| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website http://www.jcgo.org |
Case Report
Volume 8, Number 3, September 2019, pages 91-96
Fetal Heart Rate as an Indirect Indicator of Treatment Response in Fetal Hyperthyroidism Secondary to Transplacental Passage of Maternal Thyrotropin Receptor Antibodies
Alexander L. Juuselaa, c, Munir Nazirb, Zankhana Patel Batraa, Kristina Torrencea, Martin Gimovskyb
aOB/GYN Department, Newark Beth Israel Medical Center, 201 Lyons Ave., Newark, NJ 07112, USA
bMaternal-Fetal Medicine, OB/GYN Department, Newark Beth Israel Medical Center, 201 Lyons Ave., Newark, NJ 07112, USA
cCorresponding Author: Alexander L. Juusela, OB/GYN Department, Newark Beth Israel Medical Center, 201 Lyons Ave., Newark, NJ 07112, USA
Manuscript submitted July 8, 2019, accepted August 6, 2019
Short title: Fetal Hyperthyroidism
doi: https://doi.org/10.14740/jcgo564
| Abstract | ▴Top |
Maternal hyperthyroidism is prevalent in 0.2-0.4% of pregnancies. Graves’ disease accounts for 85% of these cases. Approximately 1-5% of neonates born to these mothers develop hyperthyroidism. Transplacental passage of thyrotropin receptor antibodies (TRAbs) are considered to be the likely cause of transient fetal and neonatal hyperthyroidism. An 18-year-old G1P0 with a history of Graves’ disease treated by radioactive ablation presented with persistent fetal tachycardia at 23 weeks gestational age. TRAb was elevated and fetal hyperthyroidism secondary to transplacental crossing of maternal TRAb was suspected. There was no evidence of overt fetal hyperthyroidism or goiter on ultrasound examination. Oral methimazole was initiated and the fetal tachycardia resolved. Upon decrease of the methimazole dosage, the fetal tachycardia returned. Methimazole dosing was again increased and stabilized at a higher dose and the fetal tachycardia remained resolved. At birth at 40 weeks gestation, the neonate was tachycardic with elevated TRAb. She was initiated on methimazole. At 1 month of life, the methimazole dose was halved. At 2 months of life, all medication was held. On further testing, the thyroid function test remained normal. In our patient who did not display evidence of fetal hyperthyroidism on ultrasound examination, as there were no abnormal fetal markers on ultrasound to evaluate treatment response, fetal heart rate on non-stress testing was successfully used as an indirect indicator of fetal thyroid status and guided appropriate titration of methimazole.
Keywords: Grave’s disease; Fetal hyperthyroidism; Maternal hyperthyroidism; Non-stress test; Endocrinology; Maternal-Fetal Medicine; Ultrasound
| Introduction | ▴Top |
Maternal hyperthyroidism is prevalent in 0.2-0.4% of pregnancies and Graves’ disease accounts for 85% of these cases [1, 2]. Approximately 1-5% of neonates born to these mothers develop hyperthyroidism, with the neonate developing symptoms as late as 1 week postpartum [3]. Even after thyroid radioactive iodine ablation or thyroidectomy, these women continue to produce thyrotropin receptor antibodies (TRAbs) of the immunoglobulin G (IgG) class, which can persist for years [4]. TRAbs cross the placenta, activate the fetal thyroid and cause a maternal antibody-induced hyperthyroid state [2, 4-14].
The fetuses and neonates of affected pregnant women are at risk for hyperthyroidism, especially in pregnancies not treated with thioamides. Approximately 1-5% of neonates born to these mothers develop hyperthyroidism [3]. If untreated, fetal hyperthyroidism can lead to fetal tachycardia, fetal goiter, craniosynostosis, intrauterine growth retardation (IUGR), preterm labor, high-output cardiac failure, hydrops fetalis and intrauterine fetal demise (IUFD) [1, 3, 8, 10, 12, 15, 16]. In these complex cases, fetal surveillance via serial ultrasound should be central to the management and should include measurements of thyroid size, thyroid vascular Doppler flow, cardiac size measurements, cardiac valve studies, as well as investigating evidence of accelerated bone maturation. Secondarily, it is well known that fetal tachycardia is not always present in hyperthyroidism, and it can even be a late phenomenon. In the study by Huel et al, fetal tachycardia was a good indicator of fetal hyperthyroidism; however, it was only present in 57.1% of affected fetuses [17].
In our case, there were no indicators of overt fetal hyperthyroidism on serial ultrasound investigation, and we were able to demonstrate that resolution of fetal tachycardia was an indirect indicator of appropriate medication titration, with a noted fetal heart rate response that preceded changes in maternal thyroid function testing. As the reaction was swifter than monitoring maternal thyroid function test (TFT), we posit that in patients without evidence of fetal hyperthyroidism on ultrasound, with the presence of baseline fetal tachycardia secondary to transplacental passage of maternal antibodies, the methimazole dosage may be titrated based on fetal heart rate values.
| Case Report | ▴Top |
An 18-year-old G2P0010 with an unknown last menstrual period (LMP), past obstetrical history significant for one medical termination of pregnancy, medical history of hypothyroidism treated with levothyroxine 100 µg PO daily and no past surgical history presented to our antenatal testing unit for a gestational dating. Ultrasound demonstrated a single viable fetus with biometry consistent with 23 weeks gestation, estimated fetal weight (EFW) 518 g (25th percentile), grossly normal anatomy, an unstable lie and fetal heart rate (FHR) of 180 beats per minute (bpm). A repeat thyroid panel including antibody levels was sent for analysis. Thyroid-stimulating hormone (TSH) was within normal range at 5.60, free T4 was 1.16 (normal), thyrotropin receptor antibody (TRAb) levels were elevated at 447 µU/mL (< 150 is normal) and thyroid-stimulating immunoglobulin (TSI) was elevated at 481. Fetal thyroid gland measured 17 × 8 mm (normal range). Obstetrical care was transferred to Maternal-Fetal Medicine and the patient presented for her first visit at 25 weeks of gestation. Detailed examination revealed that the patient had a history of Graves’ disease and underwent radioactive iodine ablation in 2014, resulting in a hypothyroid state for which she was taking the levothyroxine 100 µg per os (PO) daily. Levothyroxine was increased to 175 µg PO daily once pregnancy was diagnosed. Initial care included weekly fetal ultrasounds with evaluation of the FHR, fetal thyroid assessment (both size measurements and vascular flow studies), cardiac size and valvular studies via echocardiograms, assessment of bone maturation, middle cerebral artery (MCA) peak systolic flow and umbilical artery Dopplers.
The 25-week ultrasound demonstrated an FHR of 162 bpm and a normal for gestational-age thyroid gland measuring 18 × 9 mm. Repeat ultrasound at 29 weeks found a persistently elevated FHR with a baseline of 180 bpm and a thyroid measuring 19 × 10 mm. Concern was that the fetus was in a transient hyperthyroid state secondary to the transplacental crossing of maternal TRAbs which were stimulating the fetal thyroid tissue. The patient was initiated on methimazole 10 mg PO twice daily (BID) and levothyroxine 175 µg PO daily (qDay) in order to assist in FHR control. By 30 weeks gestation, the FHR had decreased to 157 bpm. There appeared to be a direct correlation between initiating methimazole and the change in FHR baseline. To test our hypothesis, we reduced the methimazole to 5 mg PO BID; to prevent maternal symptomatology the levothyroxine was increased to 200 µg PO qDay. By 33 weeks of gestation, the FHR rose to 167 bpm, supporting our hypothesis. Subsequently, the methimazole dosage was increased to 10 mg PO BID, which resulted in resolution of the fetal tachycardia (Fig. 1 and Table 1).
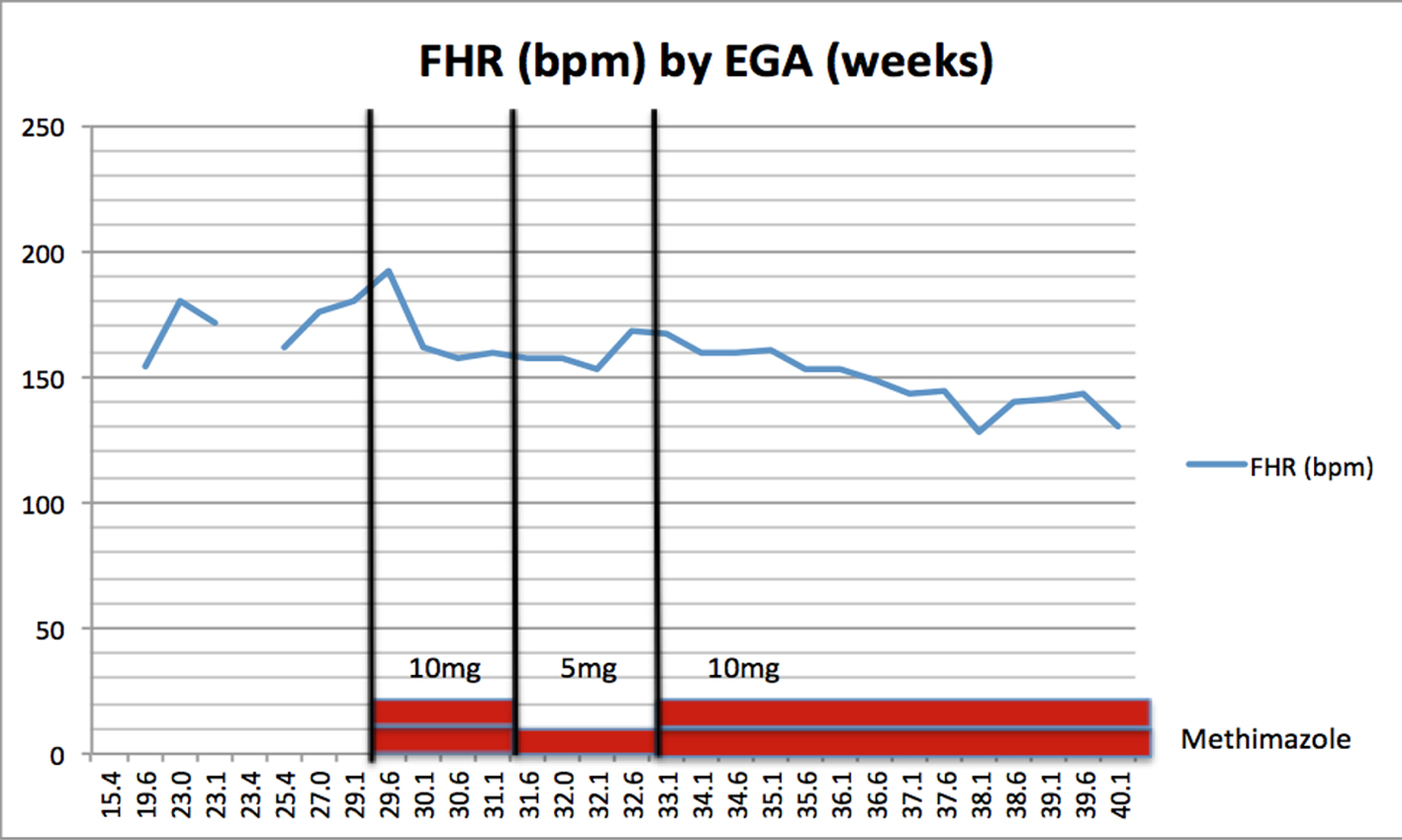 Click for large image | Figure 1. Fetal heart rate (bpm) by estimated gestational age (weeks). |
 Click to view | Table 1. Fetal and Maternal Testing |
This dosage was continued with a complete resolution of fetal tachycardia until the patient presented to labor at 40 weeks, at which point the FHR baseline was 135 bpm. Methimazole was held during the labor process. Labor was augmented with oxytocin, and the patient progressed to a spontaneous vaginal delivery without complications. Throughout the entire labor course, the FHR baseline remained 120 - 130 bpm with a category 1 tracing. Immediately after delivery, the neonate was evaluated and then taken for continuous monitoring in the neonatal intensive care unit (NICU) for suspected transient neonatal hyperthyroidism. Upon admission to the NICU, the neonatal HR was 120 - 130 bpm at rest and in the 150 s with exertion. Examination revealed mild jitteriness. Otherwise, the physical exam was benign and appropriate for the infant. Initial labs revealed a decreased TSH of < 0.03, free T4 was normal at 7.05 and TSI was elevated at 565. The neonate was initiated on methimazole 1.25 mg every 24 h. The mother was restarted on her pre-pregnancy regimen of levothyroxine 100 µg PO qDay. The hospital course for both patients was uncomplicated, and the neonate and mother were discharged on postpartum day 2, with instructions for follow-up with Pediatric Endocrinology and Maternal-Fetal Medicine, respectively. After 1 month of life, the neonate’s TFTs were normalizing with a TSH of 0.10, a free T4 of 0.69 and a TSI of 149; therefore the methimazole was decreased to 0.625 mg PO daily. At 2 months of life, the methimazole was stopped, and by 3 months of life, the TSH was 1.07, the T4 was 1.04 and the TSI was 58 (Table 2).
 Click to view | Table 2. Neonatal Thyroid Testing |
| Discussion | ▴Top |
Neonates of mothers with autoimmune thyroid diseases such as Graves’ disease are at risk of developing thyroid dysfunction secondary to maternal antibodies crossing the placental barrier [2, 5-12]. These antibodies can cause fetal tachycardia, goiter, oligohydramnios, IUGR and accelerated bone maturation [1, 3, 8, 10, 12, 15, 16]. Sustained untreated fetal hyperthyroidism can lead to IUGR, intrauterine fetal demise, preterm labor, fetal tachycardia and non-immune hydrops.
Clinical manifestations of neonatal hyperthyroidism include low birth weight, premature birth (often medically indicated), microcephaly, frontal bossing, triangular fascies, diffuse goiter, irritability, hyperactivity, restlessness, tachycardia with a bounding pulse, cardiomegaly, cardiac arrhythmias, heart failure and even neonatal demise [1, 3, 8, 10, 12, 15, 16, 18].
Neonatal hyperthyroidism may not present until a week after delivery and can last up to 8 months or more after birth, at time frames proportional to the clearance of maternal immunoglobulin from the infant’s circulation [1, 13].
The initial evaluation of thyrotoxicosis is made on the basis of the maternal TSH level, and if abnormal, a free T4 level. A history of maternal hyperthyroidism may result in persistent TRAb even after radioactive iodine ablation. These elevated levels can affect the fetus, resulting in transient fetal hyperthyroidism. Historically, serial fetal thyroid measurement via ultrasound, assessment of bone age maturation, FHR and umbilical cord blood sampling were used to diagnose fetal hyperthyroidism [1, 3, 5, 7, 8, 12, 19-22].
The most common sign of fetal thyrotoxicosis is fetal tachycardia; however, this is not always present. Typically, once a persistently elevated FHR is identified, medications that can cross the placenta are added to the maternal regimen. Since thyroxines are less likely to cross the placental barrier, carbimazoles such as methimazole are usually preferred and titrated based on the FHR response, fetal thyroid levels obtained via funipuncture and maternal thyroid levels.
After delivery, measurement of a neonate’s TFT is obtained to determine in utero exposure to maternal antibodies and the extent of their effects. Higher maternal TRAb levels during the third trimester of pregnancy may indicate a higher likelihood of a fetus developing hyperthyroidism and thyrotoxicosis [7, 11, 14, 23]. Once neonatal hyperthyroidism is confirmed, treatment should be started promptly to prevent the aforementioned long-term sequela. The goal of treatment is to normalize thyroid function quickly, avoid iatrogenic hypothyroidism and provide therapeutic relief. Treatment regimens include a combination as needed of propylthiouracil (PTU), beta-blockers, glucocorticoids, iodine and digoxin [1, 5-11, 15, 16, 19, 21]. Transient neonatal hyperthyroidism is typically self-limited and resolves in up to 8 months as the maternal antibodies are cleared from the neonates plasma; however, it requires therapy and close neonatal surveillance [1].
Conclusion
Due to persistent production of TRAb in women who are hypothyroid secondary to thyroid radioiodine ablation or surgery, these patients’ fetuses remain at risk for hyperthyroidism and thyrotoxicosis via transplacental passage of TRAb. Such women should have serial thyroid function and antibody testing, serial non-stress testing, and that fetal surveillance via serial ultrasound should be central to the management and should include measurements of thyroid size, thyroid vascular Doppler flow, cardiac size measurements, cardiac valve studies, as well as investigating evidence of accelerated bone maturation. In patients with evidence of fetal hyperthyroidism, research has shown that funipuncture for analysis of fetal thyroid levels and serial maternal thyroid function testing are methods for demonstrating treatment response.
We posit that assessing FHR via the non-stress test (NST) is less invasive than funipuncture, without the potential side effects (puncture site bleeding, cord hematoma, chorioamnionitis and transient FHR changes). As demonstrated in the case presented, in the absence of sonographic evidence of overt fetal hyperthyroidism, NST is both an equally effective and a faster method for evaluating treatment response than maternal TFT. Our data demonstrate that in a patient without evidence of fetal hyperthyroidism on ultrasound examination, as there were no others fetal markers to evaluate treatment response, FHR can be used as an indirect indicator of fetal thyroid status and that dosage of methimazole may be titrated based on FHR response. We consider the noted third-trimester progressive decrease in FHR with a stable dosage of methimazole not to be an effect of resolved hyperthyroidism; rather, we attribute it to the well-established vagal effect of the developing fetal parasympathetic nervous system. We also posit that a change in FHR baseline on an NST after initiating treatment with methimazole may be used as an early clinical indicator, preceding maternal TFT response. Therefore, we question the role of invasive fetal thyroid testing via funipuncture in the titration of antithyroid medications. Additional investigation such as a case series is required to further support our findings and it should be noted that ultrasound-guided diagnosis and surveillance should be central to the management of these complex cases.
Acknowledgments
The authors would like to thank Arlene Gimovsky and Tatiana Holway PhD for editing this case report.
Financial Support
None to declare.
Conflict of Interest
All of the authors declare no conflict of interest.
Informed Consent
As no patient identifying information is contained, patient consent was not required.
Author Contributions
Alexander L. Juusela: acquisition, analysis and interpretation of data; drafting, revising and writing of paper; preparation of graph and tables. Zankhana Patel Batra: acquisition of data and draft writing. Kristina Torrence: acquisition of data and draft writing. Martin Gimovsky: interpretation of data, draft editing and final approval. Munir Nazir: interpretation of data, draft editing and final approval.
| References | ▴Top |
- Duncombe GJ, Dickinson JE. Fetal thyrotoxicosis after maternal thyroidectomy. Aust N Z J Obstet Gynaecol. 2001;41(2):224-227.
doi - Bucci I, Giuliani C, Napolitano G. Thyroid-stimulating hormone receptor antibodies in pregnancy: clinical relevance. Front Endocrinol (Lausanne). 2017;8:137.
doi pubmed - Sato Y, Murata M, Sasahara J, Hayashi S, Ishii K, Mitsuda N. A case of fetal hyperthyroidism treated with maternal administration of methimazole. J Perinatol. 2014;34(12):945-947.
doi pubmed - Zuppa AA, Sindico P, Perrone S, Carducci C, Antichi E, Alighieri G, Cota F, et al. Different fetal-neonatal outcomes in siblings born to a mother with Graves-Basedow disease after total thyroidectomy: a case series. J Med Case Rep. 2010;4:59.
doi pubmed - Batra CM, Gupta V, Gupta N, Menon PS. Fetal hyperthyroidism: intrauterine treatment with Carbimazole in two siblings. Indian J Pediatr. 2015;82(10):962-964.
doi pubmed - Bowman ML, Bergmann M, Smith JF. Intrapartum labetalol for the treatment of maternal and fetal thyrotoxicosis. Thyroid. 1998;8(9):795-796.
doi pubmed - Matsumoto T, Miyakoshi K, Saisho Y, Ishii T, Ikenoue S, Kasuga Y, Kadohira I, et al. Antenatal management of recurrent fetal goitrous hyperthyroidism associated with fetal cardiac failure in a pregnant woman with persistent high levels of thyroid-stimulating hormone receptor antibody after ablative therapy. Endocr J. 2013;60(12):1281-1287.
doi pubmed - Polak M, Leger J, Luton D, Oury JF, Vuillard E, Boissinot C, Czernichow P. Fetal cord blood sampling in the diagnosis and the treatment of fetal hyperthyroidism in the offsprings of a euthyroid mother, producing thyroid stimulating immunoglobulins. Ann Endocrinol (Paris). 1997;58(4):338-342.
- Serup J, Petersen S. Fetal thyrotoxicosis in utero. Biol Neonate. 1979;35(3-4):175-179.
doi pubmed - Srisupundit K, Sirichotiyakul S, Tongprasert F, Luewan S, Tongsong T. Fetal therapy in fetal thyrotoxicosis: a case report. Fetal Diagn Ther. 2008;23(2):114-116.
doi pubmed - Ting MK, Hsu BR, Huang YY, Lin JD, Chen TC. Recurrent fetal thyrotoxicosis in a woman with Graves' disease: case report. Changgeng Yi Xue Za Zhi. 1999;22(3):492-497.
- Watson WJ, Fiegen MM. Fetal thyrotoxicosis associated with nonimmune hydrops. Am J Obstet Gynecol. 1995;172(3):1039-1040.
doi - Smith CM, Gavranich J, Cotterill A, Rodda CP. Congenital neonatal thyrotoxicosis and previous maternal radioiodine therapy. BMJ. 2000;320(7244):1260-1261.
doi pubmed - Hamada N, Momotani N, Ishikawa N, Yoshimura Noh J, Okamoto Y, Konishi T, Ito K, et al. Persistent high TRAb values during pregnancy predict increased risk of neonatal hyperthyroidism following radioiodine therapy for refractory hyperthyroidism. Endocr J. 2011;58(1):55-58.
doi pubmed - Maxwell KD, Kearney KK, Johnson JW, Eagan JW, Jr., Tyson JE. Fetal tachycardia associated with intrauterine fetal thyrotoxicosis. Obstet Gynecol. 1980;55(3 Suppl):18S-22S.
doi - Treadwell MC, Sherer DM, Sacks AJ, Ghezzi F, Romero R. Successful treatment of recurrent non-immune hydrops secondary to fetal hyperthyroidism. Obstet Gynecol. 1996;87(5 Pt 2):838-840.
- Huel C, et al. Use of ultrasound to distinguish between fetal hyperthyroidism and hypothyroidism on discovery of a goiter. Ultrasound Obstet Gynecol. 2009;33(4):412-420.
doi pubmed - Farrehi C. Accelerated maturity in fetal thyrotoxicosis. Clin Pediatr (Phila). 1968;7(3):134-137.
doi pubmed - Heckel S, Favre R, Schlienger JL, Soskin P. Diagnosis and successful in utero treatment of a fetal goitrous hyperthyroidism caused by maternal Graves' disease. A case report. Fetal Diagn Ther. 1997;12(1):54-58.
doi pubmed - Porreco RP, Bloch CA. Fetal blood sampling in the management of intrauterine thyrotoxicosis. Obstet Gynecol. 1990;76(3 Pt 2):509-512.
- Wallace C, Couch R, Ginsberg J. Fetal thyrotoxicosis: a case report and recommendations for prediction, diagnosis, and treatment. Thyroid. 1995;5(2):125-128.
doi pubmed - Wenstrom KD, Weiner CP, Williamson RA, Grant SS. Prenatal diagnosis of fetal hyperthyroidism using funipuncture. Obstet Gynecol. 1990;76(3 Pt 2):513-517.
- Laurberg P, Nygaard B, Glinoer D, Grussendorf M, Orgiazzi J. Guidelines for TSH-receptor antibody measurements in pregnancy: results of an evidence-based symposium organized by the European Thyroid Association. Eur J Endocrinol. 1998;139(6):584-586.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
