| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 9, Number 3, September 2020, pages 43-52
Implementation of Enhanced Recovery in Gynecologic Surgery to Improve Outcomes at an Urban Safety-Net Hospital
Mary Louise Fowlera, e, Lizette Mendeza, Shawn Whiteheadb, Bhavesh Shahb, Kristen E. Rizzac, Melissa Schaperoa, Elise P. Memmoa, Paul M. Hendessid, Ronald E. Iversond, Mallika Anandd
aDepartment of Obstetrics and Gynecology, Boston Medical Center, Boston, MA, USA
bDepartment of Pharmacy, Boston Medical Center, Boston, MA, USA
cBoston University School of Public Health, Boston, MA, USA
dDepartment of Obstetrics and Gynecology, Boston University School of Medicine, Boston, MA, USA
eCorresponding Author: Mary Louise Fowler, Department of Obstetrics and Gynecology, Boston Medical Center, 85 East Concord Street, Sixth floor, Boston, MA 02118, USA
Manuscript submitted June 15, 2020, accepted August 13, 2020, published online September 9, 2020
Short title: Enhanced Recovery in GYN to Improve Outcomes
doi: https://doi.org/10.14740/jcgo666
| Abstract | ▴Top |
Background: This study aims to implement an enhanced recovery pathway (ERP) for patients undergoing gynecologic surgery and to track clinical outcomes, including perioperative opioid use and adverse events.
Methods: Patients undergoing gynecologic surgery with a planned overnight stay were eligible. The primary outcome measure was perioperative opioid use in oral morphine milligram equivalents. Secondary outcome measures included bundle completion and length of stay. Balancing measures included rates of total and specific adverse events. Data were stratified by route of surgery and univariate analyses were performed between pre- and post-ERP groups to compare demographic factors and outcome measures. Linear regression analyses were run to assess mean differences in perioperative opioid use and length of stay when adjusting for route of surgery, age, body mass index (BMI), American Society of Anesthesiologists (ASA) status, surgical subspecialty, and postoperative hemoglobin change, and/or bundle completion score.
Results: The ERP was implemented in 16 weeks and selected in 63 eligible patients from February 1 to April 30, 2017. ERP bundle completion was significantly higher for all surgical categories following formal pathway implementation. Compared to the pre-ERP cohort, the ERP cohort demonstrated significantly decreased total opioid use in laparotomies (175.5 mg vs. 209.8 mg, P = 0.03) and minimally invasive surgeries (125 mg vs. 170.3 mg, P = 0.018). Additionally, significantly decreased intraoperative opioids were used in both laparotomies (95 mg vs. 105 mg, P = 0.03) and minimally invasive surgeries (75 mg vs. 108.5 mg, P < 0.0001), as well as significantly decreased postoperative opioid use in minimally invasive surgeries (15 mg vs. 45 mg, P = 0.04). A one-point increase in ERP bundle completion score was associated with a 9.2 mg decrease in total opioid used (P = 0.0375) as well as a 4.8 h decrease in length of stay (P < 0.0001) when adjusting for route of surgery, age, BMI, ASA status, surgical subspecialty, and case length. There were no significant differences in adverse events when ERP was used.
Conclusions: ERP implementation was rapidly accomplished at our urban, safety-net hospital. The pathway reduced perioperative opioid use without increasing adverse events. Continued monitoring of enhanced recovery quality improvement measures, including bundle completion, is essential to ensure adherence, safety, and effectiveness.
Keywords: Enhanced recovery pathway; Gynecologic surgery; Opioid reduction
| Introduction | ▴Top |
The ultimate goal of surgery is to improve the quality of life for our patients. In the current era of healthcare, one aim is to perform surgery in a way that minimizes postoperative complications and allows for faster return to preoperative function. Optimization of surgical outcomes, until recently, has largely focused on interventions within the operation itself, rather than the entire perioperative course. Advances in minimally invasive gynecologic surgery have led to decreased length of hospital stay and faster recovery times [1-3]. However, during the last two decades, interventions to improve surgical recovery have expanded to include all phases of perioperative care, termed “enhanced recovery” [4]. Enhanced recovery pathways (ERPs) are examples of evidence-based bundles of perioperative interventions designed to reduce the physiologic stress response to surgery and are aimed at improving patient satisfaction, reducing cost, and decreasing recovery time [4-6].
Clinically, ERP interventions consist of specific action items that occur within the different perioperative phases of care [7, 8]. Preoperative interventions include the selection of eligible patients for ERP, patient education, consumption of clear liquids (including an electrolyte-rich drink) until up to 2 h prior to surgery, preoperative preemptive pain medication with oral gabapentin and acetaminophen, and a warming blanket beginning 30 min prior to surgery. Intraoperative interventions include using the least invasive surgical route where feasible, short on/off anesthetics, goal-directed fluid therapy aimed at euvolemia, preemptive multimodal pain control, nausea and vomiting prophylaxis, and minimizing the use of drains and tubes. Finally, postoperative interventions include early discontinuation of intravenous (IV) fluids, early return to a regular diet, use of a bowel regimen, continued use of multimodal pain control regimen, return to oral pain medications as soon as feasible, early ambulation, and removing the Foley catheter and any other drains/tubes as early as possible [7, 8]. The end goal is to allow the patient to return to preoperative functioning as quickly as possible, as a longer length of hospital stay has been correlated with lower quality of life [9]. Pre-ERP interventions that adversely affect postoperative recovery and subsequent return to preoperative functional mobility include delayed reintroduction of oral feeding, prolonged immobilization and bed rest, intraoperative hypervolemia, excessive opioid use, and the use of drains and catheters [10].
Although ERPs are increasingly being adopted and implemented, resistance to widespread adoption may be encountered, in part due to logistical systemic barriers such as limited expertise, cost, and stakeholder reluctance to stray from pre-ERP principles of care [11, 12]. From an implementation standpoint, critical steps include achieving consensus on well-defined clinical pathway, establishing a highly-committed multidisciplinary team with a clear timeline, setting patient expectations to align with the clinical pathway, and following the outcomes on an ongoing basis for continued quality improvement [12].
In terms of clinical outcomes, other institutions have reported improved perioperative outcomes with ERP in gynecologic surgery, including decreased pain, length and cost of hospital stay, and improved quality of life [10, 13]. An additional benefit is that ERPs have demonstrated a reduction of perioperative opioid use [14], an increasingly important priority in healthcare given both the addiction potential of opioids as well as unwanted side effects such as sedation, nausea and vomiting, urinary retention, ileus and respiratory depression, which can lead to delay in hospital discharge [15]. Enhanced recovery is now considered standard of care for postoperative recovery in Great Britain, where the National Health Service has endorsed it as a quality improvement tool [16]. Nonetheless, there is limited knowledge of implementation and outcomes of ERP in gynecologic surgery at urban, safety-net hospitals in the USA. Our institution, the largest urban, safety-net hospital in Massachusetts, has previously implemented ERPs in other surgical specialties, including general surgery, colorectal surgery, bariatric surgery, and surgical oncology. We sought to implement an ERP efficiently and track outcomes for patients undergoing gynecologic surgery with a planned overnight stay compared to a pre-ERP cohort. Our primary outcome was perioperative opioid use and our secondary outcomes were length of stay, adverse events, and bundle completion.
| Materials and Methods | ▴Top |
This quality improvement (QI) project was IRB-exempt. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Implementation of the ERP process occurred over an 18-week period and began with the establishment of a departmental QI project team collaborating with an institutional steering committee. Figure 1 demonstrates the original implementation process for ERP developed by the primary investigator (MA) [17]. The timeline was adjusted to reflect a 2-week gap of inactivity during a holiday period. The ERP project team leaders within the Division of Gynecology met with the institutional ERP Steering Committee and multidisciplinary stakeholders to ensure institution-wide consistency in logistics and measurement goals. Literature and guidelines on ERP were reviewed and intradepartmental consensus regarding the ERP target population and ERP interventions was established. Preoperative and postoperative electronic order sets were developed with the assistance of the Information Technology Department. Educational sessions discussing the rationale and execution of ERP were held with stakeholders. Progress and outcomes were communicated at institutional ERP stakeholder meetings as well as at departmental and division meetings. A plan to track outcomes and provide feedback to stakeholders was developed.
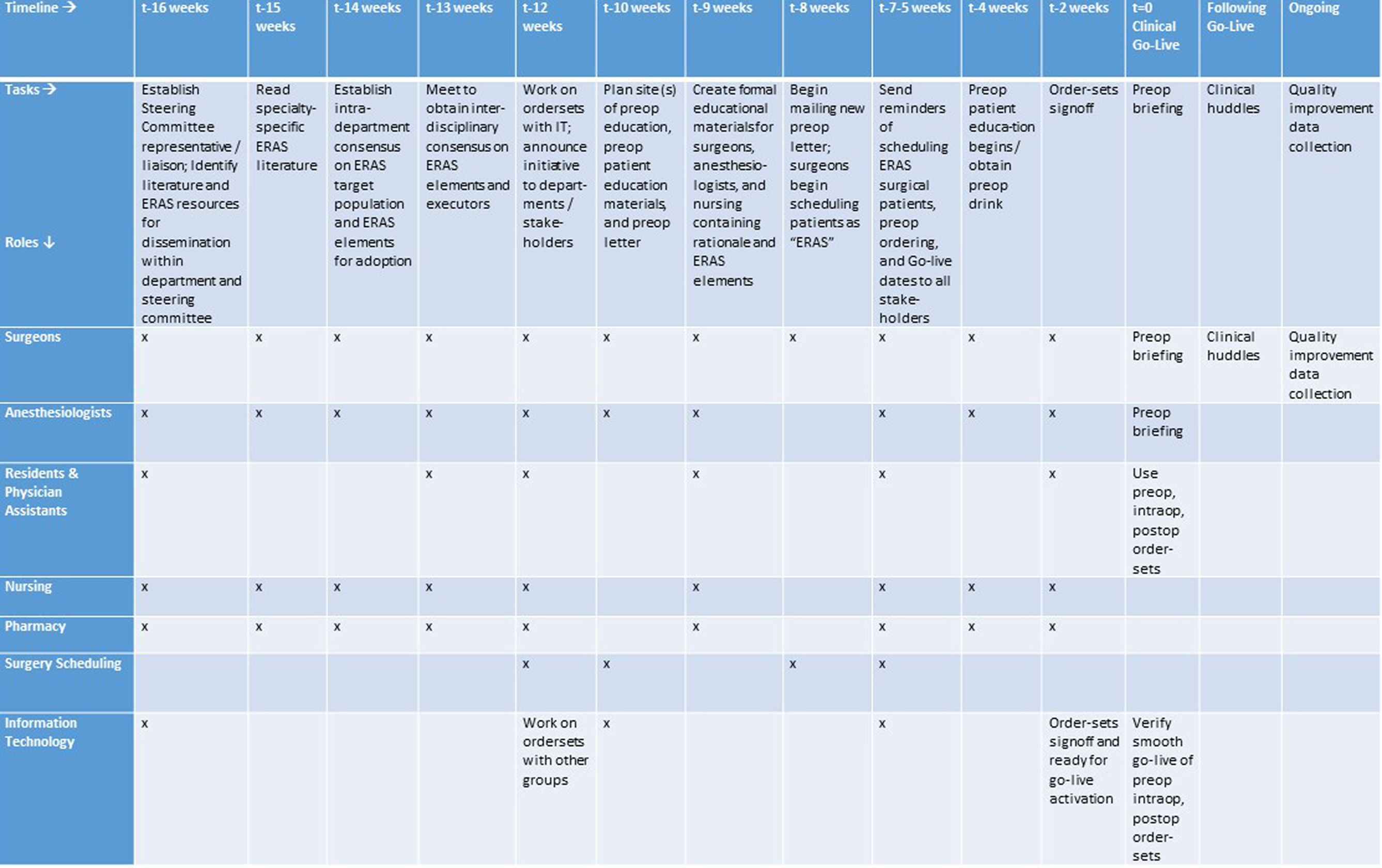 Click for large image | Figure 1. Implementation timeline for an enhanced recovery pathway in gynecologic surgery. In this implementation timeline, tasks are listed on the horizontal axis in chronologic order, and roles are listed on the vertical axis. The roles are fulfilled by members of the ERP steering committee and stakeholders to whom tasks apply. An ERP steering committee would typically meet every 1 - 4 weeks as full group or subgroup, and consists of the hospital-wide project manager, department project manager, and a representative for each division listed in the task role. A modification of this figure has previously been published. ERP: enhanced recovery pathway. |
The inclusion criteria for the ERP consisted of all patients undergoing gynecologic surgery (benign or oncologic) beginning on February 1, 2017, with a planned overnight admission (either as a bedded outpatient or inpatient). The day of surgery was defined as postoperative day 0. QI metrics were monitored on a monthly basis from the pathway implementation date of February 1, 2017 to April 30, 2017. These metrics were compared with a pre-implementation timeframe of three consecutive months: June 1, 2015 through August 31, 2015. This pre-implementation timeframe was selected to precede the earliest implementation of an ERP at our institution, as implementation of ERP at our institution in other surgical specialties began in October 2015. No patients were excluded from either cohort. Baseline, outcome, balancing, and process measures were both manually and automatically abstracted from the electronic medical records. De-identified data were stored securely in Research Electronic Data Capture (REDCap), an institutionally available web-based data repository [18].
Baseline clinical measures included patient characteristics of age, body mass index (BMI) and past medical history of type 2 diabetes mellitus. We calculated a bundle completion score, which assigned one point for each of the following: completion of preoperative patient education, preoperative consumption of clear liquids, including an electrolyte-rich drink, until 2 h preoperatively, administration of preoperative preemptive analgesics, decreasing intraoperative IV fluid administration rate/volume to ≤ 3 mL/kg/h (as determined by our Anesthesia Department for gynecologic surgery), administering intraoperative nausea and vomiting prophylaxis for patients at high risk of nausea/vomiting, performance of a transversus abdominis plane block for open procedures, intraoperative administration of bupivacaine at the surgical site by the surgeon, administering multimodal non-opioid analgesia perioperatively, discontinuation of IV fluids by postoperative day 1, initiation of a regular diet on postoperative day 0, ambulation beginning on postoperative 0, and removal of Foley catheter or any other drains as early as feasible. Bundle completion scores were calculated differently based on route of surgery and phase of care. For route of surgery, the bundle completion scores for laparoscopic surgical cases did not include performance of a transversus abdominis plane block. For vaginal surgical cases, the bundle completion score did not incorporate performance of a transversus abdominis plane block or Foley removal.
The primary outcome measure was perioperative opioid use, including total opioid use during the hospital stay, opioid use in the operating room, post-anesthesia care unit (PACU), and postoperative inpatient unit, measured in milligrams of oral morphine equivalents. Secondary outcome measures were: length of hospital stay, adverse events, and bundle completion score. Specifically, adverse events included total and specific adverse event rates, including 30-day readmission, emergency department visits, culture-proven urinary tract infection (UTI), ileus, pre-renal renal failure, hyponatremia, adverse reaction(s) to any ERP medication or intervention, and Clavien-Dindo complication grades [19].
Differences between groups for continuous variables were analyzed using the two-sample Student’s t-test and Wilcoxon’s rank sum test. Differences between groups in categorical variables were analyzed with the Chi-square test and Fisher’s exact test. Multivariate linear regression analyses were used to model mean differences in total opioid use, opioid use in the operating room, opioid use in the PACU, opioid use postoperatively, and length of stay. Linear regression models were adjusted for route of surgery, age, BMI, American Society of Anesthesiologists (ASA) status, surgical subspecialty, case length, bundle completion score, and postoperative hemoglobin change. Similarly, after stratifying by route of surgery, multivariate regression analyses were used to assess mean differences in opioid use and length of stay. For the stratified regression models, backward elimination was performed to identify potential predictors for opioid use and length of stay. The following variables were included in the backward elimination models: age, BMI, ASA status, surgical subspecialty, case length, and postoperative hemoglobin change. A P value of < 0.05 was considered statistically significant for all statistical comparisons. Analysis was conducted in SAS (SAS Institute Inc. 2013. Release: 9.4. Cary, NC, USA).
| Results | ▴Top |
The ERP development and implementation occurred over a 16-week period (18-week calendar period minus a 2-week inactive period) (Fig. 1). This resulted in successfully achieving the target date for clinical execution of the pathway beginning February 1, 2017.
Table 1 displays the baseline characteristics of patients, grouped by open versus minimally invasive transabdominal surgery (MIS) versus vaginal surgery and further subdivided into the ERP (post-implementation) and pre-ERP (pre-implementation) cohorts. ERP selection occurred for 63 eligible patients undergoing gynecologic surgery from February 1, 2017 to April 30, 2017 requiring a planned overnight stay. These patients were compared to a pre-ERP cohort of 96 patients who underwent surgery between June 1, 2015 and August 31, 2015.
 Click to view | Table 1. Baseline Characteristics of Patients Undergoing Gynecologic Surgery With a Planned Overnight Stay |
Table 2 depicts key outcomes in the intraoperative and PACU phases of care. A temporal shift was noted with respect to the planned early administration of nonopioids. Rather than waiting to administer nonopioids postoperatively, there was a non-significant increase in intraoperative nonopioid administration (open: 13 (68.4%) ERP vs. 21 (50.0%) pre-ERP, P = 0.18; MIS: 17 (68%) ERP vs. 20 (62.5%) pre-ERP, P = 0.67) as well as a significant increase in intraoperative nonopioid administration for vaginal cases (11 (57.9%) ERP vs. 6 (27.3%) pre-ERP, P = 0.047). This was accompanied by a statistically significant decrease in the need for PACU nonopioid administration among both open and MIS cases (open: 6 (31.6%) ERP vs. 31 (73.8%) pre-ERP, P = 0.0001; MIS: 7 (28%) ERP vs. 20 (62.5%) pre-ERP, P = 0.009) as well as a non-significant decrease in PACU nonopioid administration in vaginal cases (9 (47.4%) vs. 13 (59.1%), P = 0.45).
 Click to view | Table 2. Intraoperative and PACU Outcomes of Patients Undergoing Gynecologic Surgery With a Planned Overnight Stay |
Table 3 depicts key outcomes in the postoperative phase of care (inpatient unit following release from the PACU) and total hospital course. Significant decreases were seen in intraoperative opioid administration for both open (95 mg ERP vs. 105 mg pre-ERP, P = 0.03) and MIS cases (75 mg ERP vs. 108.5 mg pre-ERP, P < 0.001). There was also a significant decrease in postoperative opioids used in MIS cases (15 mg ERP vs. 45 mg pre-ERP, P = 0.04) and a nonsignificant decrease in open (52.5 mg ERP vs. 67.5 mg pre-ERP, P = 0.19) and vaginal (15 mg ERP vs. 35.8 mg pre-ERP, P = 0.20) cases. Finally, there was a significant decrease in the number of patients requiring IV opioid use after open surgery (8 (42.1%) ERP vs. 30 (71.4%) pre-ERP, P = 0.03). In looking at the combined opioid use for postoperative day 0 plus postoperative day 1, there was significantly decreased opioid use in open (134.5 mg vs. 185.5 mg, P = 0.016) and MIS (125 vs. 146.5, P = 0.02) cases.
 Click to view | Table 3. Postoperative Outcomes and Perioperative Opioid Use Among Patients Undergoing Gynecologic Surgery With a Planned Overnight Stay |
A multivariate linear regression was performed and found that when controlling for route of surgery, age, BMI, ASA status, surgical subspecialty, case length, and postoperative hemoglobin change, on average, ERP patients used 30.4 mg less opioids overall (P = 0.0135) and 19.4 mg less opioids intraoperatively (P = 0.0023) than pre-ERP patients. Additionally, a 1-min increase in case length was associated with a 0.24 mg increase in total opioids (P = 0.0006) and a 0.16 mg increase in intraoperative opioids (P < 0.0001). Interestingly, on average, a 1-year increase in age was associated with a 0.9 mg decrease in postoperative opioids when adjusting for the above listed variables (P = 0.0416). When stratified by route of surgery, for open surgery, the difference in intraoperative opioids used between ERP and pre-ERP patients was 30.7 mg (P = 0.0243) and the difference in total opioids used between ERP and pre-ERP patients was 48.7 mg (P = 0.0195). A 1-min increase in open case length was associated with a 0.19 mg increase in intraoperative opioids (P = 0.0092). For minimally invasive surgery, the difference in intraoperative opioids used between ERP and pre-ERP patients was 22.2 mg (P = 0.0159). For vaginal surgery, a 1-min increase in case length was associated with a 0.23 mg increase in intraoperative opioids (P = 0.0165). Figure 2 depicts the decline in opioid consumption by perioperative phases of care following the implementation of ERP.
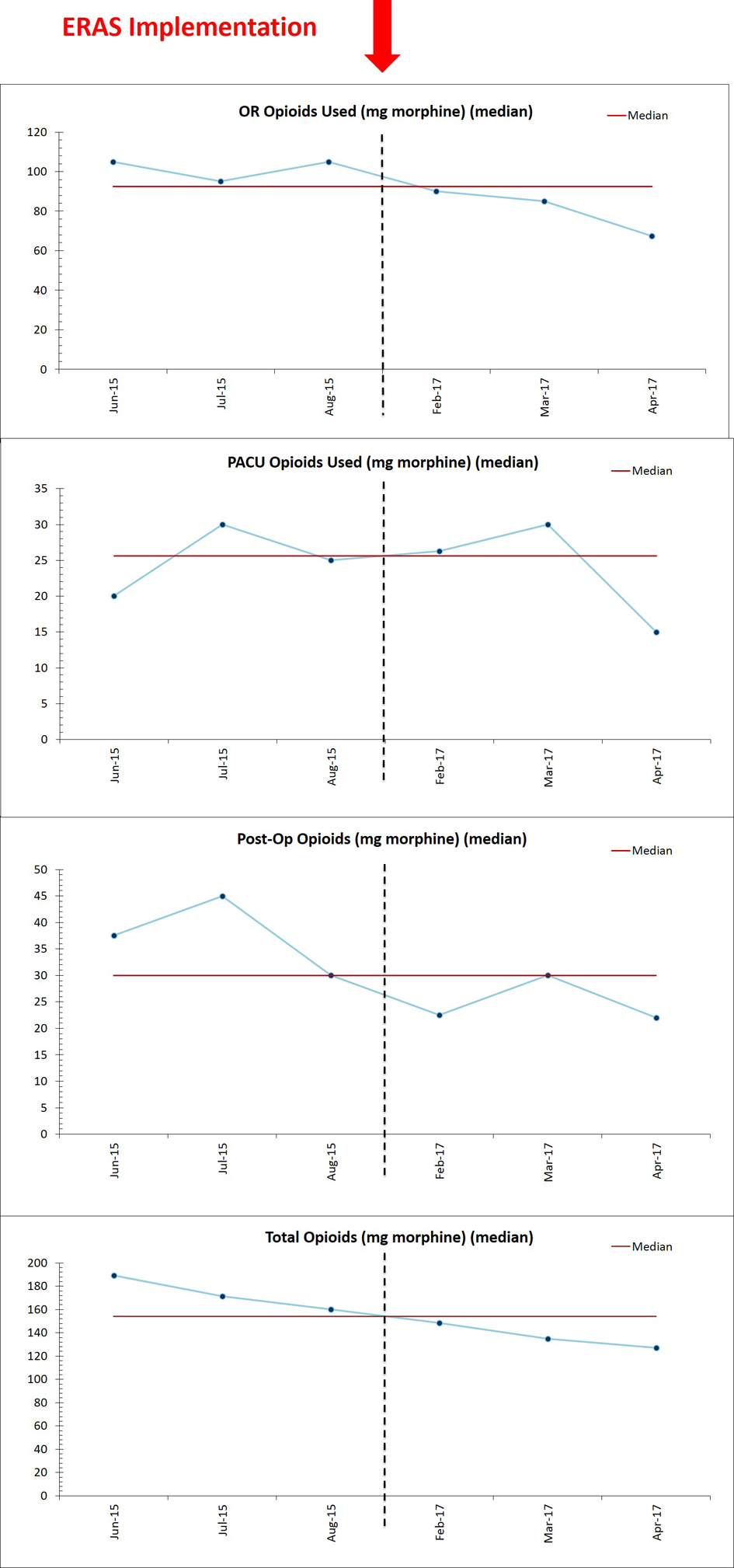 Click for large image | Figure 2. Opioid use by phase of care, before and after ERP implementation. Opioid use is depicted for each perioperative phase of care before and after ERP implementation. The red arrow at the top of the figure denotes the date of ERP implementation (February 1, 2017). ERP: enhanced recovery pathway. |
In terms of secondary outcomes, following implementation of the ERP, there was a significant decrease in length of stay in hours for vaginal cases (29 h ERP vs. 31.4 h pre-ERP, P = 0.04). A concomitant decrease in hospital length of stay was not seen in the other routes of surgery (open: 57 h ERP vs. 54 h pre-ERP, P =0.12; MIS: 31 h ERP vs. 29.1 h pre-ERP, P = 0.41). However, regression analyses revealed other clinical metrics associated with length of stay. A simple linear regression was performed with ASA, and, overall, a one-unit increase in ASA was associated with an 8.3 h increase in length of stay (P = 0.0445). Additionally, a 1-min increase in case length was associated with a 6-min increase in length of stay (P < 0.0001) overall. A 1-min increase in case length was associated with a 10.8 min increase in length of stay for open surgery (P < 0.0001) and a 3-min increase in length of stay for MIS (P = 0.0006). There was no significant difference in total adverse events, 30-day readmissions, emergency department visits, UTIs, ileus, prerenal renal failure or Clavien-Dindo grade 3+ complications between the ERP versus pre-ERP cohorts for open, MIS or vaginal routes of surgery (Table 3).
In terms of bundle completion, the overall bundle completion percent was 81.9% for ERP and 49.3% for pre-ERP patients, a 32.6% increase in bundle completion (P < 0.0001). Bundle completion for open cases was 67.9% for ERP versus 38.6% for pre-ERP (P < 0.0001); for MIS it was 83.4% for ERP versus 56.0% for pre-ERP (P < 0.0001), and for vaginal cases it was 73.7% for ERP vs 40.9% pre-ERP (P < 0.0001). Among ERP patients, 0% of open cases, 20.0% of MIS cases, and 0% of vaginal cases received all possible elements of the bundle (P = 0.02). Importantly, a one-point increase in ERP bundle completion score was associated with a 9.2 mg decrease in total opioids (P = 0.0375) as well as a 4.8 h decrease in length of stay (P < 0.0001) when adjusting for route of surgery, age, BMI, ASA status, surgical subspecialty, and case length.
| Discussion | ▴Top |
This study demonstrates that in the setting of a large, complex, and primarily government-funded hospital, rapid and successful implementation of ERP is possible. Early in its implementation, the pathway has demonstrated positive outcomes, including statistically significantly reduced intraoperative and total opioid use in both open and minimally invasive surgery on univariate analysis, without an increase in total adverse events. Furthermore, we found a significant decrease of IV opioid use postoperatively among ERP patients undergoing open surgery and postoperative opioids among ERP patients undergoing minimally invasive surgery. With the use of multimodal analgesia, we expected decreased total opioid use during the hospital stay [20]. Although there was a trend towards decreased opioid use in all phases of care, the differences were not statistically significant for every group and every phase of care. Nonetheless, we found that ERP bundle completion matters: our formal implementation of an ERP enabled significantly improved uptake of ERP best practices for all routes of surgery and, in turn, greater ERP bundle completion, even by one point, led to a significant decrease in opioid use. This is in line with other studies demonstrating the implementation of such a program was associated with significantly decreased opioid use [21, 22]. One study did demonstrate reduction of opioids after implementation of an ERP at a safety-net hospital; however, this study only looked at minimally invasive surgeries [23].
In terms of our secondary outcome, length of stay, for our patients, a significant reduction in length of stay was only seen in the vaginal surgery group. This differs from the findings of Kalogera et al, where the pathway was associated with reduced opioids as well as a significant reduction in length of stay among gynecologic oncologic and urogynecologic surgical patients [10]. We might have been underpowered to detect such a difference owing to our relatively short baseline length of stay for a planned overnight admission (30 h for minimally invasive surgery and 54 h for open surgery). In a randomized controlled trial of patients undergoing laparotomy in gynecologic oncology by Dickson et al, the study authors also did not find a significantly reduced length of stay after implementation of an Enhanced Recovery After Surgery (ERAS) program [24]. In addition to clinical factors such as ASA status and operative time, multiple systemic factors contribute to time of discharge, including acquisition of prescription medications, staffing shift changes, and availability of an early ride home. To that end, ongoing additional system-based QI efforts have been initiated to improve hospital length of stay and the discharge process at our hospital.
Strengths of this study include its collection of data from an urban, safety-net hospital representing a diverse patient population. Another strength of the study is stratification of patients who underwent open versus minimally-invasive surgeries, since route of surgery plays an important role in postoperative outcomes. Finally, the use of bundle completion scores enabled us to account for pre-ERP use of ERP elements and determine the impact of rapid ERP implementation.
Limitations of this study include its retrospectively assessed outcomes. Other institutions and other surgical specialties outside our department had begun implementing ERP previously. Therefore, in order to minimize potential confounding, we used a pre-ERP comparison cohort from 18 months prior to initiation of the ERP in the Division of Gynecology that preceded any implementation of ERP at our institution. Our use of a bundle completion score enabled us to account for any prior diffusion of discrete ERP elements. Another limitation was difficulty in detecting preoperative opioid dependence, as there was not a standardized method of recording this within patient’s electronic medical records. Finally, although we found no significant differences in adverse events or complications among the three groups or in reduction of length of stay for open and MIS routes of surgery, this study may have been underpowered to detect such differences.
This QI project demonstrated that ERP implementation was feasible and rapidly accomplished at our urban, safety-net hospital. Participating in multidisciplinary steering committee meetings, using a unique timeline, developing consensus-based electronic order sets, educating all stakeholders, and tracking bundle completion enabled us to achieve this goal with positive outcomes in our patients.
While initial success post-implementation of ERP has been demonstrated, continued long-term monitoring of ERP measures is essential to ensure further improvement in adherence to process measures, additional improvement in clinical outcomes and effectiveness, and continued safety. Specifically, we would like to increase patient selection for ERP, decrease IV fluid administration rate, continue to decrease opioid use, and continue to increase bundle completion. We are continuing to encourage universal surgical site injection with bupivacaine hydrochloride in an effort to continue to decrease opioid use. Another area for improvement is hospital length of stay, which is not solely impacted by the ERP. Therefore, we are exploring additional systems-based processes to improve this outcome. Furthermore, we are sharing our implementation timeline with other surgical divisions. Our timeline was purposefully written in a general way to demystify our own implementation process and could possibly be adapted and individualized for use at other institutions as well. Finally, additional future directions include determining post-discharge opioid use and studying the cost-effectiveness and patient satisfaction of ERP in gynecologic surgery.
Acknowledgments
The authors would like to thank Pamela Rosenkranz, R.N., B.S.N., M.Ed., Director of Clinical Quality and Patient Safety in the Department of Surgery at Boston University School of Medicine and Boston Medical Center, and Beth A. O’Donnell, M.P.H., former Quality Improvement Project Manager, Department of Surgery, for providing their institutional expertise in enhanced recovery pathway administration. The authors would like to acknowledge Gerardo Rodriguez, M.D., Director of the Surgical Intensive Care Unit at Boston Medical Center, for providing anesthesiology expertise in pathway development. The authors would like to thank Temitope Awosogba, MD, and Amma Agyemang, MD, PhD, former residents in Obstetrics and Gynecology, for their contribution to data collection. The authors would like to thank Stephanie D. Talutis, M.D., M.P.H., resident in the Department of Surgery at Boston Medical Center, for her assistance with database setup while she was a fellow in the Study of Quality and Patient Safety.
Financial Disclosure
None to declare.
Conflict of Interest
Dr. Paul M. Hendessi is a consultant for Medtronic Corp; Dr. Mallika Anand receives royalties from UpToDate, Inc; The remaining authors declare that there is no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
MLF contributed to data collection, data analysis, and manuscript writing/editing. LM, KR and MS contributed to data analysis. SW and BS contributed to data collection. EM, PH and RI contributed to project implementation and management. MA contributed with protocol/project development, implementation, data management, and manuscript writing/editing.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Miller TE, Thacker JK, White WD, Mantyh C, Migaly J, Jin J, Roche AM, et al. Reduced length of hospital stay in colorectal surgery after implementation of an enhanced recovery protocol. Anesth Analg. 2014;118(5):1052-1061.
doi pubmed - Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbolle P, Hendel HW, Rosenberg J, et al. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89(4):446-453.
doi pubmed - Lawrence JK, Keller DS, Samia H, Ermlich B, Brady KM, Nobel T, Stein SL, et al. Discharge within 24 to 72 hours of colorectal surgery is associated with low readmission rates when using Enhanced Recovery Pathways. J Am Coll Surg. 2013;216(3):390-394.
doi pubmed - Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78(5):606-617.
doi pubmed - Ren L, Zhu D, Wei Y, Pan X, Liang L, Xu J, Zhong Y, et al. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36(2):407-414.
doi pubmed - Horres CR, Adam MA, Sun Z, Thacker JK, Miller TJ, Grant SA, Huang J, et al. Proceedings of the American Society for enhanced recovery/evidence based peri-operative medicine 2016 annual congress of enhanced recovery and perioperative medicine. Perioper Med (Lond). 2016;5(Suppl 1):21.
doi - Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, et al. Guidelines for pre- and intraoperative care in gynecologic/oncology surgery: enhanced recovery after surgery (ERAS®) Society recommendations - Part I. Gynecol Oncol. 2016;140(2):313-322.
doi pubmed - Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations - Part II. Gynecol Oncol. 2016;140(2):323-332.
doi pubmed - Sharma A, Sharp DM, Walker LG, Monson JR. Predictors of early postoperative quality of life after elective resection for colorectal cancer. Ann Surg Oncol. 2007;14(12):3435-3442.
doi pubmed - Kalogera E, Bakkum-Gamez JN, Jankowski CJ, Trabuco E, Lovely JK, Dhanorker S, Grubbs PL, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 Pt 1):319-328.
doi pubmed - Bona S, Molteni M, Rosati R, Elmore U, Bagnoli P, Monzani R, Caravaca M, et al. Introducing an enhanced recovery after surgery program in colorectal surgery: a single center experience. World J Gastroenterol. 2014;20(46):17578-17587.
doi pubmed - Barber EL, Van Le L. Enhanced recovery pathways in gynecology and gynecologic oncology. Obstet Gynecol Surv. 2015;70(12):780-792.
doi pubmed - Wodlin NB, Nilsson L, Kjolhede P. Health-related quality of life and postoperative recovery in fast-track hysterectomy. Acta Obstet Gynecol Scand. 2011;90(4):362-368.
doi pubmed - Wick EC, Grant MC, Wu CL. Postoperative Multimodal Analgesia Pain Management With Nonopioid Analgesics and Techniques: A Review. JAMA Surg. 2017;152(7):691-697.
doi pubmed - Tan M, Law LS, Gan TJ. Optimizing pain management to facilitate enhanced recovery after surgery pathways. Can J Anaesth. 2015;62(2):203-218.
doi pubmed - Bell A, Relph S, Sivashanmugarajan V, Yoong W. Enhanced recovery programmes: do these have a role in gynaecology? J Obstet Gynaecol. 2013;33(6):539-541.
doi pubmed - Anand M, Trabuco EC. Enhanced recovery after gynecologic surgery: components and implementation. Eckler K, Joshi GP, Falcone T, eds. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on November 1, 2017).
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) - a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
doi pubmed - Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213.
doi pubmed - Maund E, McDaid C, Rice S, Wright K, Jenkins B, Woolacott N. Paracetamol and selective and non-selective non-steroidal anti-inflammatory drugs for the reduction in morphine-related side-effects after major surgery: a systematic review. Br J Anaesth. 2011;106(3):292-297.
doi pubmed - Meyer LA, Lasala J, Iniesta MD, Nick AM, Munsell MF, Shi Q, Wang XS, et al. Effect of an enhanced recovery after surgery program on opioid use and patient-reported outcomes. Obstet Gynecol. 2018;132(2):281-290.
doi pubmed - Schwartz AR, Lim S, Broadwater G, Cobb L, Valea F, Marosky Thacker J, Habib A, et al. Reduction in opioid use and postoperative pain scores after elective laparotomy with implementation of enhanced recovery after surgery protocol on a gynecologic oncology service. Int J Gynecol Cancer. 2019;29(5):935-943.
doi pubmed - Jalloul RJ, Simpson I, Lin AS, Cotton S, Elshatanoufy S. Effect of enhanced recovery after surgery (ERAS) Implementation on surgical outcomes and opioid prescription patterns in patients undergoing minimally invasive hysterectomy: a safety-net teaching hospital experience. JMIG. 2019;26(7):S119.
doi - Dickson EL, Stockwell E, Geller MA, Vogel RI, Mullany SA, Ghebre R, Witherhoff BJ, et al. Enhanced recovery program and length of stay after laparotomy on a gynecologic oncology service: a randomized controlled trial. Obstet Gynecol. 2017;129(2):355-362.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
