| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 12, Number 1, March 2023, pages 19-23
Solitary Fibrous Tumor in the Vulva
Ikuo Kudawaraa, c, Naohiro Yasudaa, Atsuhiko Okagakib
aDepartment of Orthopaedic Surgery, National Hospital Organization Osaka National Hospital, Chuo-ku, Osaka 540-0006, Japan
bDepartment of Obstetrics and Gynecology, National Hospital Organization Osaka National Hospital, Chuo-ku, Osaka 540-0006, Japan
cCorresponding Author: Ikuo Kudawara, Department of Orthopaedic Surgery, National Hospital Organization Osaka National Hospital, Chuo-ku, Osaka 540-0006, Japan
Manuscript submitted January 7, 2023, accepted February 6, 2023, published online March 31, 2023
Short title: Solitary Fibrous Tumor in the Vulva
doi: https://doi.org/10.14740/jcgo852
| Abstract | ▴Top |
Solitary fibrous tumor (SFT) is a rare soft tissue tumor that is composed of fibroblastic tumor cells and collagenous stroma with NAB2-STAT6 gene rearrangement. Vulvar SFTs are extremely rare. We report a case of vulvar SFT and review the current literature. A 71-year-old woman presented with a slow-growing mass in her left labia majora. The patient also complained of urinary stream diversion. Physical examination revealed a tumor in the left labia majora, which manifested as a rubbery, immobile, and tender mass. Computed tomographic images showed an enhanced vulvar mass. Magnetic resonance imaging (MRI) demonstrated a well-defined tumor with heterogeneous intermediate signal intensity on T1-weighted images and heterogeneous high signal intensity on T2-weighted images. The tumor measured 68 × 45 × 57 mm on MRI and was located in the labia majora. Percutaneous needle biopsy revealed an SFT. Immunohistochemically, the tumor cells were positive for STAT6 and CD34. Local excision was performed with negative surgical margins. Preoperative needle biopsy was useful for diagnosis and planning a surgical strategy. Local resection of the vulvar SFT improved symptoms of urinary stream diversion. There was no tumor recurrence during the 4 years following the surgery.
Keywords: Solitary fibrous tumor; Vulva; Surgery; Older patient; MRI
| Introduction | ▴Top |
Solitary fibrous tumors (SFTs) are rare soft tissue tumors composed of fibroblastic tumor cells and collagenous stroma and exhibit NAB2-STAT6 gene rearrangement [1]. SFTs are usually benign; however, recurrence or metastasis can occur in some cases [1]. The most frequent sites of SFT are the thorax, head, and abdomen [2, 3]. SFT arising in the vulva is extremely rare. The most common histology of the vulvar tumor is squamous cell carcinoma; mesenchymal tumors at this site are uncommon [4]. We herein present a rare case of SFT of the vulva successfully treated with surgery by the orthopedic oncologic service.
| Case Report | ▴Top |
Investigations
A 71-year-old postmenopausal woman presented with a slow-growing mass in the left labia majora that had persisted for several years. She also complained of the urine stream deviating towards the left. Her primary clinical history included being diabetic and a hepatitis C virus carrier. Physical examination revealed a tumor in the left labia majora, which manifested as a rubbery, immobile, and tender mass (Fig. 1a). Redness, ulceration, or pigmentation of the bulging skin was not observed. There were no abnormalities in the labia minora, glans of the clitoris or vulva vestibule.
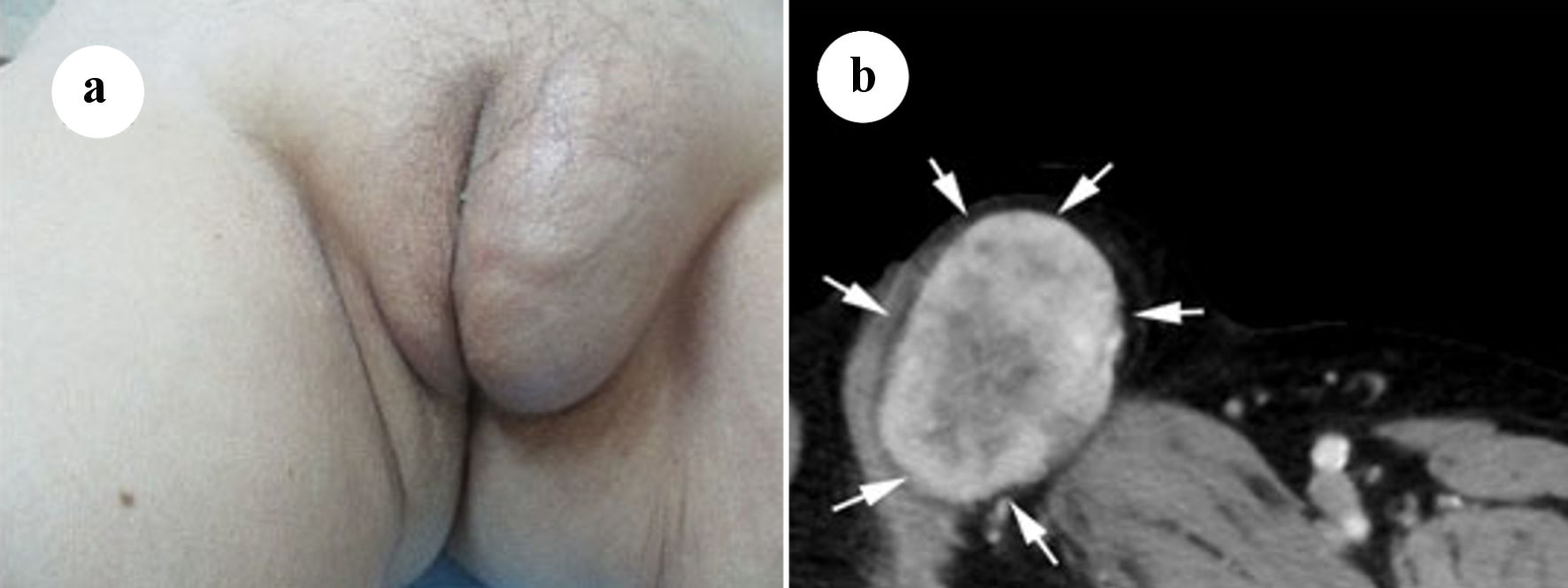 Click for large image | Figure 1. (a) Soft tissue mass on the left labia majora. (b) Axial computed tomography (CT) scan with intravenous contrast showing a mass in the left labia majora with intermediate density and enhancement (arrows). |
Diagnosis
Enhanced computed tomography (CT) images of the vulva showed an oval-shaped tumor, which was well circumscribed and enhanced (Fig. 1b). No other abnormal lesions were observed in the pelvis on CT. Axial non-enhanced T1-weighted magnetic resonance imaging (MRI) of a well-defined mass in the left labia majora revealed marginally higher signal intensity in the peripheral region and partially intermediate signal intensity in the central region than that in the surrounding muscle (Fig. 2a). T2-weighted imaging revealed high signal intensity in the peripheral region and markedly high signal intensity in the central region (Fig. 2b). Moreover, focally short linear low intensities were observed in both on T1- and T2-weighted images. The tumor measured 68 × 45 × 57 mm on MRI and was located in the subcutis of the labia major adjacent to the crus of the clitoris and pectineus muscle. Soft tissue sarcoma was suggested based on the above radiological findings.
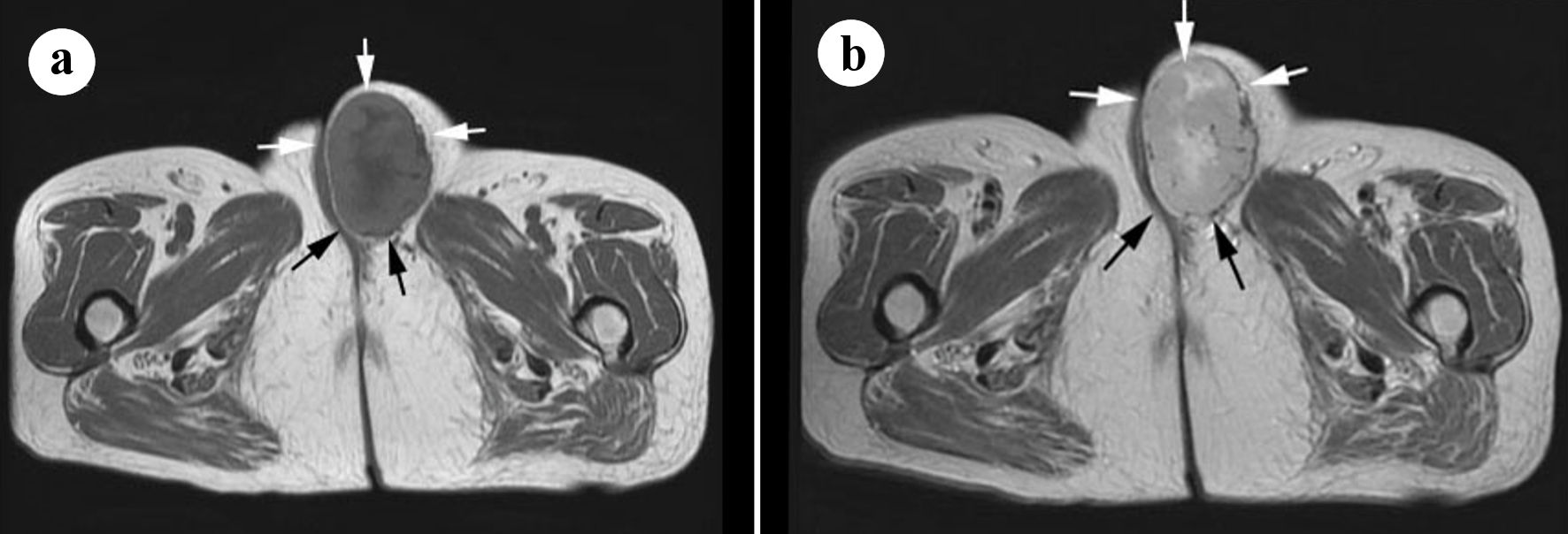 Click for large image | Figure 2. (a) Axial T1-weighted magnetic resonance imaging (MRI) of the tumor in the left labia majora revealed marginally higher signal intensity in the peripheral region and partially intermediate signal intensity in the central region than that in the surrounding muscle (arrows). (b) T2-weighted image revealed high signal intensity in the peripheral region and marked high signal intensity in the central region (arrows). |
Percutaneous needle biopsy using Monopty® (BD) was performed under local anesthesia using 1% lidocaine. Microscopically, the tumor was composed of short spindle cells with a collagenous matrix forming a storiform or hemangiopericytomatous pattern. Immunohistochemically, the tumor cells were positive for STAT6 and partially positive for CD34. Thus, SFT was diagnosed, based on these histological findings (Fig. 3).
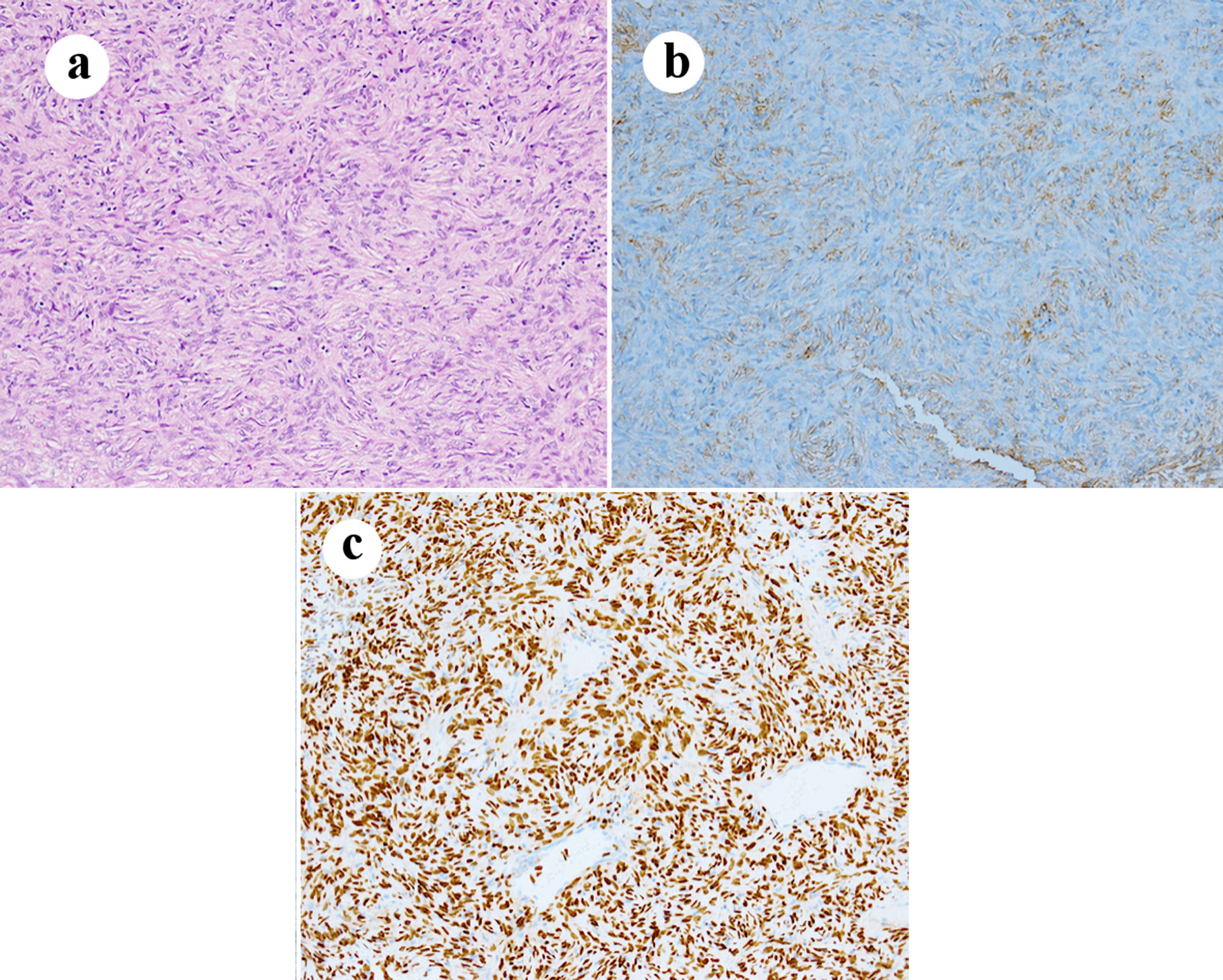 Click for large image | Figure 3. Photomicrograph. The tumor is composed of spindle cells without nuclear atypia and fibrous stroma forming hemangiopericytomatous pattern (a) Hematoxylin and eosin stain (× 200). The tumor cells are focally positive for CD34 (b) and strongly positive for STAT6 (c) (× 200 immunohistochemical staining). |
Treatment
Tumor resection was performed under general anesthesia soon after a definitive diagnosis of SFT was made. During surgery, we dissected the tumor in the subcutis of the labia majora and mons pubis using dissecting forceps. Around the urethral side of the tumor, we did careful blunt dissection while pressing by the fingers the crus of the clitoris underlying the urethral catheter. Monopolar and bipolar coagulation for oozing after removal of the tumor was performed. Consequently, the tumor, with a short cuff of the surrounding fatty tissue, was completely resected. Pathological evaluation revealed negative surgical margins (R0). Histologically, the short spindle cells were arranged partially in a fascicular or storiform pattern. They showed mainly hemangiopericytomatous pattern. Ischemic necrosis occurred in the central portion. The number of mitotic counts was 1 per 10 high-power fields. From these findings, the diagnosis was SFT in the vulva at the time of biopsy.
The patient provided informed consent for biopsy and surgery, and the according to the local institutional review board guidelines.
Follow-up and outcomes
The patient’s postoperative course was uneventful. The deviation of urine towards the left was resolved after surgery. There was no tumor recurrence in the 4 years following the surgery.
| Discussion | ▴Top |
SFTs are rare soft tissue tumors, often occurring in the pleura, extremities, abdominal cavity, pelvis, and retroperitoneum [1]. Vulvar SFT is extremely unusual, and only 30 such tumors have been described as single or several case reports in the literature [5-14]. Based on clinical data from 24 patients, the median patient age reported is 50 years old (range: 22 - 75 years old) and the mean tumor size is 4.7 cm in diameter (range: 1.0 - 18.0 cm) [5-14].
Due to its rarity, it is often difficult to distinguish vulvar SFTs from the other vulvar tumors such as squamous cell carcinoma on physical or radiological findings. Vulvar carcinoma demonstrates an ill-defined mass with low signal intensity on T1-weighted images and intermediate-high signal intensity on T2-weighted MRI images, and contrast enhancement [15]. In contrast, SFT generally presents as a well-defined mass with intermediate signal intensity on T1-weighted images and heterogeneously high signal intensity on T2-weighted images. The present case was suggestive of a malignant tumor, including sarcoma, based on the hypervascularity of the tumor on enhanced CT and heterogeneous signal intensities on MRI. A heterogeneous signal corresponds to fibrosis, high cellularity, and myxoid changes in a tumor [1, 16]. However, these findings are similar to those of soft tissue sarcomas such as leiomyosarcoma and fibrosarcoma, which occasionally arise in the vulva [4, 17, 18].
Therefore, it is important to perform histological diagnosis by biopsy before treatment.
We did the percutaneous needle biopsy for the vulvar lesion, which is less invasive and safe. Histological findings of the biopsy specimen revealed that the tumor was composed of short spindle cell proliferation with hemangiopericytomatous pattern. Immunohistochemically, tumor cells were positive for CD34 and STAT6. A recent pathological study has demonstrated that STAT6 is positive in all cases, and CD34 has been detected in 88% of the cases [14]. Thus, immunohistochemical analysis is essential for SFT diagnosis.
The standard treatment for localized STSs is thought to be surgery. However, there is no clear definition of surgical procedure for each case. Of the 16 cases with described surgical procedures in the literature, vulvectomy was done in two cases [7, 11], wide excision with skin graft in one case [9], and marginal excision in 13 cases [8, 10, 11]. Out of the 13 cases with marginal excision, five cases with positive margins were observed. Since most of the cases mentioned above did not have follow-up data regarding local control or metastasis, we consider that we could not decide on an appropriate surgical procedure for vulvar SFT. Recently, the surgical treatment for vulvar cancer is changing from radical vulvectomy to conservative surgery. Namely, evaluating tumor size, location, and grade, less invasive approach with preserving the surrounding urethra, clitoris, and anal sphincter was investigated to avoid postoperative problems such as physical and cosmetic impairment of the vulvar region [19]. These personalized treatments will result in satisfactory outcomes in women with vulvar cancers.
In the present case, the tumor was located within the subcutis, not extending to the urethra, crus clitoris, and underlying muscle layer, based on visual inspection and radiological findings. Moreover, the histologically, the case was judged to be a benign SFT. Therefore, a vulvectomy was not performed, and the tumor with a fatty cuff was resected. We consider that safety surgical margins are achieved according to the histological examination of the surgical specimen.
SFTs have a clinical spectrum from benign to malignant for 10-30% of recurrences [1, 2]. Two studies have reported that 27% and 43% of all vulvar SFTs are malignant. [11, 14]. Moreover, clinically distant metastases have developed in rare cases [14].
Demicco et al advocated a risk stratification model that includes age (< 55 years, ≥ 55 years), tumor size (< 5 cm, 5 to < 10 cm, 10 to < 15, ≥ 15 cm), and mitotic figures (10 high-power fields, 0, 1 - 3, ≥ 4) after applying Cox proportional hazards regression method on 110 cases with SFT. Three risk groups (low, moderate, and high) based on the above three factors were indicated [20]. In later years, they published a modified four-variable risk stratification model adding tumor necrosis and reported that the revised model was more significantly associated with metastasis [21]. The present case is considered at moderate risk according to the criteria. There was no tumor recurrence 4 years after surgery. However, to be more careful, long-term follow-up is necessary.
The efficacy of adjuvant radiotherapy and chemotherapy for localized SFT is controversial. Moreover, targeted therapy for NAB2-STAT6 is not yet established. Currently, the relationship between molecular subtypes of NAB2-STAT 6 fusion and prognosis remain to be determined [22]. Future translational research to identify subtype-specific clinical approaches will contribute to the selection of appropriate treatment options for the SFT patients.
Learning points
Vulvar SFT is extremely rare. However, SFT should be considered in the differential diagnosis of a vulvar tumor on CT or MRI.
A preoperative percutaneous needle biopsy was useful in making a diagnosis and planning a surgical strategy.
Tumor cells positive for CD34 and STAT6 ensured accurate histological diagnosis of SFT.
Conservative surgery for the vulvar tumor achieved the good functional and oncological outcomes.
Acknowledgments
Our thanks to Dr. Ikuko Sawada (Department of Gynecology, Osaka Police Hospital) providing clinical information and Dr. Mana Taki (Department of Gynecology, Kyoto University) for her reference.
Financial Disclosure
None of the authors has any financial support to disclose.
Conflict of Interest
The authors declare that there is no conflict of interests.
Informed Consent
Informed consent was obtained from the patient for publication.
Author Contributions
Ikuo Kudawara: conceptualization, methodology, data curation, writing and preparing the original draft, and software analyses. Naohiro Yasuda: writing, reviewing, and editing. Atsuhiko Okagaki: writing, reviewing, and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author.
| References | ▴Top |
- Demicco EG, Fritche KJ, Han A. Solitary fibrous tumour. In: WHO classification of tumours. WHO Classification of Tumours Editorial Boards, eds. Soft tissue and bone tumours. 5th edition. IARC, Lyon, 2020; p. 104-108.
- Miettinen M. Solitary fibrous tumor, hemanigiopericytoma, and related tumors. In: Miettinen M. eds. Modern soft tissue pathology: tumors and non-neoplastic conditions. Cambridge, New York, 2010; p. 335-347.
doi - Nielsen GP, O'Connell JX, Dickersin GR, Rosenberg AE. Solitary fibrous tumor of soft tissue: a report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical, and ultrastructural data. Mod Pathol. 1997;10(10):1028-1037.
- Ulutin HC, Zellars RC, Frassica D. Soft tissue sarcoma of the vulva: A clinical study. Int J Gynecol Cancer. 2003;13(4):528-531.
doi pubmed - Fukunaga M. Atypical solitary fibrous tumor of the vulva. Int J Gynecol Pathol. 2000;19(2):164-168.
doi pubmed - Biedrzycki OJ, Singh N, Habeeb H, Wathen N, Faruqi A. Solitary fibrous tumor of the female genital tract a case report and review of the literature. Int J Gynecol Pathol. 2007;26(3):259-264.
doi pubmed - Burnett LA, Christopherson WA, Olawaiye AB. Vulvar hemangiopericytoma: A case report and literature review. Gynecol Oncol Case Rep. 2012;2(4):146-149.
doi pubmed pmc - Taki M, Baba T, Mandai M, Suzuki A, Mikami Y, Matsumura N, Konishi I. Solitary fibrous tumor arising slowly in the vulva over 10 years: case report and review. J Obstet Gynaecol Res. 2012;38(5):884-888.
doi pubmed - Nag G, Rao SR. A rare mesenchymal neoplasm at unusual location: Solitary fibrous tumor of vulva. Gynecol Oncol Rep. 2015;12:52-54.
doi pubmed pmc - Pearre DC, Federspiel JJ, Grumbine FC. Solitary fibrous tumor of the vulva resulting in spinal metastasis: A case report. Gynecol Oncol Rep. 2017;22:97-99.
doi pubmed pmc - Yang EJ, Howitt BE, Fletcher CDM, Nucci MR. Solitary fibrous tumour of the female genital tract: a clinicopathological analysis of 25 cases. Histopathology. 2018;72(5):749-759.
doi pubmed - Tardio JC, Machado I, Alemany I, Lopez-Soto MV, Nieto MG, Llombart-Bosch A. Solitary fibrous tumor of the vulva: report of 2 cases, including a de novo dedifferentiated solitary fibrous tumor diagnosed after molecular demonstration of NAB2-STAT6 gene fusion. Int J Gynecol Pathol. 2018;37(6):547-553.
doi pubmed - Tandon N. Solitary Fibrous Tumor of the Vulva: Case Report of a Rare Entity and Review of Literature. Int J Gynecol Pathol. 2021;40(3):234-239.
doi pubmed - Devins KM, Young RH, Croce S, Burandt E, Bennett JA, Pesci A, Zannoni GF, et al. Solitary fibrous tumors of the female genital tract: a study of 27 cases emphasizing nonvulvar locations, variant histology, and prognostic factors. Am J Surg Pathol. 2022;46(3):363-375.
doi pubmed - Kim KW, Shinagare AB, Krajewski KM, Howard SA, Jagannathan JP, Zukotynski K, Ramaiya NH. Update on imaging of vulvar squamous cell carcinoma. AJR Am J Roentgenol. 2013;201(1):W147-157.
doi pubmed - Wignall OJ, Moskovic EC, Thway K, Thomas JM. Solitary fibrous tumors of the soft tissues: review of the imaging and clinical features with histopathologic correlation. AJR Am J Roentgenol. 2010;195(1):W55-62.
doi pubmed - Meyers SP. MRI of bone and soft tissue tumors and tumorlike lesions. Differential diagnosis and atlas. Thieme, New York. 2008. p. 433-437.
doi - Meyers SP. MRI of bone and soft tissue tumors and tumorlike lesions. Differential diagnosis and atlas. Thieme, New York. 2008. p. 525-542.
doi - Giannini A, D'Oria O, Chiofalo B, Bruno V, Baiocco E, Mancini E, Mancari R, et al. The giant steps in surgical downsizing toward a personalized treatment of vulvar cancer. J Obstet Gynaecol Res. 2022;48(3):533-540.
doi pubmed pmc - Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, Lazar AJ, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298-1306.
doi pubmed - Demicco EG, Wagner MJ, Maki RG, Gupta V, Iofin I, Lazar AJ, Wang WL. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30(10):1433-1442.
doi pubmed - Smrke A, Thway K, Huang PH, Jones RL, Hayes AJ. Solitary fibrous tumor: molecular hallmarks and treatment for a rare sarcoma. Future Oncol. 2021;17(27):3627-3636.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
