| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 11, Number 2, June 2022, pages 47-52
Tubal Stump Ectopic Pregnancy Following Two Previous Ectopic Pregnancies
Brittany Deryndaa, b, Victoria Griffitha, Rahil Malika
aDr. Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
bCorresponding Author: Brittany Derynda, Dr. Kiran C. Patel College of Osteopathic Medicine, Nova Southeastern University, Fort Lauderdale, FL, USA
Manuscript submitted February 25, 2022, accepted April 2, 2022, published online April 23, 2022
Short title: Three Consecutive Ectopic Pregnancies
doi: https://doi.org/10.14740/jcgo798
| Abstract | ▴Top |
We present the case of a 36-year-old G4P1031 Caucasian female with a history of three consecutive ectopic pregnancies following a successful cesarean section of her first child. The first ectopic pregnancy was located on the left fallopian tube and was managed with methotrexate treatment with inadequate beta-human chorionic gonadotropin decline leading to a therapeutic salpingectomy. The second was located in the right adnexa and was managed medically with methotrexate. The third was a ruptured left-sided fallopian stump ectopic pregnancy 24 days following an embryo transfer and was managed surgically via emergency laparoscopy resulting in removal of the left fallopian tube remnants. Ectopic pregnancy is an obstetrical emergency and the leading cause of maternal morbidity and mortality in the first trimester. Women with a history of prior ectopic pregnancy have an approximately eightfold increase in risk for a future ectopic pregnancy and there remains a gap in knowledge regarding prevention of recurrent ectopic pregnancies. Ectopic pregnancies are rare, but multiple recurrent ectopic pregnancies are much more rare and through this case we shed light on the importance of appropriate individualized discussions regarding risks of future pregnancy following a previous ectopic pregnancy. Furthermore, tubal stump ectopic pregnancies pose a surgical challenge as an ectopic pregnancy not visualized on ultrasound can lead to erroneous excision of the unaffected contralateral tube intraoperatively due to expectations that an ectopic pregnancy would not likely recur on the side that is surgically absent due to prior salpingectomy. We also highlight the necessity for investigation of strategies for management of pregnancies following a prior ectopic pregnancy and preventing recurrent ectopic pregnancies.
Keywords: Tubal stump ectopic pregnancy; Ectopic pregnancy; In vitro fertilization; Recurrent ectopic pregnancy; Obstetrical emergency; Hemoperitoneum
| Introduction | ▴Top |
Ectopic pregnancy is an obstetrical emergency and the leading cause of maternal morbidity and mortality in the first trimester [1]. Ectopic pregnancies occur in 2% of all pregnancies, and women with a history of prior ectopic pregnancy have approximately an eightfold increase in risk for a future ectopic pregnancy [2]. Rates of a third ectopic pregnancy have been shown to be significantly higher after expectant management of the second ectopic pregnancy compared to treatment with methotrexate or surgical intervention [3]. In addition, rates of ectopic pregnancy following in vitro fertilization-embryo transfer (IVF-ET) are two- to three-fold greater compared to the general population [4]. Classification of ectopic pregnancy varies based on location. Interstitial ectopic pregnancies are defined as products of conception in the interstitial area surrounded by a continuous rim of myometrium while cornual ectopic pregnancies occur as a result of implantation in the rudimentary horn of a unicornuate uterus, and stump ectopic pregnancies defined as implantation in the isthmic portion of the remnant tube after previous salpingectomy [5, 6].
While risk factors for ectopic pregnancy have been studied, difficulty still exists in early identification of tubal stump ectopic pregnancies. To emphasize the necessity for investigation of strategies for management of pregnancies following an ectopic pregnancy and preventing recurrent ectopic pregnancies, we report a case of a woman with three consecutive ectopic pregnancies.
| Case Report | ▴Top |
Investigations
The patient was a 36-year-old G4P1031 Caucasian female with a history of ovarian cysts treated with left oophorectomy and fimbriectomy at the age of 15, and hypothyroidism controlled on levothyroxine. Obstetric history was significant for cesarean section of her first child followed by three consecutive ectopic pregnancies. The patient was presenting for follow-up after her third ectopic pregnancy.
In February of 2019, the patient presented to an unaffiliated outside institution emergency room complaining of moderate abdominal pain but was clinically stable. The patient was diagnosed with an ectopic pregnancy clinically due to lack of intrauterine pregnancy (IUP) with an extrauterine adnexal mass and beta-human chorionic gonadotropin (β-hCG) above the discriminatory zone. The patient was managed with methotrexate treatment with inadequate β-hCG decline leading to a therapeutic left salpingectomy which resolved her symptoms.
In March of 2019, the patient again presented to the emergency room complaining of mild abdominal pain rated a three out of ten and vaginal spotting. The patient was clinically stable but had a β-hCG of 2,000 mIU/mL. An ultrasound was completed showing no IUP or sac, but instead a persistent right adnexal lesion posterior to the right ovary was identified measuring 2.3 × 1.9 cm with mild free pelvic fluid. Given the β-hCG value, the absence of IUP, and pelvic ultrasound findings, the patient was treated for a second ectopic pregnancy. The patient was treated with an initial dose of methotrexate therapy followed by an additional dose three days later. Repeat β-hCG in office one week and one month following the emergency room visit measured 2,211 and 27 mIU/mL, respectively, representing clinical resolution.
In November of 2021, the patient presented to emergency room with intractable back, shoulder and abdominal pain status post embryo transfer 24 days prior. On arrival, the patient was pale, hypotensive with a blood pressure of 74/62 mm Hg and tachycardic with a heart rate in the 160s.
Diagnosis
The diagnosis for all three emergency room visits was ectopic pregnancy. Each of these diagnoses included a high index of suspicion and associated clinical findings. During the third presentation, a pelvic ultrasound was completed showing a 1.3 cm endometrial stripe and mild fluid collection posterior to the uterus and adnexa, with no intra- or extrauterine pregnancy visualized and hemoperitoneum extending up to the liver surface. Labs revealed a β-hCG of 2,601.4 mIU/mL. Due to hemodynamic instability and high suspicion for intra-abdominal hemorrhage, the patient was taken for emergency laparoscopy for suspected ruptured ectopic pregnancy.
Treatment
Treatment of this patient’s three cases of ectopic pregnancies included a combination of both surgical intervention and medical management. During treatment for the third ectopic pregnancy, the patient was taken for emergency laparoscopy for planned drainage of hemoperitoneum, removal of ectopic pregnancy, and possible salpingectomy. During operative laparoscopy, the right tube and ovary appeared grossly normal (Fig. 1). The left ovary was absent, as a result of prior oophorectomy and salpingectomy, and there were left sidewall adhesions present which were lysed. The left adnexal region had remnants of fallopian tube with bulbous outpouching and hemorrhage consistent with a likely ruptured tubal stump ectopic pregnancy (Fig. 2). The residual left fallopian tube with ectopic pregnancy was excised and sent to pathology and 1,700 cc of blood was evacuated from a large hemoperitoneum. Pressure applied with blunt graspers caused further extrusion of the products of conception which were collected and sent for processing. The fallopian tube remnant base opening into the uterine cornua was sealed for hemostasis with bipolar diathermy without need for sutures or stitches. Oxidized regenerated cellulose product was placed for adjunct hemostasis. No active oozing was noted upon application of the cellulose product. She received two units of packed red blood cells for hemoglobin < 7 g/dL. Labs completed 2 days later revealed a β-hCG of 59 mIU/mL.
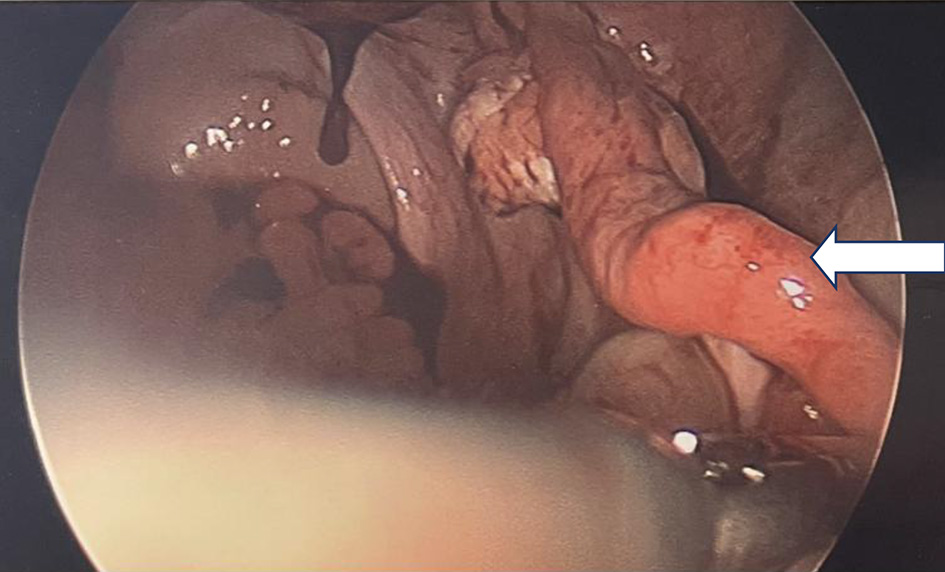 Click for large image | Figure 1. Diagnostic laparoscopy demonstrating normal right fallopian tube (arrow). |
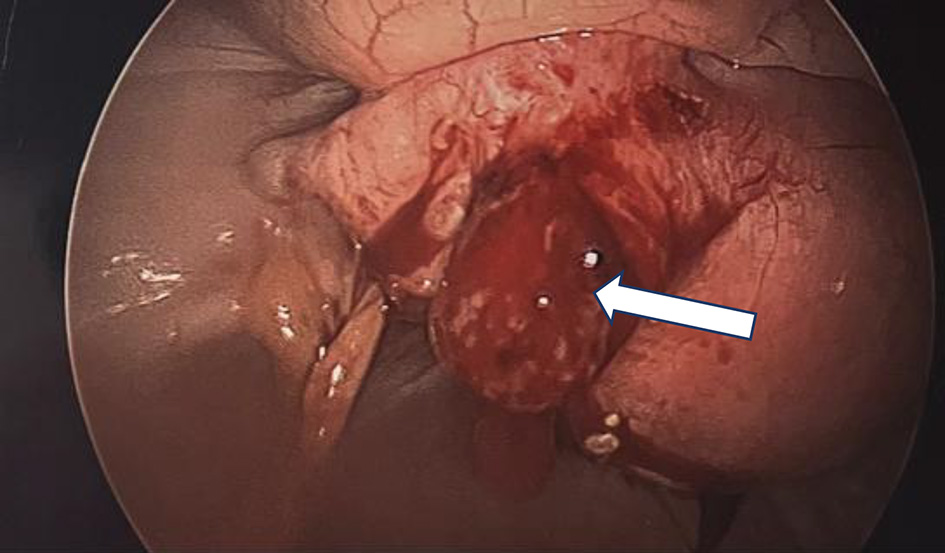 Click for large image | Figure 2. Diagnostic laparoscopy demonstrating bulging left fallopian tube with ectopic pregnancy located in residual tube vs. corneal (arrow). |
Follow-up and outcomes
Following all three treatments of the patient’s ectopic pregnancies down trending β-hCG levels were followed in an outpatient setting representing clinical resolution. Following the patient’s third ectopic pregnancy, she was advised to consider alternative methods of conception prior to attempting pregnancy again. To date the patient has not been known to attempt pregnancy again.
| Discussion | ▴Top |
Tubal stump pregnancies are rare and account for 0.4-1.2% of ectopic pregnancies [7]. The nomenclature for tubal stump ectopic pregnancies is inconsistent but is usually defined as implantation in the isthmic portion of the remnant tube after previous salpingectomy [6]. Ipsilateral ectopic pregnancy on a tubal remnant after salpingectomy is associated with mortality rates higher than other ectopic pregnancies due to ability of remnant position of the tube to distend and increased vascularity to the area [6]. Tubal stump ectopic pregnancies are a unique subgroup with an increased risk of early rupture and thus, diagnosis after rupture has occurred requiring rapid surgical intervention to prevent serious complications [8].
The current accepted paradigm for clinical diagnosis and management of ectopic pregnancy is serial quantitative measurements of β-hCG until complete resolution in combination with transvaginal ultrasound [9]. Measurement of β-hCG was highly predictive of IVF-ET outcomes and is a respected method in monitoring ongoing versus failing pregnancies including ectopic pregnancies [10]. The unique anatomic location of tubal stump pregnancy sometimes leads to delayed diagnosis. In some cases, the diagnosis of tubal stump pregnancy is difficult due to close proximity to the ovary and possibility of mistaking the tubal stump pregnancy for an ovarian follicle [11]. Specific sonographic methods for diagnosing interstitial and tubal stump pregnancies include the “interstitial line sign” which has a sensitivity of 80% and specificity of 98%. The “interstitial line sign” represents the visualization of an echogenic line extending into the abutting interstitial ectopic mass of the tubal mid-portion [12]. Although sonographic guidance exists for tubal stump pregnancies, most tubal stump pregnancies rupture early, prior to identification, and are difficult to identify following rupture.
Surgical management of ectopic pregnancies requires advanced surgical skills due to significant time constraints and risk of ongoing hemorrhage if timely interventions are not deployed. Either laparoscopy or laparotomy may be used based on surgeon comfort, patient’s body habitus and medical history. For ectopic pregnancy located in the interstitial zone, an incision is carried downward from the serosa into the myometrium. The products of conception may spontaneously extrude through the serosal layer, as seen in our patient, or require removal by means of blunt, sharp, suction, or hydrodissection. Expeditious removal of products of conception will help reduce blood loss. Vasopressin can be used to minimize blood loss with varying success. Additional control can be obtained with electrosurgical coagulation as done with our patient and sometimes, sutures can be placed in a figure-eight fashion with a 2-0 absorbable or delayed-absorbable suture [13].
There is an increased risk of extra-uterine pregnancy following cesarean sections, which our patient had [12]. In addition, there is an increased risk of ectopic pregnancy following IVF-ET, with rates ranging from 2.1% to 8.6% of all clinical pregnancies following IVF-ET [14]. Rates of ectopic pregnancy were found to be significantly higher following IVF-ET (1.4%) compared to intrauterine insemination (1.1%) [15]. Several factors increase the risk of ectopic pregnancy following IVF-ET including previous ectopic pregnancy, previous tubal surgery, uterine abnormalities, tubal infertility, endometriosis, smoking, and pelvic inflammatory disease, two of which our patient had [16].
Various maternal and fetal risks exist for future pregnancies following an ectopic including a 1.27 times higher risk of preterm birth, 1.21 times higher risk of placental abruption, 1.45 times higher risk of placenta previa [17], in addition to an eightfold increased risk of a future ectopic pregnancy compared to mothers with no history of ectopic pregnancy [2]. Because of these possible complications, it is important to have discussions regarding the following detailed topics with women who desire future pregnancy after an ectopic pregnancy.
Regarding fertility following surgical treatment for ectopic pregnancy, there is a steady increase in ability to become pregnant during the first 12 months following treatment for ectopic pregnancy, after which the slope flattens but continues to rise gradually during the next 36 months [18]. The US Food and Drug Administration recommends women avoid pregnancy during treatment and for at least one ovulatory cycle after methotrexate therapy; however, some experts continue to recommend that women delay pregnancy for at least 3 months after methotrexate therapy to ensure clearance of systemic methotrexate [19]. Additionally, prospective observational studies noted no difference in anti-Mullerian hormone levels or reproductive outcomes after administration of methotrexate [20]. Bennetot et al found that IUP rates following ectopic pregnancy were significantly lower for patients older than 35 years old or with a history of infertility or tubal disease. However, IUP was significantly higher after conservative treatment with salpingostomy compared to salpingectomy [21].
A concern for future pregnancies is uterine rupture, specifically through a defective area of the superolateral portion of the uterus. Uterine rupture was described in a woman at 20 weeks after spontaneous resolution of the ectopic pregnancy [22] and another at 24 weeks with previous cornual pregnancy treated with salpingectomy [23]. Some suggest suturing the uterine wall after surgical management for reinforcement of the defective area, specifically in cases where the ectopic pregnancy extends into the endometrial cavity though there are reports of successful full-term deliveries following laparoscopic treatment of cornual pregnancy without reinforcing sutures [24]. Regardless of surgical management, it is imperative patients are advised that cesarean section is the optimum mode of delivery following previous ectopic pregnancy to avoid uterine rupture [25].
Another concern for future pregnancy is recurrence of ectopic pregnancy after surgical intervention as the risk of recurrent ectopic pregnancy will continue, despite careful surgical management [26]. This increased risk of recurrent ectopic pregnancies has been shown to be due various etiologies including tubal pathology, assisted contraception [27], and uterine fibroids [28]. Recurrence of ectopic pregnancies has also been associated with smoking, age, prior spontaneous abortion [29] and history of voluntary termination of pregnancy [21]. Preventing recurrence of ectopic pregnancies is difficult due to the diminished ability to modify many of the contributing risk factors. It is therefore important to raise awareness among both women and clinical staff on the signs and symptoms of ectopics with the goal to prevent poor outcomes of ectopic pregnancies [30].
A limitation of this study includes our inability to directly distinguish between interstitial or tubal stump ectopic pregnancy in our patient due to the edema and hemorrhage surrounding the sac during intraoperative diagnosis. Hemoperitoneum presents a diagnostic challenge as the collection may obscure vital structures and confound diagnosis of an ectopic pregnancy [31]. Ideally, one would be able to visualize the ectopic pregnancy using ultrasound prior to hemorrhage; however, in our patient that was not the case due to early hemorrhage. To better visualize the whole field, Mausener Geffen et al recommended using both a transabdominal and transvaginal ultrasound in patients with suspected ectopic pregnancy [32]. In addition, tubal pregnancies prove difficult to diagnose due to possible confusion with ovarian findings such as corpus luteum cysts and ovarian follicles due to similar color Doppler appearances. Recommendations include demonstrating the that tubal ring is extraovarian and moving separate from the ovary, known as the sliding organ sign, during real time examination to confirm the diagnosis [32].
Current methods for prevention of ectopic pregnancies are limited. One method of prevention of ectopic pregnancies following IVF includes extending in vitro cultures until blastocyst stage prior to embryo transfer, which has led to increased pregnancy rates and decreased ectopic pregnancy rates [15]. In addition, ectopic pregnancy rates were significantly lower in patients with prescribed contraceptive use compared to those without [32]. Various groups have demonstrated effectiveness of single prophylactic intratubal injection of methotrexate following laparoscopic linear salpingostomy for prevention of persistent ectopic pregnancy [33, 34].
Future research on preventative strategies for recurrent ectopic pregnancies includes development of a diagnostic biomarker to predict pregnancy outcomes prior to the completion of IVF to properly manage those who are at increased risk for ectopic pregnancy [15]. In addition, Mol et al tried to implement a screening protocol for women at risk of ectopic pregnancy involving transvaginal sonography and serum β-hCG but found that it was debatable whether the possible benefits of prevention of complications outweighed the possible determinants of cost, false-positives, and emotional stress induced by screening [35].
Conclusion
Ectopic pregnancies, specifically tubal stump ectopic pregnancies, are rare, life-threatening events. The diagnosis of tubal stump ectopic pregnancy is often delayed due to early occurrence of rupture and difficulty with visualization with prompt and proper diagnosis being essential to life saving treatment. Presumptive absence of an ectopic pregnancy due to history of a prior salpingectomy can lead to erroneously excision of contralateral tube that may have inflammation or edema noted during laparascopy which may mislead the surgeon towards excising the unaffected tube. In women with previous salpingectomies, it is imperative to monitor for and hold a high index of suspicion for tubal stump ectopic pregnancy. It is important to be aware of future pregnancy recommendations and possible outcomes following ectopic pregnancies while discussing options with patients. Appropriate individualized discussions regarding risks of future pregnancy are essential following a previous tubal stump ectopic pregnancy. Additionally, there is a need for future research regarding management of pregnancies following ectopic pregnancies and prevention of future ectopic pregnancies.
Learning points
History of an ectopic pregnancy increases the risk of a future ectopic pregnancy eightfold.
This report highlights a case of recurrent ectopic pregnancy on the same side as a prior ectopic pregnancy treated via a salpingectomy.
Individualized physician-patient discussions should be facilitated concerning future pregnancies after an ectopic pregnancy is experienced.
Further research is needed to develop strategies to prevent recurrent ectopic pregnancies.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was taken from the patient after clearly explaining the benefits and risks of the usage of the patient’s data in this case report.
Author Contributions
BD and VG: literature search, introduction, discussion, abstract, and references. RM: case report, images, and literature search.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
IUP: intrauterine pregnancy; β-hCG: beta-human chorionic gonadotropin; IVF-ET: in vitro fertilization-embryo transfer
| References | ▴Top |
- Yoder N, Tal R, Martin JR. Abdominal ectopic pregnancy after in vitro fertilization and single embryo transfer: a case report and systematic review. Reprod Biol Endocrinol. 2016;14(1):69.
doi - Farquhar CM. Ectopic pregnancy. Lancet. 2005;366(9485):583-591.
doi - Karavani G, Gutman-Ido E, Herzberg S, Chill HH, Cohen A, Dior UP. Recurrent tubal ectopic pregnancy management and the risk of a third ectopic pregnancy. J Minim Invasive Gynecol. 2021;28(8):1497-1502.e1491.
doi - Chang HJ, Suh CS. Ectopic pregnancy after assisted reproductive technology: what are the risk factors? Curr Opin Obstet Gynecol. 2010;22(3):202-207.
doi - Kirk E. Ultrasound in the diagnosis of ectopic pregnancy. Clin Obstet Gynecol. 2012;55(2):395-401.
doi - Takeda A, Manabe S, Mitsui T, Nakamura H. Spontaneous ectopic pregnancy occurring in the isthmic portion of the remnant tube after ipsilateral adnexectomy: report of two cases. J Obstet Gynaecol Res. 2006;32(2):190-194.
doi - Ko PC, Liang CC, Lo TS, Huang HY. Six cases of tubal stump pregnancy: complication of assisted reproductive technology? Fertil Steril. 2011;95(7):2432.e2431-2434.
doi - Melcer Y, Naaman HZ, Hausman R, Vaknin Z, Levinsohn-Tavor O, Maymon R, Smorgick N. Tubal stump pregnancy after salpingectomy-Does the time interval from surgical intervention to conception matter? J Obstet Gynaecol Res. 2021;47(7):2509-2514.
doi - Jurkovic D, Wilkinson H. Diagnosis and management of ectopic pregnancy. BMJ. 2011;342:d3397.
doi - Wu G, Yang J, Xu W, Yin T, Zou Y, Wang Y. Serum beta human chorionic gonadotropin levels on day 12 after in vitro fertilization in predicting final type of clinical pregnancy. J Reprod Med. 2014;59(3-4):161-166.
- Nishida M, Miyamoto Y, Kawano Y, Takebayashi K, Narahara H. A case of successful laparoscopic surgery for tubal stump pregnancy after tubectomy. Clin Med Insights Case Rep. 2015;8:1-4.
doi - Timor-Tritsch IE, Monteagudo A, Matera C, Veit CR. Sonographic evolution of cornual pregnancies treated without surgery. Obstet Gynecol. 1992;79(6):1044-1049.
- Hoffman BL, Schorge JO, Halvorson LM, Hamid CA, Corton MM, Schaffer JI. Chapter 43: Surgeries for benign gynecologic disorders. In: Williams Gynecology, 4e. McGraw Hill; 2020.
- Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107(3):595-604.
doi - Santos-Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod. 2016;31(2):393-402.
doi - Refaat B, Dalton E, Ledger WL. Ectopic pregnancy secondary to in vitro fertilisation-embryo transfer: pathogenic mechanisms and management strategies. Reprod Biol Endocrinol. 2015;13:30.
doi - Chouinard M, Mayrand MH, Ayoub A, Healy-Profitos J, Auger N. Ectopic pregnancy and outcomes of future intrauterine pregnancy. Fertil Steril. 2019;112(1):112-119.
doi - al-Nuaim L, Bamgboye EA, Chowdhury N, Adelusi B. Reproductive potential after an ectopic pregnancy. Fertil Steril. 1995;64(5):942-946.
doi - Hackmon R, Sakaguchi S, Koren G. Effect of methotrexate treatment of ectopic pregnancy on subsequent pregnancy. Can Fam Physician. 2011;57(1):37-39.
- Oriol B, Barrio A, Pacheco A, Serna J, Zuzuarregui JL, Garcia-Velasco JA. Systemic methotrexate to treat ectopic pregnancy does not affect ovarian reserve. Fertil Steril. 2008;90(5):1579-1582.
doi - de Bennetot M, Rabischong B, Aublet-Cuvelier B, Belard F, Fernandez H, Bouyer J, Canis M, et al. Fertility after tubal ectopic pregnancy: results of a population-based study. Fertil Steril. 2012;98(5):1271-1276.e1271-1273.
doi - Downey GP, Tuck SM. Spontaneous uterine rupture during subsequent pregnancy following non-excision of an interstitial ectopic gestation. Br J Obstet Gynaecol. 1994;101(2):162-163.
doi - Weissman A, Fishman A. Uterine rupture following conservative surgery for interstitial pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;44(3):237-239.
doi - Moon HS, Choi YJ, Park YH, Kim SG. New simple endoscopic operations for interstitial pregnancies. Am J Obstet Gynecol. 2000;182(1 Pt 1):114-121.
doi - Faraj R, Steel M. Can we reduce the recurrence of cornual pregnancy? A case report. Gynecol Surg. 2009;6:57-59.
doi - Gleicher N, Karande V, Rabin D, Pratt D. Laparoscopic removal of twin cornual pregnancy after in vitro fertilization. Fertil Steril. 1994;61(6):1161-1162.
doi - van der Weiden RM, Karsdorp VH. Recurrent cornual pregnancy after heterotopic cornual pregnancy successfully treated with systemic methotrexate. Arch Gynecol Obstet. 2005;273(3):180-181.
doi - Wittich AC. Recurrent cornual ectopic pregnancy in a patient with leiomyomata uteri. J Am Osteopath Assoc. 1998;98(6):332-333.
- Bouyer J, Coste J, Shojaei T, Pouly JL, Fernandez H, Gerbaud L, Job-Spira N. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case-control, population-based study in France. Am J Epidemiol. 2003;157(3):185-194.
doi - Knight M. Preventing ectopic pregnancy and its complications - what next? Paediatr Perinat Epidemiol. 2017;31(1):11-13.
doi - Mausner Geffen E, Slywotzky C, Bennett G. Pitfalls and tips in the diagnosis of ectopic pregnancy. Abdom Radiol (NY). 2017;42(5):1524-1542.
doi - Raine-Bennett T, Fassett MJ, Chandra M, Armstrong MA, Shi JM, Chiu VY, Alabaster A, et al. Ectopic pregnancy prevention: Further evidence of benefits of prescription contraceptives. Contraception. 2022;105:19-25.
doi - Akira S, Negishi Y, Abe T, Ichikawa M, Takeshita T. Prophylactic intratubal injection of methotrexate after linear salpingostomy for prevention of persistent ectopic pregnancy. J Obstet Gynaecol Res. 2008;34(5):885-889.
doi - Kaya H, Babar Y, Ozmen S, Ozkaya O, Karci M, Aydin AR, Ozbasar D. Intratubal methotrexate for prevention of persistent ectopic pregnancy after salpingotomy. J Am Assoc Gynecol Laparosc. 2002;9(4):464-467.
doi - Mol BW, van der Veen F, Bossuyt PM. Symptom-free women at increased risk of ectopic pregnancy: should we screen? Acta Obstet Gynecol Scand. 2002;81(7):661-672.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
