| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 11, Number 2, June 2022, pages 39-46
Comparative Study Between Using Only Vaginal Misoprostol and Using Vaginal Misoprostol and Estradiol Cream for Induction of Labor: A Randomized Controlled Trial
Mortada E. Ahmeda, Fekria A. Salamaa, Amira M. Ahmeda, b, Rania G. El-Skaana
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
bCorresponding Author: Amira Maher Ahmed Hassan, Department of Obstetrics and Gynecology, Faculty of Medicine, Ain Shams University, Cairo, Egypt
Manuscript submitted May 2, 2022, accepted May 26, 2022, published online June 16, 2022
Short title: Misoprostol and Estradiol Cream for IOL
doi: https://doi.org/10.14740/jcgo807
| Abstract | ▴Top |
Background: The aim of the study was to evaluate the effectiveness of vaginal misoprostol versus vaginal misoprostol and estradiol cream for ripening of the very unfavorable cervix in patients requiring induction of labor (IOL) to shorten induction delivery interval.
Methods: This study was a randomized controlled trial conducted on 120 women with unfavorable cervix during the period from April 2021 to October 2021. Patients were randomized into two equal groups as follows: group I included 60 patients who were given only vaginal misoprostol 25 µg, and group II included 60 patients in which women were given vaginal misoprostol 25 µg with vaginal estradiol 150 mg.
Results: Thirty-two patients (53.3%) in the misoprostol group and 38 patients (63.3%) in the estradiol group reached the active phase. According to the mode of delivery, 29 patients (48.3%) in the misoprostol and 24 patients (40%) in the estradiol group underwent cesarean section (CS). The most common causes of CS were failed induction and fetal distress. With exception of the first minute Apgar score, no statistically significant difference in IOL between both groups was reported.
Conclusion: We found that a combination of the misoprostol and estradiol does not achieve a significant difference in IOL compared to vaginal misoprostol alone.
Keywords: Misoprostol; Estradiol; Induction of labor
| Introduction | ▴Top |
Induction of labor (IOL) is a common procedure that occurs in nearly 25% of term pregnancies [1]. IOL can decrease the frequency of stillbirths, reduce risks of infection, and lower cesarean section (CS) rates without increasing adverse pregnancy outcomes [2].
Cervical preparation is one of the most substantial factors in the success of IOL. Attempting induction with an unripe cervix is difficult and rarely successful as unfavorable cervix is less likely to be affected by uterine muscle contractility and pressure of the fetal presenting compared to the favorable cervix. Inducing labor with an unripened cervix can result in induction failure or prolonged labor and childbirth with the use of instruments. This will contribute to low levels of satisfaction of delivery, and also to negative psychological and physical effects [3].
While several methods of cervical ripening before induction have been proposed, prostaglandins are the current agents of choice [4].
Misoprostol, a prostaglandin E1 analog, has gained popularity as an IOL agent in recent years [5]. Misoprostol has some potential benefits over other prostaglandins. It is stable at room temperature, cheap, and can be given orally, vaginally, sublingually, and buccal. To this day, no unique dosage or administration method has been recorded without causing such side effects [3].
Estradiol was proposed that acts synergistically with misoprostol vaginally and significantly hastens the process of cervical ripening, initiation of active labor, and vaginal delivery [6].
The purpose of this study was to evaluate the effectiveness of vaginal misoprostol versus vaginal misoprostol and estradiol cream for ripening of the very unfavorable cervix in patients requiring IOL aiming to initiate active phase of labor for shortening induction delivery interval.
| Materials and Methods | ▴Top |
Our study was registered on Clinical trial.gov. with the following number: NCT05306405. This study was a randomized controlled trial conducted on 120 women with unfavorable cervix during the period from April 1, 2021 to October 31, 2021 at Ain Shams University Maternity Hospital in Egypt to compare the safety and effectiveness of vaginal misoprostol with combined vaginal misoprostol and estradiol for IOL in unfavorable cervix.
Eligible patients (according to our inclusion criteria which were female patients with gestational age from 36 to 41 weeks (gestational age was confirmed by sure last menstrual period of the patient or serial ultrasound if she did not have sure dating), with singleton living fetus < 4 kg (confirmed by pregnancy ultrasound before IOL), with cephalic presentation, with no labor pain, or any amniotic fluid abnormalities (either oligohydramnios with deepest vertical pocket (DVP) of less than 2 cm or polyhydramnios with DVP of more than 8 cm), with Bishop score < 5) were randomly allocated to one of two treatment arms in a single-blind manner by the computer-generated system. While we excluded pregnant female patients who had multiple gestation, abnormal umbilical artery Doppler indices (lost or reversed umbilical artery results) or non-reassuring non-stress test (e.g., fetal heart rate (FHR) more than 160 bpm or less than 100 bpm), fetal weight > 4 kg, non-vertex presentation, intrauterine fetal death, and previous uterine surgery.
After taking informed written consent, the recruited patients were subjected to detailed history taking and thorough examination, including pelvic examination to demonstrate the presenting part and to assess cervical dilatation, effacement, consistency and station using Bishop score [7]. In addition, laboratory tests, including complete blood picture, Rh, and urine analysis were performed. Ultrasound was done trans-abdominally using MEDISON R5 ultrasound machine equipped with a 3.5 MHz Convex probe to evaluate the fetal biometry, placental site, fetal weight, and amount of liquor.
Patients were randomized into two equal groups as follows: group I (control group) included 60 patients who were given only vaginal misoprostol 25 µg (Vagiprost manufactured by ADWia Pharmaceutical Company), and group II included 60 patients in which women were given vaginal misoprostol 25 µg (Vagiprost) with vaginal estradiol 150 mg (Premarine cream manufactured by Aly and Aly Pharmacy). Every 1 g of premarine cream contained 150 mg estradiol and the given dose was adjusted by a digital scale. Misoprostol was repeated every 4 h in both groups for maximum five doses [8], reaching Bishop score > 8, rupture of membrane (ROM) or occurrence of labor pain. The repeated doses, evaluation and labor were done by the supervisors and expert staff. Neither women nor the staff knew whether the woman under observation was assigned to only misoprostol or misoprostol with estradiol group.
Cervical evaluation was done using Bishop score. A score < 5 was taken as unfavorable. End point of the study was initiation of active phase of first stage of labor which commenced from 6 cm to full cervical dilatation.
Allocation concealment mechanism is using consecutive numbers on opaque sealed envelopes having a letter of “A” or “B” according to the sequence generated through the computer sequentially numbered opaque sealed envelope system with each envelope containing a letter corresponding to a number in the randomization list. Participating women were allocated to each group according to the letter inside the envelope.
Ethical considerations
The study was approved by the Ethics Committee of the Department of Obstetrics and Gynecology, Faculty of Medicine Ain Shams University. The research protocols used in this research were approved by the ethical standards of the responsible institution on human subjects and in accordance with the Declaration of Helsinki.
Sample size justification
Sample size calculation was done using PASS 11 program, setting power at 80% and alpha error at 5%. Reviewing results from a previous study [6] showed that time from initiation to active labor in misoprostol versus misoprostol and estradiol groups was 15.33 ± 3.76 versus 12.97 ± 5.27. According to these findings, sample size of at least 120 pregnant females (60/group) was needed.
Statistical methods
Data were collected, coded, revised, and entered into the Statistical Package for Social Science (Rstudio) version 2.3.2. The data were presented as numbers and percentages for the qualitative data, mean, standard deviations (SDs), and ranges for the quantitative data with parametric distribution and median with interquartile range (IQR) for the quantitative data with the non-parametric distribution. Shapiro test was used to verify the normality of distribution. The Chi-square test, Fisher exact test or Wilcoxon Mann-Whitney tests were used in the comparison between the two groups. P-value was considered significant as P < 0.05 (significant (S)) and P < 0.01 (highly significant (HS)) (Fig. 1).
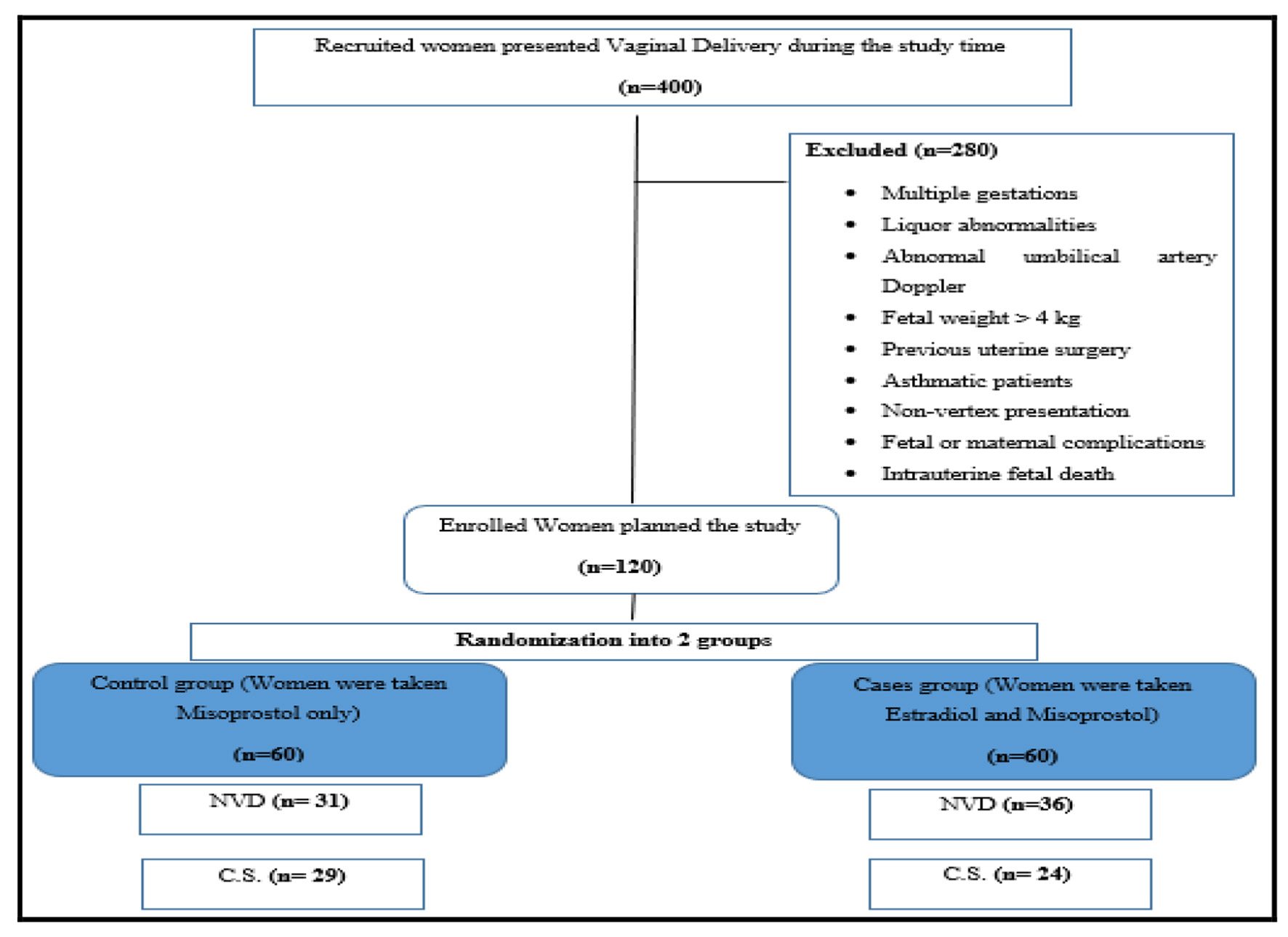 Click for large image | Figure 1. CONSORT flow diagram showing the recruitment and handling of the study population during the study. NVD: normal vaginal delivery; CS: cesarean section. |
| Results | ▴Top |
Baseline characteristics are summarized in Table 1. No statistically significant differences were found between the two studied groups as regards age, parity, gestational age, abortion times, and medical and surgical history.
 Click to view | Table 1. Comparison Between Two Studied Groups According to Baseline Characteristics |
The most common causes of induction were decreased fetal kicks (less than 10 kicks per day) in 12 patients (20%) in the misoprostol group and 22 patients (36.7%) in the estradiol group, severe preeclampsia toxemia (SPET) in 17 patients (28.3%) in misoprostol group and 15 patients (25%) in estradiol group while ROM in 11 patients (18.3%) in the misoprostol group and 13 patients (21.7%) in estradiol group. There was no statistically significant difference between the two groups (P = 0.151).
The minimum Bishop score in both groups was 3, while the maximum was 5 with mean ± SD of 3.38 ± 0.56 in the misoprostol group and 3.28 ± 0.52 in the estradiol group. There was no statistically significant difference between the two groups according to Bishop score (P = 0.06).
Maternal and fetal complications are presented in Table 2. No females had postpartum hemorrhage and uterus rupture in both groups, while two patients (3.3%) in the misoprostol group and only one patient (1.7%) in the estradiol group showed hyperstimulation. All patients in both groups showed no fetal hypoxia.
 Click to view | Table 2. Comparison Between Two Studied Groups According to Maternal Complications and Fetal Outcome |
According to the mode of delivery, 29 patients (48.3%) in the misoprostol and 24 patients (40%) in the estradiol group underwent cesarean section (CS) with no statistically significant difference between the two groups. The most common causes of CS were failed induction and fetal distress (Table 3).
 Click to view | Table 3. Comparison Between Two Studied Groups According to Mode of Delivery |
The number of doses of misoprostol ranged between 1 and 3 doses with a mean ± SD of 2.19 ± 0.64 in the misoprostol group, while ranged between 1 and 5 doses with a mean ± SD of 2.5 ± 0.83 in the estradiol group, with no statistically significant difference (P = 0.201).
Among 32 patients in the active phase in the misoprostol group, 22 patients (68.8%) received oxytocin with a median time of 5.0 (4.0 - 6.0) h, while among 38 patients in the active phase in the estradiol group, 25 patients (65.8%) received oxytocin with a median time of 5.0 (4.1 - 6.8) h. There was no statistically significant difference between the two groups regarding oxytocin intake and time of taking oxytocin (P = 0.994 and 0.315, respectively).
The occurrence of active phase, the time needed to reach the active phase, and the induction delivery time in the active phase are illustrated in Table 4. Thirty-two patients (53.3%) in the misoprostol group and 38 patients (63.3%) in the estradiol group were in the active phase. It takes into our consideration that not all females who reached the active phase delivered by normal delivery. There was no statistically significant difference between both groups as regards the occurrence of the active phase, the time needed to enter the active phase, and induction delivery time.
 Click to view | Table 4. Comparison Between Two Studied Groups According to Active Phase |
| Discussion | ▴Top |
IOL should be used when the benefits of delivery outweigh the risks of continuing, for example, in the setting of maternal or fetal medical complications. These decisions should always be made in conjunction with the patient and their desires [9]. In the current study, the most common causes of IOL were decreased fetal kicks, severe preeclampsia, and premature ROM. There was no statistically significant difference between the two groups (P = 0.151).
Our findings as regards the causes of induction are consistent with Walker et al’s who conducted a randomized controlled trial involving primiparous women who were randomly assigned to IOL. They found the most common causes of IOL were post-term, preeclampsia, and premature ROM [10].
Misoprostol is a prostaglandin that is used for cervical ripening and IOL along many decades. Although we only find a few works in the literature linking misoprostol’s cervical ripening effect to the presence or absence of estrogen, we believe that there is evidence to suggest a connection [6, 11].
Estrogen appears to be essential for cervical ripening to take place. Pregnant women with placental sulphatase deficiency (resulting in low circulating estrogens) do not show ripening of their cervix. The inflammatory cascade during the cervical ripening process involves leucocytes, and the presence of estrogen receptors on cervical leucocytes suggests that estrogen may directly regulate leucocyte function in the cervix. The local application of estrogen for the IOL has been tried, and estrogen does enhance cervical ripening. However, estrogens appear to be less effective than prostaglandins for the IOL and delivery, and there are insufficient data to draw any conclusions [11].
In Norwegian University Teaching Hospital, Oppegaard et al performed a randomized, double-blind, placebo-controlled trial on 67 postmenopausal women to determine the effect of a combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women. They concluded that 1,000 µg of vaginal misoprostol, 12 h prior to day-care hysteroscopy, after 14 days of pretreatment with vaginal estradiol, has a significant cervical ripening effect compared with placebo in postmenopausal women [11].
Several studies [12, 13] indicated that participants who were treated with misoprostol were suffering from gastrointestinal experiences, tachysystole, and hyperstimulation which were the results of misoprostol dosage.
On the contrary, in Dasgupta and Singh’s study, there were no significant adverse effects seen with the use of vaginal 25 µg misoprostol on either fetus or mother in both protocols (misoprostol alone and misoprostol with estradiol) [6].
In the current study, no uterine rupture was recorded, but uterine hyperstimulation was reported in three patients (one patient in the estradiol group and two patients in the misoprostol group) which differed from Dasgupta and Singh’s study, who reported no incidence of uterine hyperstimulation.
As regards fetal complications in the current study, no fetal hypoxia was reported, but neonatal infections occurred in one patient in the misoprostol group. Meconium staining was higher in the misoprostol group than the estradiol group (21.7% vs. 10%) with no significant difference between both groups (P = 0.134). Six patients in the estradiol group and 10 patients in the misoprostol group were admitted to neonatal intensive care unit (NICU) with no statistically significant difference (P = 0.421). Although the first minute Apgar score in the misoprostol group was significantly lower than that in the estradiol group (P = 0.009*), the fifth minute Apgar score was also lower in the misoprostol group than in the estradiol group but with no statistically significant difference (P = 136). Our findings regarding the fetal outcomes were in agreement with Dadashaliha et al’s study [3].
In this study, although not statistically significant, the percentage of spontaneous labor in the misoprostol group (51.7%) was lower than in the estradiol group (60%), and the CS rate in the misoprostol group was higher than in the estradiol group (48.9% vs. 40%, respectively). The causes of CS were failed induction and fetal distress with no statistically significant difference between both groups (P = 0.825 and 0.63).
The rate of CS in our study was notably higher than in previous studies by Souizi et al [14], Dasgupta and Singh [6], and Roudsari et al [15], where the CS rate was 7%, 10%, and 10%, respectively]. This difference may be due to the difference in demographic data between patients’ characters in both studies and the frequent use of operative vaginal delivery in Souizi et al. However, in the studies by Dasgupta and Singh and Roudsari et al, the difference is related to the difference in sample size between both studies (our study had 120 patients and their study had 90 patients); also there were different indications of CS between both studies.
In this study, the mean ± SD of misoprostol doses in the estradiol group was higher than that in the misoprostol group (2.5 ± 0.83 versus 2.19 ± 0.64) with no statistically significant difference (P = 0.201).
In contrast to our results, on average, 4 - 5 doses of misoprostol were required in Dasgupta and Singh’s study for cervical ripening or initiation of active labor; however, the dose required in the combined group (vaginal misoprostol and vaginal estradiol) was significantly less than that in the misoprostol group (P = 0.017) [6].
Various studies have found an induction delivery interval with vaginal misoprostol of 16 - 20 h, which is in agreement with our study (median (IQR) of 15.0 (12.5 - 18.6) in the misoprostol group and 16.8 (13.1 - 19.8) in the estradiol group) [6, 16].
In terms of oxytocin intake and time of taking oxytocin, no statistically significant difference between the two groups was found (P = 0.994 and 0.315, respectively). Also, as regards the active phase, the time needed to enter the active phase, and the induction delivery time in the active phase, all were comparable in both groups with no statistically significant difference (P = 0.355, 0.701, and 0.519, respectively).
However, the findings of the current study do not support the previous research by Dasgupta and Singh. They reported significant differences between vaginal misoprostol versus vaginal misoprostol with estradiol for IOL regarding induction initiation to cervical ripening interval, induction initiation to active labor initiation, and induction initiation to delivery (induction to cervical ripening (P = 0.017), the time required for cervical ripening (P = 0.042), the time required for starting of active labor (P = 0.017), and time required for delivery in vaginal delivery cases (P = 0.047)) [6].
Another study that differs from our work was Raksha et al’s study. This was a randomized study conducted to compare the safety and effectiveness of vaginal misoprostol vs. combined vaginal misoprostol with estradiol for priming an unfavorable cervix. They found that the time interval between the administration of the first dose to cervical ripening (P < 0.001) and vaginal delivery (P < 0.001), the number of doses of misoprostol required for cervical ripening (P < 0.001), and the number of cases of failure of cervical ripening (P = 0.009) were found to be less in the misoprostol and estradiol group when compared to the misoprostol only group. They concluded that estradiol acts synergistically with misoprostol vaginally and significantly hastens the process of cervical ripening, vaginal delivery and also decreases the number of doses of misoprostol required to achieve this [17].
Overall, our data suggested that a combination between vaginal misoprostol and vaginal estradiol does not achieve a significant difference in IOL compared to vaginal misoprostol alone with exception of the first minute Apgar score. This was contradictory to the previous conclusion by Dasgupta and Singh and Raksha et al, which proved that estradiol acts synergistically with misoprostol vaginally and significantly hastens the process of cervical ripening, initiation of active labor, and vaginal delivery [6, 17]. Accordingly, further studies are required to validate the contradictory findings.
Conclusion
This study was a randomized controlled trial to evaluate the effectiveness of vaginal misoprostol versus vaginal misoprostol and estradiol cream for ripening of the unfavorable cervix in patients requiring IOL. We found that although the spontaneous labor was slightly more frequent in patients who received combined vaginal misoprostol and vaginal estradiol, this combination does not achieve a significant difference in IOL regarding the number of misoprostol doses, the mode of delivery and the time needed to enter the active phase compared to vaginal misoprostol alone with exception of the first minute Apgar score with no significant results between fetal or maternal complications and use of combined vaginal misoprostol and vaginal estradiol.
We recommend complementary studies to evaluate more than one method whether pharmacological or mechanical in IOL to establish best model to be used safely in the clinical practice and validate the contradictory finding as regards the use of estradiol in IOL.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed written consent was taken from all participants before recruitment in the study, and after explaining the purpose and procedures of the study.
Author Contributions
Rania G. El-Skaan: protocol design; Amira M. Ahmed: data collection and analysis; Mortada E. Ahmed and Fekria A. Salama: manuscript writing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Pierce S, Bakker R, Myers DA, Edwards RK. Clinical insights for cervical ripening and labor induction using prostaglandins. AJP Rep. 2018;8(4):e307-e314.
doi pubmed - Tarimo CS, Mahande MJ, Obure J. Prevalence and risk factors for caesarean delivery following labor induction at a tertiary hospital in North Tanzania: a retrospective cohort study (2000-2015). BMC Pregnancy Childbirth. 2020;20(1):173.
doi pubmed - Dadashaliha M, Fallah S, Mirzadeh M. Labor induction with randomized comparison of cervical, oral and intravaginal misoprostol. BMC Pregnancy Childbirth. 2021;21(1):721.
doi pubmed - Patte C, Deruelle P. A critical appraisal of the misoprostol removable, controlled-release vaginal delivery system of labor induction. Int J Womens Health. 2015;7:889-899.
doi pubmed - Bolla D, Weissleder SV, Radan AP, Gasparri ML, Raio L, Muller M, Surbek D. Misoprostol vaginal insert versus misoprostol vaginal tablets for the induction of labour: a cohort study. BMC Pregnancy Childbirth. 2018;18(1):149.
doi pubmed - Dasgupta E, Singh G. Vaginal misoprostol vs vaginal misoprostol with estradiol for labor induction: a prospective double blind study. J Obstet Gynaecol India. 2012;62(1):47-51.
doi pubmed - Bishop EH. Pelvic Scoring for Elective Induction. Obstet Gynecol. 1964;24:266-268.
- Chatsis V, Frey N. Misoprostol for cervical ripening and induction of labour: a review of clinical effectiveness, cost-effectiveness and guidelines. Ottawa (ON), 2018.
- Coates D, Homer C, Wilson A, Deady L, Mason E, Foureur M, Henry A. Induction of labour indications and timing: A systematic analysis of clinical guidelines. Women Birth. 2020;33(3):219-230.
doi pubmed - Walker KF, Bugg GJ, Macpherson M, McCormick C, Grace N, Wildsmith C, Bradshaw L, et al. Randomized trial of labor induction in women 35 years of age or older. N Engl J Med. 2016;374(9):813-822.
doi pubmed - Oppegaard KS, Lieng M, Berg A, Istre O, Qvigstad E, Nesheim BI. A combination of misoprostol and estradiol for preoperative cervical ripening in postmenopausal women: a randomised controlled trial. BJOG. 2010;117(1):53-61.
doi pubmed - Gupta H, Singh U, Mehrotra S. Comparative evaluation of 25 µg and 50 µg of intravaginal misoprostol for induction of labor. The Journal of Obstetrics Gynecology of India. 2010;60(1):51-54.
doi - Girija S, Manjunath AP. Comparison of two dosing regimens of vaginal misoprostol for labour induction: a randomised controlled trial. J Turk Ger Gynecol Assoc. 2009;10(4):220-225.
- Souizi B, Mortazavi F, Haeri S, Borzoee F. Comparison of vaginal misoprostol, laminaria, and isosorbide dinitrate on cervical preparation and labor duration of term parturient: a randomized double-blind clinical trial. Electron Physician. 2018;10(5):6756-6763.
doi pubmed - Vahid Roudsari F, Ayati S, Ghasemi M, Hasanzadeh Mofrad M, Shakeri MT, Farshidi F, Shahabian M. Comparison of vaginal misoprostol with foley catheter for cervical ripening and induction of labor. Iran J Pharm Res. 2011;10(1):149-154.
- Hall R, Duarte-Gardea M, Harlass F. Oral versus vaginal misoprostol for labor induction. Obstet Gynecol. 2002;99(6):1044-1048.
doi pubmed - Raksha M, Rao AA, Kamath A, Rao B, Shameem V. Induction of labor in unfavourable cervix: vaginal misoprostol versus vaginal misoprostol with estradiol. International Journal of Pharmaceutical and Biomedical Research. 2013;4(4):202-205.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
