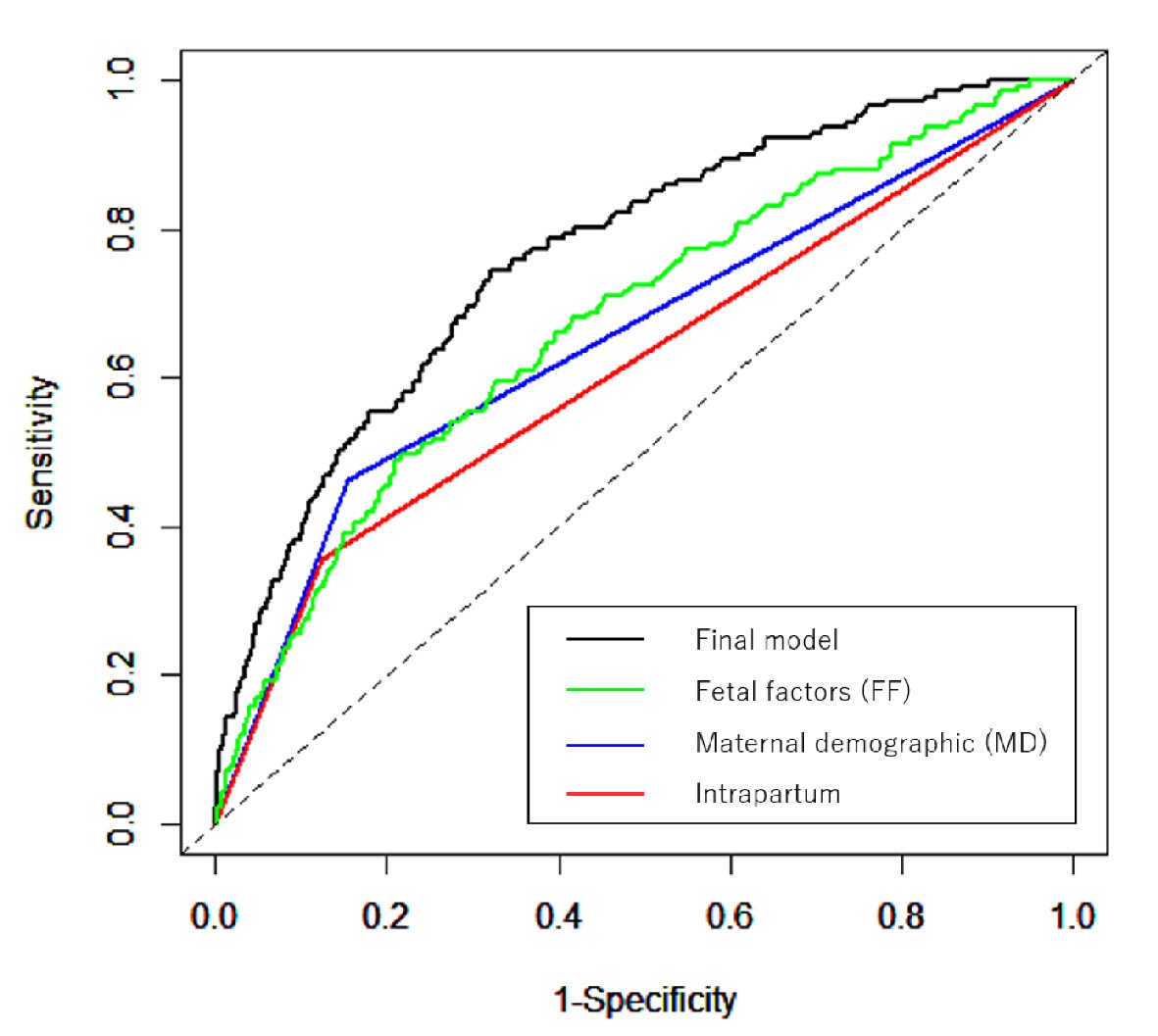 Click for large image | Figure 1. NRI and IDI of final model versus other factors. A multivariate logistic regression (stepwise selection method) was used to establish the parsimonious model for bleeding risk prediction, which was adjusted for background information. Predictive abilities were assessed using ROC and AUC. The NRI and IDI between the final model and other models (FF model, MD model and intrapartum model) were calculated. |
The NRI and IDI between the FF model and final model were 0.733 (95% CI: 0.565 - 0.901; P < 0.0001) and 0.073 (95% CI: 0.051 - 0.90; P < 0.0001), 0.057 (95% CI: -0.117 - 0.230; P = 0.288) and 0.015 (95% CI: -0.004 - 0.034; P = 0.521) between the MD model and final model and -0.146 (95% CI: -0.319 - 0.027; P = 0.098) and -0.003 (95% CI: -0.021 - 0.014; P = 0.700) between the intrapartum model and final model. The final model reflects the risk of atonic bleeding better.
| Discussion | ▴Top |
Globally, the incidence of PPH has recently increased [4-7]. It results in 150,000 maternal deaths per year and PPH has become the leading cause of maternal death. The reasons for this are not yet well understood. Predicting PPH and thus the establishment of an appropriate preventive treatment method are extremely important for minimizing maternal mortality [8]. International standards regarding what constitutes abnormal bleeding after delivery have not been established and the differences resulting from racial and social backgrounds have not been considered [9].
The risk factors for PPH that have been previously reported are fetal weight over 4 kg, maternal obesity, anemia, myoma of the uterus, history of cesarean section surgery, post-term delivery, a history of PPH, antidepressant drugs, being over 35 years old, excessive amniotic fluid, abnormal bleeding during pregnancy, pregnancy-induced hypertension, accreta/percreta/increta, multipara over four times, and multiple births [10-18].
Other PPH risks, such as prolonged first and second stages of labor, prolonged third stage of labor, chorioamnionitis, induction of labor, IL, rest of placenta, anomaly of rotation, and perineal laceration have also been reported [19-24]. In this study, the above-mentioned factors, which may have led to PPH, were excluded. These factors suggest a risk of PPH; however, PPH can occur even in the absence of these factors.
Prior preparation could be useful if atonic bleeding is expected. Our results may have a meaningful impact on small clinics that do not have enough facilities.
It cannot be denied that bias can be a problem in these studies. Results are subject factors influencing the delivery, such as the hospital facilities, and the experience and ability of doctors and nurses looking after the patient. Studies that are able to standardize these factors are required. It is difficult to measure the exact amount of bleeding but bias can be removed if comparisons are made under the same delivery conditions and environment. Duration of labor and length of the second and third stages in this study was assessed under the same monitoring of the delivery and same circumstances of delivery.
We found that IVF/ICSI pregnancies, new-born weight and IL were independent factors contributing to atonic bleeding.
Based on the results of this study, the weight of the new-born at delivery influenced atonic bleeding [25]. A large baby has been reported to be an independent factor contributing to PPH [12]. The reason for this is that a large baby causes excessive stretching of the uterine muscles, resulting in poor contractions.
In addition, IVF/ICSI pregnancies having been reported to be independently involved in PPH; and PPH still occurs with singleton births after IVF/ICSI/gamete intrafallopian transfer (GIFT). Exploratory analyses of factors in the IVF/ICSI group showed associations with fresh embryo transfers in stimulated cycles, endometriosis, and hormone treatments, suggesting that events around the time of implantation may be responsible, and that suboptimal endometrial function is the reason for PPH [25]. In terms of increased PPH after IVF, Aziz et al reported that patients who conceived from oocyte donation who did not receive controlled ovarian hyper-stimulation were at an increased risk of manual placental extraction, and this association was not influenced by age group. However, this may be one reason for an increase in PPH [26]. Our study focused on PPH as it relates to atonic bleeding. There are many other causes of PPH and the placental factor has been excluded.
We previously reported there to be a great amount of medical intervention at deliveries following IVF-induced pregnancies, even in women younger than 40 years of age, and have also suggested that muscle weakness in IVF patients is one of the factors causing atonic bleeding (in press). We believe that human bodily functions may decrease when things become convenient because of scientific development. It is reasonable to suggest that muscle hypofunction may be the reason for a global increase in PPH, especially atonic bleeding. Further studies are needed to determine the cause of increased atonic bleeding after IVF/ICSI-induced pregnancies.
From the ROC results, the AUC for the final model was 0.733 (95% CI: 0.565 - 0.901; P < 0.0001), and it was found that atonic bleeding could be predicted when the three independent factors occurred together.
As there are racial differences in weights of new-borns and sizes of the pelvis, it is necessary to investigate hereafter whether these ratios should be applied without modification. The items used as predictors of atonic bleeding must be individually applied; for instance, the cut-off values of new-born weight would be different for different races.
Other than the factors used in this study, we also considered the degree of anemia, fundus of uterus, and abdominal circumference as factors that may improve foresight precision. It may be necessary to perform a prospective study [17, 27].
Conclusions
Although our study was limited in statistical power due to the small sample size, our study found that IVF/ICSI pregnancy, new-born weight and IL were independent factors causing atonic bleeding. The coincidence of these three factors significantly predicts the likelihood of atonic bleeding.
Further studies are necessary to determine the causes of atonic bleeding and thus improve the efficiency of treatment and decrease the occurrence of atonic bleeding.
Acknowledgments
The authors acknowledge the assistance of Editage, a Division of Cactus Communications, for proofreading this manuscript.
Financial Disclosure
There is no funding related to this study.
Conflict of Interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. The authors have no competing financial interests related to this study.
Informed Consent
Written informed consent was obtained from the patients for publication of this study and any accompanying images.
Author Contributions
AY drafted the manuscript. SO, TY and AH decided a parturient policy and participated in delivery. SH performed statistically analysis. TY helped to draft the manuscript.
| References | ▴Top |
- Perinatology Committee, Japan Society of Obstetrics and Gynecology. 2013.
- Obstetrical Hemorrhage. Stanford: Appleton & Lange. 2010.
- Perinatology Committee, Japan Society of Obstetrics and Gynecology. 2009.
- Ford JB, Patterson JA, Seeho SK, Roberts CL. Trends and outcomes of postpartum haemorrhage, 2003-2011. BMC Pregnancy Childbirth. 2015;15:334.
doi pubmed - Mehrabadi A, Hutcheon JA, Lee L, Liston RM, Joseph KS. Trends in postpartum hemorrhage from 2000 to 2009: a population-based study. BMC Pregnancy Childbirth. 2012;12:108.
doi pubmed - Mehrabadi A, Hutcheon JA, Lee L, Kramer MS, Liston RM, Joseph KS. Epidemiological investigation of a temporal increase in atonic postpartum haemorrhage: a population-based retrospective cohort study. BJOG. 2013;120(7):853-862.
doi pubmed - Mehrabadi A, Liu S, Bartholomew S, Hutcheon JA, Kramer MS, Liston RM, Joseph KS, et al. Temporal trends in postpartum hemorrhage and severe postpartum hemorrhage in Canada from 2003 to 2010. J Obstet Gynaecol Can. 2014;36(1):21-33.
doi - Weeks A. The prevention and treatment of postpartum haemorrhage: what do we know, and where do we go to next? BJOG. 2015;122(2):202-210.
doi pubmed - Knight M, Callaghan WM, Berg C, Alexander S, Bouvier-Colle MH, Ford JB, Joseph KS, et al. Trends in postpartum hemorrhage in high resource countries: a review and recommendations from the International Postpartum Hemorrhage Collaborative Group. BMC Pregnancy Childbirth. 2009;9:55.
doi pubmed - Kramer MS, Berg C, Abenhaim H, Dahhou M, Rouleau J, Mehrabadi A, Joseph KS. Incidence, risk factors, and temporal trends in severe postpartum hemorrhage. Am J Obstet Gynecol. 2013;209(5):449 e441-447.
- Yasmeen S, Danielsen B, Moshesh M, Gilbert WM. Is grandmultiparity an independent risk factor for adverse perinatal outcomes? J Matern Fetal Neonatal Med. 2005;17(4):277-280.
doi pubmed - Lu MC, Korst LM, Fridman M, Muthengi E, Gregory KD. Identifying women most likely to benefit from prevention strategies for postpartum hemorrhage. J Perinatol. 2009;29(6):422-427.
doi pubmed - Wong SF, Ho LC. Labour outcome of low-risk multiparas of 40 years and older. A case-control study. Aust N Z J Obstet Gynaecol. 1998;38(4):388-390.
doi pubmed - Henry A, Birch MR, Sullivan EA, Katz S, Wang YA. Primary postpartum haemorrhage in an Australian tertiary hospital: a case-control study. Aust N Z J Obstet Gynaecol. 2005;45(3):233-236.
doi pubmed - Robinson HE, O'Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstet Gynecol. 2005;106(6):1357-1364.
doi pubmed - Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(3):561-568.
doi pubmed - Tsu VD. Postpartum haemorrhage in Zimbabwe: a risk factor analysis. Br J Obstet Gynaecol. 1993;100(4):327-333.
doi pubmed - Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal and obstetric complications of pregnancy are associated with increasing gestational age at term. Am J Obstet Gynecol. 2007;196:155.e1-6.
doi pubmed - Magann EF, Evans S, Chauhan SP, Lanneau G, Fisk AD, Morrison JC. The length of the third stage of labor and the risk of postpartum hemorrhage. Obstet Gynecol. 2005;105(2):290-293.
doi pubmed - Bais JM, Eskes M, Pel M, Bonsel GJ, Bleker OP. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women. A Dutch population-based cohort study on standard (> or = 500 ml) and severe (> or = 1000 ml) postpartum haemorrhage. Eur J Obstet Gynecol Reprod Biol. 2004;115(2):166-172.
doi - Rueangchainikhom W, Srisuwan S, Prommas S, Sarapak S. Risk factors for primary postpartum hemorrhage in Bhumibol Adulyadej Hospital. J Med Assoc Thai. 2009;92(12):1586-1590.
pubmed - Selo-Ojeme DO, Okonofua FE. Risk factors for primary postpartum haemorrhage. A case control study. Arch Gynecol Obstet. 1997;259(4):179-187.
doi pubmed - Sosa CG, Althabe F, Belizan JM, Buekens P. Risk factors for postpartum hemorrhage in vaginal deliveries in a Latin-American population. Obstet Gynecol. 2009;113(6):1313-1319.
doi pubmed - Begley CM, Gyte GM, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2015;3:CD007412.
doi - Healy DL, Breheny S, Halliday J, Jaques A, Rushford D, Garrett C, Talbot JM, et al. Prevalence and risk factors for obstetric haemorrhage in 6730 singleton births after assisted reproductive technology in Victoria Australia. Hum Reprod. 2010;25(1):265-274.
doi pubmed - Aziz MM, Guirguis G, Maratto S, Benito C, Forman EJ. Is there an association between assisted reproductive technologies and time and complications of the third stage of labor? Arch Gynecol Obstet. 2016;293(6):1193-1196.
doi pubmed - Soltan MH, Ibrahim EM, Tawfek M, Hassan H, Farag F. Raised nitric oxide levels may cause atonic postpartum hemorrhage in women with anemia during pregnancy. Int J Gynaecol Obstet. 2012;116(2):143-147.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
