| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 9, Number 3, September 2020, pages 73-77
Endometrial Stromal Sarcoma in the Young
Mikaela Erlinda G. Martineza, b, Elizabeth K. Jacintoa
aDepartment of Obstetrics and Gynecology, Philippine General Hospital, University of the Philippines Manila, College of Medicine, Manila, Philippines
bCorresponding Author: Mikaela Erlinda G. Martinez, Department of Obstetrics and Gynecology, Philippine General Hospital, Taft Avenue Ermita, Barangay 670 Zone 72, Manila, 1000 Metro Manila, Philippines
Manuscript submitted May 12, 2020, accepted July 3, 2020, published online September 9, 2020
Short title: ESS in the Young
doi: https://doi.org/10.14740/jcgo647
| Abstract | ▴Top |
Endometrial stromal sarcoma (ESS) represents a very rare group of malignant tumors comprising less than 10% of all uterine sarcomas but only around 0.2% of all uterine cancer. In developing countries, the prevalence of ESS is approximately two in a million perimenopausal women between ages of 45 and 50 years. The occurrence in younger women is rare and the diagnosis frequently delayed due to low index of suspicion. Two cases of ESS diagnosed in women in the 20s age group were documented in a tertiary hospital. Both patients presented with abnormal vaginal bleeding associated with hypogastric pain and rapid abdominal enlargement. Surgery was the primary treatment modality and histopathologic examination confirmed the diagnosis. Adjuvant therapy remains controversial. ESS is a rare pathological entity, more so in the young. However, the diagnosis of a malignancy should not be missed despite rarity of occurrence in this age group.
Keywords: Endometrial stromal sarcoma; Uterine sarcoma; Malignancies of the female genital tract
| Introduction | ▴Top |
Endometrial stromal sarcoma (ESS) represent a very rare group of malignant tumors comprising less than 10% of all uterine sarcomas but only around 0.2% of all uterine cancer [1-6]. In developing countries, the prevalence of ESS is approximately two in a million women, occurring primarily in perimenopausal women between ages of 45 and 50 years [2, 7]. Its incidence in younger women is rare.
In a local tertiary hospital, 19 patients were diagnosed with ESS during 2001 - 2012 with an average age of 42 years. Among these women, two cases occurred in women in their 20s, the youngest at 20 years of age. This report discusses the diagnostic and therapeutic dilemmas of ESS in the young.
| Case Reports | ▴Top |
Case 1
A 28-year-old nulligravid presented with irregular menstrual bleeding, rapid abdominal enlargement and oliguria. There was a solid abdominopelvic mass on physical examination. The cervix was anterosuperiorly deviated. The corpus and adnexa were difficult to assess due to the mass. Ultrasound revealed endometrial and cul-de-sac masses with malignant features. The patient underwent extra fascial hysterectomy with bilateral salpingo-oophorectomy. Operative findings showed a large uterus with a 12 × 10 × 10 cm polypoid, friable, necrotic mass occupying the endometrial cavity with full thickness myometrial involvement (Fig. 1a). The right ovary was converted into an 8 × 8 × 10 cm solid mass adherent to the adjacent structures (Fig. 1b). The pelvic lymph nodes were solid, fixed, highly vascular and markedly enlarged. Final histopathologic diagnosis was high-grade ESS (HG-ESS) (Fig. 1c-f). The plan was for adjuvant chemotherapy, but the patient succumbed to the disease 1 month after surgery.
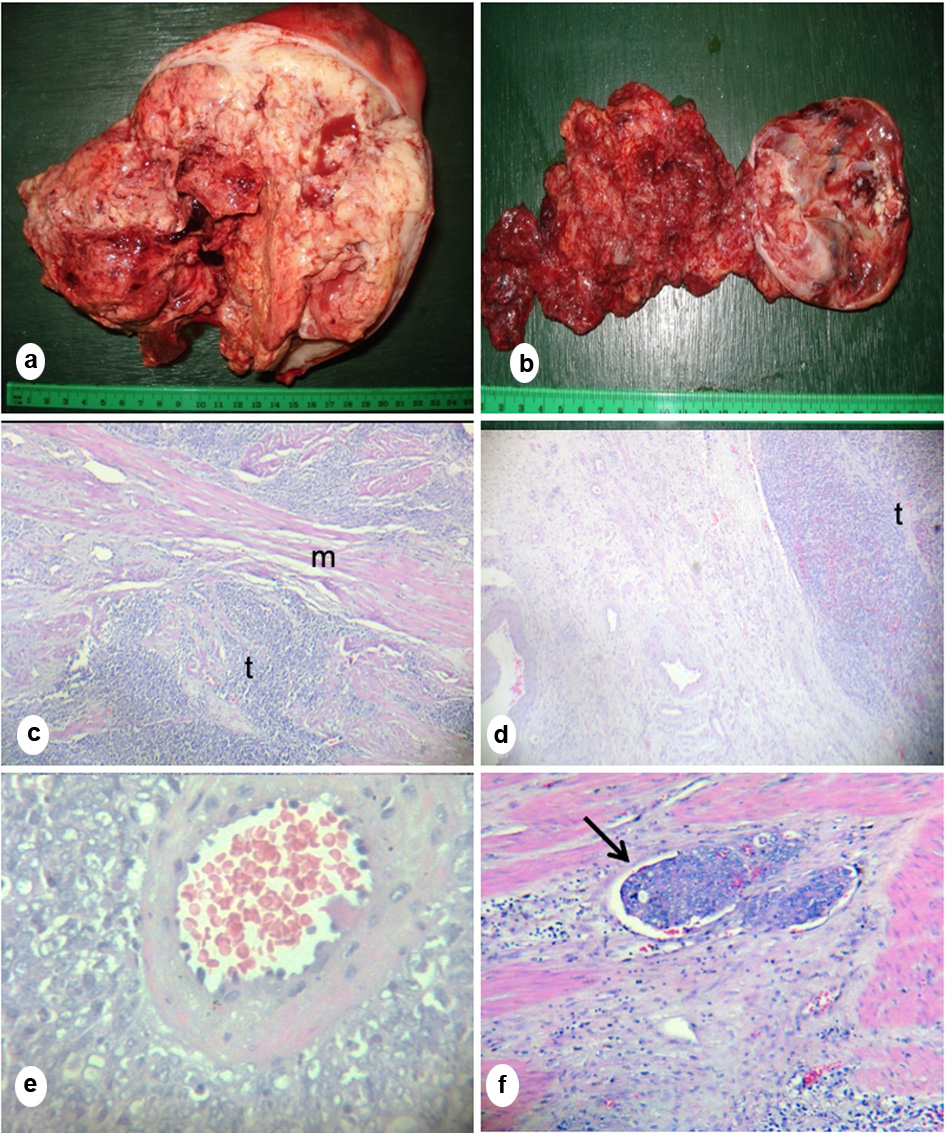 Click for large image | Figure 1. (a) Gross section of the uterus showed a polypoid, necrotic mass occupying the entire endometrial cavity with full thickness myometrial invasion. (b) Gross section of the right ovary showed friable necrotic mass. (c) Microscopic section of the mass showed sheaths of tumor cells (t) infiltrating smooth muscle bundles (m). (d) Microscopic section of the right ovary showed medullary tumor invasion (t). (e) Microscopic section of the tumor showing characteristic whirling of tumor cells around the spiral arteriole and (f) Lymphovascular space invasion (arrow) showing presence of tumor inside a blood vessel (c-f, hematoxylin eosin stain; c, d, f, × 20; e, × 100). |
Case 2
A 20-year-old nulligravid consulted for heavy menstrual bleeding associated with hypogastric pain, pallor and weakness. A 3 × 3 cm prolapsing fleshy mass was palpated at the cervical os. Endometrial biopsy revealed malignancy; hence, the patient underwent extra fascial hysterectomy with bilateral salpingo-oophorectomy. Intraoperatively, there was a 4 × 3 × 3 cm necrotic, friable, pedunculated endometrial mass attached to the lower uterine segment and upper cervical lip (Fig. 2a). The final histopathologic diagnosis was low-grade ESS (LG-ESS) (Fig. 2b-f). The patient was started initially on high-dose progesterone therapy with poor compliance. A month after surgery, there was a solid abdominal mass suggestive of recurrence. Pelvic external beam radiation therapy was done but patient was eventually lost to follow-up.
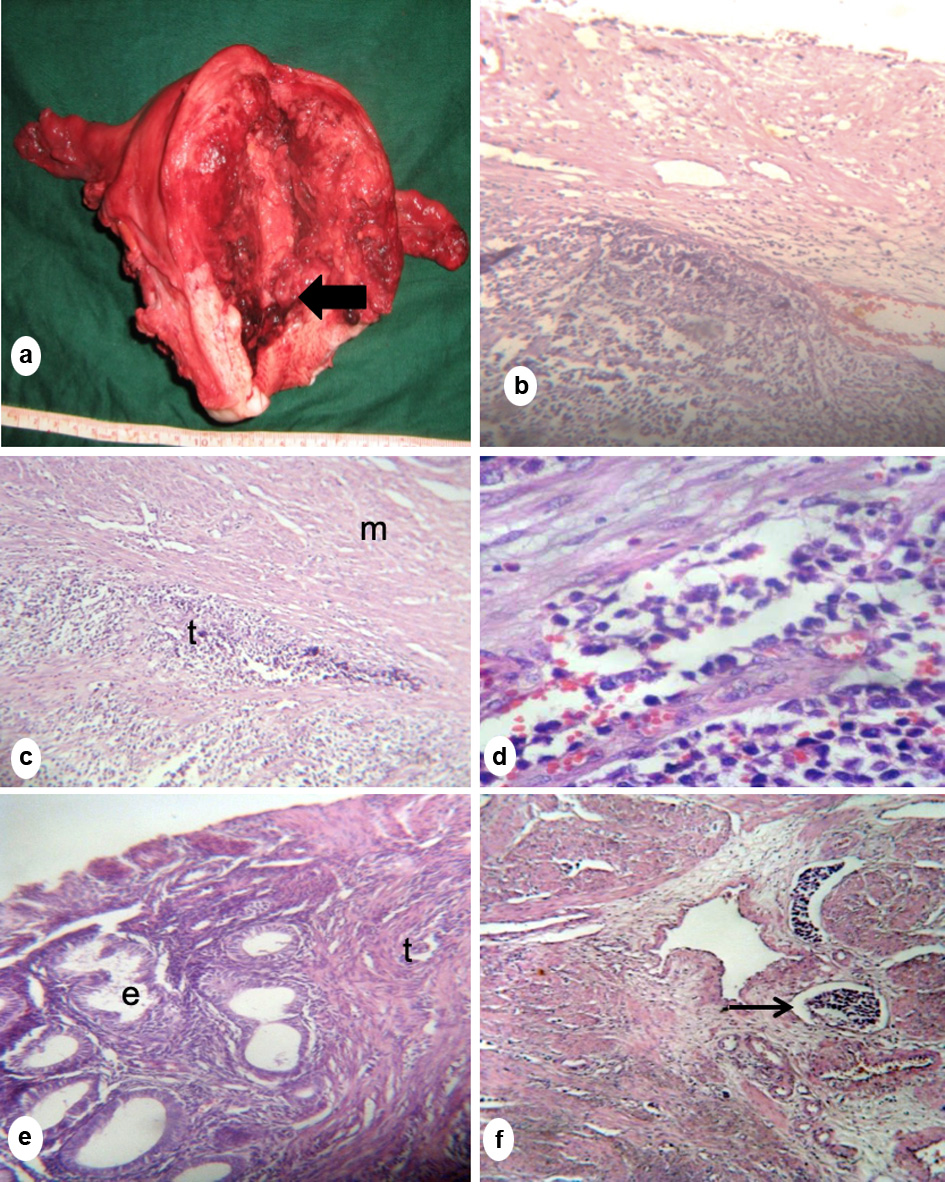 Click for large image | Figure 2. (a) Gross section of the uterus showed a necrotic friable pedunculated mass attached to the lower uterine segment (arrow). (b) Microscopic section showing tumor infiltration extending into the posterior cervix. (c, d) Microscopic section of the uterine mass showing sheets of tumor (t) invading smooth muscle bundles of the myometrium (m), separating them sheath by sheath. (e) Endometrial glands (e) were scanty, located at the periphery of the mass and surrounded by neoplastic stromal cells (t). (f) Lymphovascular space invasion (arrow) (b-f, hematoxylin eosin stain; b, c, f, × 20; d, × 40). |
| Discussion | ▴Top |
ESS was first reported by Norris and Taylor in 1966, who stratified ESS into LG and HG types based on mitotic index. LG-ESS has less than 10 mitoses per 10 high-power field and is known to be an indolent tumor. There is no nuclear atypia or pleomorphism. Distant metastases are rare, and recurrences are reported to be approximately 5 to 25 years from diagnosis. Conversely, HG-ESS infiltrates the myometrium to a greater extent and is more aggressive than LG-ESS. Distant metastases occur frequently in this group of tumors and recurrence occurs only a few months from diagnosis [5, 7].
Since then, nomenclature and classification of ESS have evolved. Currently, the World Health Organization (WHO) classification divides these tumors into four different subsets namely, endometrial stromal nodule, LG-ESS, HG-ESS and uterine undifferentiated sarcoma (UUS). This new classification was thought to better reflect the unique clinicopathologic features, “undifferentiated” appearance and aggressive biological potential of the HG tumors [5].
ESS is unusual in young women, with 16 cases reported in women under 30 years old since the 1990s [8-23]. Majority were nulliparas with symptom duration ranging from several days to 1 year. Two cases were reported during pregnancy, both of which were terminated upon the diagnosis of ESS [14, 22]. Three cases conceived spontaneously after conservative surgery with adjuvant hormonal therapy [15, 16] and chemotherapy [12]. All three resulted in livebirths. Five cases presented with rapid progression and distant metastasis [10, 13, 18, 19, 23]. These cases illustrate the unpredictable, varied and drastic nature of ESS in this age group (Supplementary Material 1, www.jcgo.org).
Little is known regarding risk factors, optimal therapy and outcomes because of the non-specific characteristics and rarity of ESS. Pathogenesis remains unknown, although specific cytogenetic aberrations and molecular changes have been recently elucidated. Almost all ESS are characterized by an overexpression of estrogen and progesterone receptors [6]. Among the index cases, common factors were their age, race and nulliparity (Table 1).
 Click to view | Table 1. Clinicopathological Profile of the Two Cases of ESS in the Young |
ESS presents with abnormal uterine bleeding as in the index patients. Other common symptoms include uterine enlargement and pelvic pain. Physical findings may vary, such as palpation of an abdominopelvic mass, an enlarged corpus or appreciation of a fleshy prolapsing mass on internal examination.
Since ESS is not typical in the young, diagnosing this disease can be problematic. Only 37.2% were diagnosed pre-operatively [24]. Benign etiologies predominate in the younger age group, hence ESS is commonly mistaken for a rapidly enlarging myoma [9, 11, 12, 15-18, 23] or a polyp [22]. Unfortunately, there are no existing guidelines for pre-operative diagnostics [24]. It is therefore imperative to elicit a thorough history and perform a complete physical and pelvic examination. These coupled with imaging studies, such as transvaginal ultrasound, CT scan or MRI, should be helpful in diagnosing ESS.
Definitive diagnosis of ESS is still made by histopathologic examination of the specimen following hysterectomy. Macroscopically, ESS presents as a yellow, fleshy, polypus tumor, sometimes as a single nodule which may grow into the cervix [7, 11]. It may also present as a poorly demarcated lesion with occasional cystic degeneration (case 2). HG-ESS can present as multiple, soft tan masses that bulge into and often fill the entire endometrial cavity [25] (case 1). 75% have early infiltration of the myometrium [7].
Microscopically, features of ESS recapitulate the gross appearance with cords of tumor cells infiltrating and separating smooth muscle sheaths [26]. Lymphovascular space invasion is pathognomonic. The neoplastic stromal cells resemble either those of proliferative endometrium or hyperplastic endometrial stromal cells, but with scanty cytoplasm and indistinct cell borders. Sparse endometrial glands are usually noted. Proliferation of small vessels resembling endometrial spiral arterioles is also characteristic finding [25, 26]. These typical features were evident in the histologic examination of case 2 and the diagnosis of LG-ESS was made based on morphology alone. But what happens when these histological features become subdued or distorted as in HG, undifferentiated ESS?
HG-ESS can be differentiated from LG-ESS by the presence of hemorrhage and necrosis. The neoplastic cells are spindle to polygonal shaped with marked nuclear pleomorphism and nuclear atypia. HG-ESS has larger, more vesicular nuclei in which chromatin clumps are coarser and more prominent [25]. They bear little resemblance to proliferative phase endometrium, justifying the new term “undifferentiated”. Myometrial infiltration is more extensive, and the vascular pattern of the low-grade tumor is typically absent.
The provisional readings for case 1 were initially reported as lymphoma due to histological similarities. Histologically, lymphoma presents as a monotonous, round cell neoplasm with marked pleomorphism and prominent nucleoli [25], similar to HG-ESS which presents with round, anaplastic, undifferentiated cells. Although in lymphoma, cytoplasm is abundant whereas in ESS, cytoplasm is scanty.
Immunohistochemical staining plays a vital role in differentiating ESS from such histological mimics. Cluster of differentiation 10 (CD10) is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stromal neoplasms. CD10 can distinguish these tumors from histological mimics such as leiomyoma, leiomyosarcoma and adult granulosa cell tumor which generally stain negative [27]. Cytokeratin, actin and myosin may also be used to further differentiate ESS from the latter tumors. Conversely, CD10 is also expressed in hematopoietic neoplasms such as lymphoma. To distinguish ESS from lymphoma, leukocyte common antigen (LCA) CD45, CD3 or CD20 is commonly used. LCA stains lymphocytes in general. CD20 and CD3 specifically identify B cells and T cells in normal and neoplastic tissues, respectively. Negative results for the latter immunostainings coupled with a positive result for CD10 strengthen the diagnosis of ESS. A positive vimentin stain, which connotes mesenchymal tumors, would have supported the diagnosis as well [27].
Hysterectomy remains the cornerstone of treatment in ESS although fertility-sparing surgery has been reported in cases of LG-ESS and results have been promising [6]. In our index patients, the question of whether there is a need to perform bilateral oophorectomy is relevant, considering the detrimental effects of surgical menopause in the young. However, the effects of estrogen in the persistence and early recurrence of ESS presented by several studies favor the removal of the ovaries regardless of age [28].
Lymphatic invasion is pathognomonic for ESS, formerly designated as endolymphatic stromal myosis [2]. However, the role of lymphadenectomy has not been fully established. Recent data suggested the incidence of lymph node metastasis to be higher than suspected and in some cases nodal involvement was the only evidence of extrauterine disease [28], suggesting the need for more extensive lymph node sampling. Lymph node dissection clearly provides prognostic information and treatment guidance; however, its potential therapeutic value remains to be determined.
Several studies on adjuvant hormonal therapy have been conducted with evidence of regression or periods of stable disease in cases of ESS. Progestin therapy may have a positive effect on the disease, causing the inhibition of endometrial epithelium proliferation. Anti-estrogenic agents such as megestrol, aromatase inhibitors and gonadotropin-releasing hormone (GnRH) agonist have also been used [6, 12, 14, 16-18, 29].
Since ESS has the propensity for early hematogenous spread, the use of systemic therapy may prove to be appealing. Available evidence showed use of the single agent therapy with doxurubicin, ifosfamide, trabectedin and gemcitabine [5, 8, 10, 29]. Combination therapy with doxorubicin plus ifosfamide can be used for rapid palliation, stopping rapidly progressing disease or for neoadjuvant chemotherapy [5].
Adjuvant radiation therapy was reported to be an effective treatment for patients with ESS due to excellent local control in all stages and good disease-specific survival in early stages as seen in case 2. Adjuvant radiation therapy clearly reduces the incidence of pelvic recurrence; however, in the majority of the studies, it has no effect on overall survival.
LG-ESS has an estimated overall survival ranging 69-84% at 5 years. In contrast, HG-ESS has a worse prognosis despite treatment at an early stage, with a 5-year survival rate of 55%. Patients usually succumb to the disease within 3 years of initial diagnosis [7], as in case 1. Among potential prognostic factors, surgical pathologic stage seems to be the most important. Age, parity, race, menopausal status, degree of nuclear atypia, mitotic index and tumor size were potential clinicopathologic risk factors in ESS [12]. However, the impact of these other prognostic factors on survival remains unclear or controversial, and still needs to be validated by larger studies. Nonetheless, it is important to note that LG-ESS should be identified from HG-ESS as the prognosis of the latter is dismal in contrast to the relatively indolent nature of typical LG-ESS.
Conclusions
Endometrial stromal sarcoma is a rare tumor, more so in the young. It remains a diagnostic and therapeutic challenge to gynecologists worldwide. Although benign etiologies predominate in the young, the diagnosis of a malignancy should not be missed; especially an aggressive disease entity such as ESS.
| Supplementary Material | ▴Top |
Suppl 1. Summary of Previously Published Cases of ESS in Women Under 30 Years of Age.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained from the patients.
Author Contributions
All authors certify that they have participated sufficiently in the intellectual content of this manuscript. Each author has reviewed the final version of the manuscript and has approved it for publication.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Livi L, Paiar F, Shah N, Blake P, Villanucci A, Amunni G, Barca R, et al. Uterine sarcoma: twenty-seven years of experience. Int J Radiat Oncol Biol Phys. 2003;57(5):1366-1373.
doi - Ashraf-Ganjoei T, Behtash N, Shariat M, Mosavi A. Low grade endometrial stromal sarcoma of uterine corpus, a clinico-pathological and survey study in 14 cases. World J Surg Oncol. 2006;4:50.
doi pubmed - Berceanu S, Patrascu A, Berceanu C, Tica AA, Badulescu A. Endometrial stromal sarcoma: clinico-pathological report of four cases and review of the literature. Rom J Morphol Embryol. 2008;49(2):251-255.
- Ali RH, Rouzbahman M. Endometrial stromal tumours revisited: an update based on the 2014 WHO classification. J Clin Pathol. 2015;68(5):325-332.
doi pubmed - Horng HC, Wen KC, Wang PH, Chen YJ, Yen MS, Ng HT, Taiwan Association of Gynecology Systematic Review G. Uterine sarcoma Part II-Uterine endometrial stromal sarcoma: The TAG systematic review. Taiwan J Obstet Gynecol. 2016;55(4):472-479.
doi pubmed - Noventa M, Gizzo S, Conte L, Dalla Toffola A, Litta P, Saccardi C. Fertility sparing surgery in young women affected by endometrial stromal sarcoma: an oncologic dilemma or a reliable option? review of literature starting from a peculiar case. Onco Targets Ther. 2015;8:29-35.
doi pubmed - DiSaia PJ, Creaseman W. Clinical Gynecologic Oncology, 8th edition. Philadelphia: Mosby; 2012.
- Bellone F, Nicolo G, Remorgida V. A case of sarcoma originating from the endometrial stroma in a 16-year-old girl. Adolesc Pediatr Gynecol. 1990;3:212-216.
doi - Mustaphi R, Sawhney H, Dey P. Endometrial stromal sarcoma with retroperitoneal metastasis in a young patient. Int J Gynaecol Obstet. 1994;47(3):303-304.
doi - Zalameda C. Endometrial stromal sarcoma in the young. Philippine Journal of Obstetrics and Gynecology. 2008;32(2):97-103.
- Guzin K, Yigit A, Afsar S, Ozerden E, Doganyilmaz S, Gucluer B. A young patient with low grade endometrial stromal sarcoma: a case report. Turkiye Klinikeri J Gynecol Obst. 2010;20(6):407-410.
- Yan L, Tian Y, Fu Y, Zhao X. Successful pregnancy after fertility-preserving surgery for endometrial stromal sarcoma. Fertil Steril. 2010;93(1):269 e261-263.
doi pubmed - Amant F, Van Calsteren K, Debiec-Rychter M, Heyns L, De Beeck KO, Sagaert X, Bollen B, et al. High-grade endometrial stromal sarcoma presenting in a 28-year-old woman during pregnancy: a case report. J Med Case Rep. 2010;4:243.
doi pubmed - Delaney AA, Gubbels AL, Remmenga S, Tomich P, Molpus K. Successful pregnancy after fertility-sparing local resection and uterine reconstruction for low-grade endometrial stromal sarcoma. Obstet Gynecol. 2012;120(2 Pt 2):486-489.
doi pubmed - Jassal CD, Patnaik BL, Divya A, Prasad S. Low-grade endometrial stromal sarcoma in young age: a clinicopathological report. J Obstet Gynaecol India. 2012;62(1):73-75.
doi pubmed - Dong R, Pang Y, Mao H, Yang N, Liu P. Successful pregnancy following conservative management of low-grade endometrial stromal sarcoma: A case report. Oncol Lett. 2014;7(4):1039-1042.
doi pubmed - Dong R, Mao H, Zhang P. Conservative management of endometrial stromal sarcoma at stage III: A case report. Oncol Lett. 2014;8(3):1234-1236.
doi pubmed - Chhabra S, Bhutani N, Singh S, Sangwan M, Sen R. Pulmonary metastases of uterine endometrial stromal sarcoma in a young patient: An extreme rarity. Human Pathology: Case Reports. 2017;10:98-101.
doi - Eamudomkarn N, Itarat Y, Kleebkaow P, Kietpeerakool C. A case report of high-grade endometrial stromal sarcoma: a rare cause of abnormal uterine bleeding in a young woman. Case Rep Obstet Gynecol. 2018;2018:5906760.
doi pubmed - Calin FD, Gheorghiu D, Ionescu CA, Neacsu A, Navolan DB, Dimitriu MCT, Ionescu A, et al. Endometrial stromal sarcoma in a 27-year-old woman. Case report and literature review. Rom J Morphol Embryol. 2018;59(3):933-938.
- Chang Y, Li J, Fan Y, Lan Y, Shi H, Zhou J. Ovarian function preservation of low grade endometrial stromal sarcoma at stage II: A case report and review of literature. Res Rep Gynaecol Obstet. 2018;2(1):5-8.
- Wong JWH, Fox KR, Casamina C, Lai TS, Killeen JL, Carney ME. A case of high-grade endometrial stromal sarcoma with concurrent pregnancy. Journal of Gynecologic Surgery. 2018;34(2):105-108.
doi - Sohail R, Kanwal S, Murtaza A, Haq B. Endometrial stromal sarcoma in a 20-year-old woman. BMJ Case Rep. 2019;12(12).
doi pubmed - Jin Y, Pan L, Wang X, Dai Z, Huang H, Guo L, Shen K, et al. Clinical characteristics of endometrial stromal sarcoma from an academic medical hospital in China. Int J Gynecol Cancer. 2010;20(9):1535-1539.
- Kurman RJ, Ellenson LH, Ronnett BM. Blaustein's pathology of the female genital tract, 6th edition. Boston, MA: Springer. 2011.
doi - Lee CH, Nucci MR. Endometrial stromal sarcoma - the new genetic paradigm. Histopathology. 2015;67(1):1-19.
doi pubmed - Chu PG, Arber DA, Weiss LM, Chang KL. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001;14(5):465-471.
doi pubmed - Thomas MB, Keeney GL, Podratz KC, Dowdy SC. Endometrial stromal sarcoma: treatment and patterns of recurrence. Int J Gynecol Cancer. 2009;19(2):253-256.
doi pubmed - Nakamura K, Nakayama K, Ishikawa M, Ishikawa N, Katagiri H, Katagiri A, Ishibashi T, et al. Letrozole as second-line hormonal treatment for recurrent low-grade endometrial stromal sarcoma: A case report and review of the literature. Oncol Lett. 2016;12(5):3856-3860.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
