| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 12, Number 2, August 2023, pages 52-58
Screening for Thyroid Disorder in First Trimester of Pregnancy: A Cross-Sectional Study
Sumnima Mainalia, Sanita Kayasthaa, Sagar Devkotab, g, Priyanka Yadava, Bishal Bharatia, Sristi Upadhyayc, Sailuja Maharjand, Sarbagya Manandhare, Laxman Timilsinaf
aDepartment of Obstetrics and Gynecology, Nepal Medical College Teaching Hospital, Attarkhel, Kathmandu, Nepal
bDepartment of Anesthesiology and Critical Care, Kulhudhuffushi Regional Hospital, Maldives
cDepartment of Pediatrics, Kulhudhuffushi Regional Hospital, Maldives
dDepartment of Pathology, Kulhudhuffushi Regional Hospital, Maldives
eDepartment of Cardiology, Baidya & Banskota Hospital, Gwarko, Lalitpur, Nepal
fDepartment of Neurosurgery, Chitwan Medical College, Bharatpur, Nepal
gCorresponding Author: Sagar Devkota, Department of Anesthesiology and Critical Care, Kulhudhuffushi Regional Hospital, Maldives
Manuscript submitted April 27, 2023, accepted June 17, 2023, published online August 7, 2023
Short title: Screening for TD in First Trimester of Pregnancy
doi: https://doi.org/10.14740/jcgo880
| Abstract | ▴Top |
Background: Alteration in thyroid function is very common among females, in particular during pregnancy. The majority of them are clinically veiled, and with considerable differences in outcome of mother and fetus, even more serious maternal and fetal outcome if present during the first trimester. So, we conducted this study to explore the magnitude of thyroid problems during first trimester of pregnancy at a tertiary health care facility in Nepal and highlight the benefits of its early diagnosis.
Methods: This was a cross-sectional study conducted over a period of 1 year among 160 pregnant females in their first trimester, attending to the outpatient department of a tertiary care center. Blood samples were collected from all the patients for thyroid function test which included measurement of serum levels of thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free tri-iodothyronine (fT3). Pregnant females with previously diagnosed thyroid disorders or currently under medications were excluded.
Results: Among the 160 patients included in the study, 25.83 years was the mean age. The mean body mass index (BMI) was 24.62 kg/m2 and most of the patients were overweight (40.625%). Of the patients 53.12% were primigravida and 46.87% were multigravida. Based on the thyroid function tests, 91.25% were found to be euthyroid, 3.125% were hyperthyroid and 5.625% were hypothyroid. The prevalence of subclinical hyperthyroidism and overt hyperthyroidism was found to be 60% and 40% respectively and that of subclinical hypothyroidism and overt hypothyroidism was 55.6% and 44.4% respectively. None of the patients had symptoms of thyroid disorder (neither hyperthyroidism nor hypothyroidism). The correlation between BMI and TSH among the pregnant females included in the study was statistically significant when tested by using Karl Pearson correlation coefficient (r = 0.164, P = 0.038).
Conclusions: Based on this study, around one in every 12 pregnant women had thyroid disorder. Early identification of thyroid disorders and prompt initiation of treatment is very essential for the health of mother and fetus due to the significant maternal or fetal morbidity and mortality associated with thyroid disorders. Thus, routine screening for thyroid disorder should be considered during pregnancy.
Keywords: First trimester; Overt hyperthyroidism; Overt hypothyroidism; Subclinical hypothyroidism; Subclinical hyperthyroidism; Thyroid disorders
| Introduction | ▴Top |
Thyroid disorders have been identified as one of the major health problems in Nepal [1], which may be attributed to various factors like congenital factors, genetic predisposition, poor dietary iodine intake, pregnancy, radiotherapy, viral infections, surgery, underlying diseases such as infiltrative disorders, or even autoimmunity [2-4]. One of the studies done in eastern part of Nepal has highlighted the impact of thyroid disorders showing prevalence of hyperthyroidism (13.68%) and hypothyroidism (17.19%) [5]. Various studies have been done across the world to show the magnitude of thyroid disorders in the general population [6-8].
Thyroid disorders have been categorized as subclinical and overt disorders. Subclinical hypothyroidism is defined as elevated serum thyroid-stimulating hormone (TSH) level with normal serum thyroxine level and overt/clinical hypothyroidism is defined as a high serum TSH with low serum thyroxine level. Subclinical hyperthyroidism is defined as low serum TSH level with normal serum thyroxine level and overt/clinical hyperthyroidism is defined as low serum TSH level with elevated serum thyroxine level [9].
Pregnancies in women with subclinical hypothyroidism were three times more likely to be complicated by placental abruption (relative risk 3.0, 95% confidence interval 1.1 - 8.2). Preterm birth, defined as delivery at or before 34 weeks of gestation, was almost two-fold higher in women with subclinical hypothyroidism (relative risk 1.8, 95% confidence interval 1.1 - 2.9) [10]. The presence of overt hypothyroidism in pregnancy is known to be associated with significant maternal complications like arrhythmias, gestational hypertension, anemia, myopathies, congestive heart failure and postpartum hemorrhage [11]. It is also known to be associated with significant fetal morbidity and mortality including spontaneous/threatened abortion, pre-eclampsia, preterm delivery, low birth weight, intra-uterine growth retardation and high perinatal mortality [12]. There have been reports of neonatal hyperbilirubinemia and neonatal hypo/hyperthyroidism as well [13, 14]. Newborns from mothers with hypothyroidism may also develop attention deficit disorder and hyperactivity syndrome [15].
Similarly, overt hyperthyroidism during pregnancy is known to be associated with various complications like pre-eclampsia, preterm labor, low birth weight, and fetal and perinatal loss [10]. Subclinical hyperthyroidism in pregnancy is not related with adverse maternal and fetal outcomes [16].
Various studies done regarding thyroid disorders during pregnancy worldwide have shown significant morbidity and mortality in pregnant females. The aftermath of thyroid disorders when left untreated, even when clinically, is shown to be remarkable, sometimes hazardous to both mother and child. Estimation of the burden of the thyroid disorder in pregnant women in our region is necessary. So, we conducted this study to estimate the burden of these disorders in our population and highlight the need of screening and appropriate the treatment in our population.
| Materials and Methods | ▴Top |
Subjects
A descriptive cross-sectional study was done at a tertiary care center in Nepal among 160 pregnant females in their first trimester attending the outpatient department over a period of 1 year. Blood samples were taken from all the patients enrolled for the study to perform thyroid function test and to find out the prevalence of hypothyroidism and hyperthyroidism. Every third patient within the first 4 months of pregnancy coming to the outpatient department was included based on simple randomization. Pregnant females more than 12 weeks of gestation, not willing to participate and those with pre-existing thyroid disorders, were excluded from the study.
Ethical issues and informed consent
Approval was taken from the Institutional Review Committee of the hospital and informed written consent was taken from all the patients before enrolling them for the study. Ethical Compliance with Human/Animal Study is not applicable for this study as no intervention was done in the patients.
Experimental procedure
Patient demographic details were recorded. History was taken and clinical examination was done after which venous blood sample was taken in a plain vial and sent to laboratory for measurement of serum TSH, free tri-iodothyronine (fT3) and free thyroxine (fT4). Weight of the patient was taken by pre-pregnancy weight as said by the patient. The height was taken using blue bird measuring tape.
Sample size was estimated using the relation n = (z2×p×q)/d2 taking into consideration the findings of the study done among pregnant females in India which showed a prevalence of 11.6% of thyroid disorders [17].
The data collected from clinical and laboratory examinations were recorded in pre-formed pro forma and then entered into SPSS 16.0 software. The findings of clinical tests and laboratory examinations were tabulated and analyzed. The prevalence was calculated and presented along with 95% confidence interval. The demographic pattern in terms of age and parity was presented in tabulated form. Karl Pearson correlation coefficient was used to establish the correlation between BMI and TSH level with level of significance of 5%.
The normal thyroid function test (TFT) ranges based on the laboratory were as follows: TSH: 0.465 - 4.68 µIU/mL, fT4: 0.78 - 2.19 ng/dL, fT3: 2.77 - 5.27 pg/mL.
| Results | ▴Top |
Out of the total 160 patients included in the study, the majority were in the age group 24 - 49 years (n = 79, 49.4%) followed by the age group 18 - 23 years (Table 1). Most of the patients were overweight (n = 65, 40.625%) followed by obese (26.25 %) (Fig. 1). The mean BMI was 24.62 ± 4.54 kg/m2. Out of the total 160 patients, most of the patients were primigravida (53.125%) followed by multigravida (46.875 %) (Fig. 2).
 Click to view | Table 1. Distribution Based on Age Group |
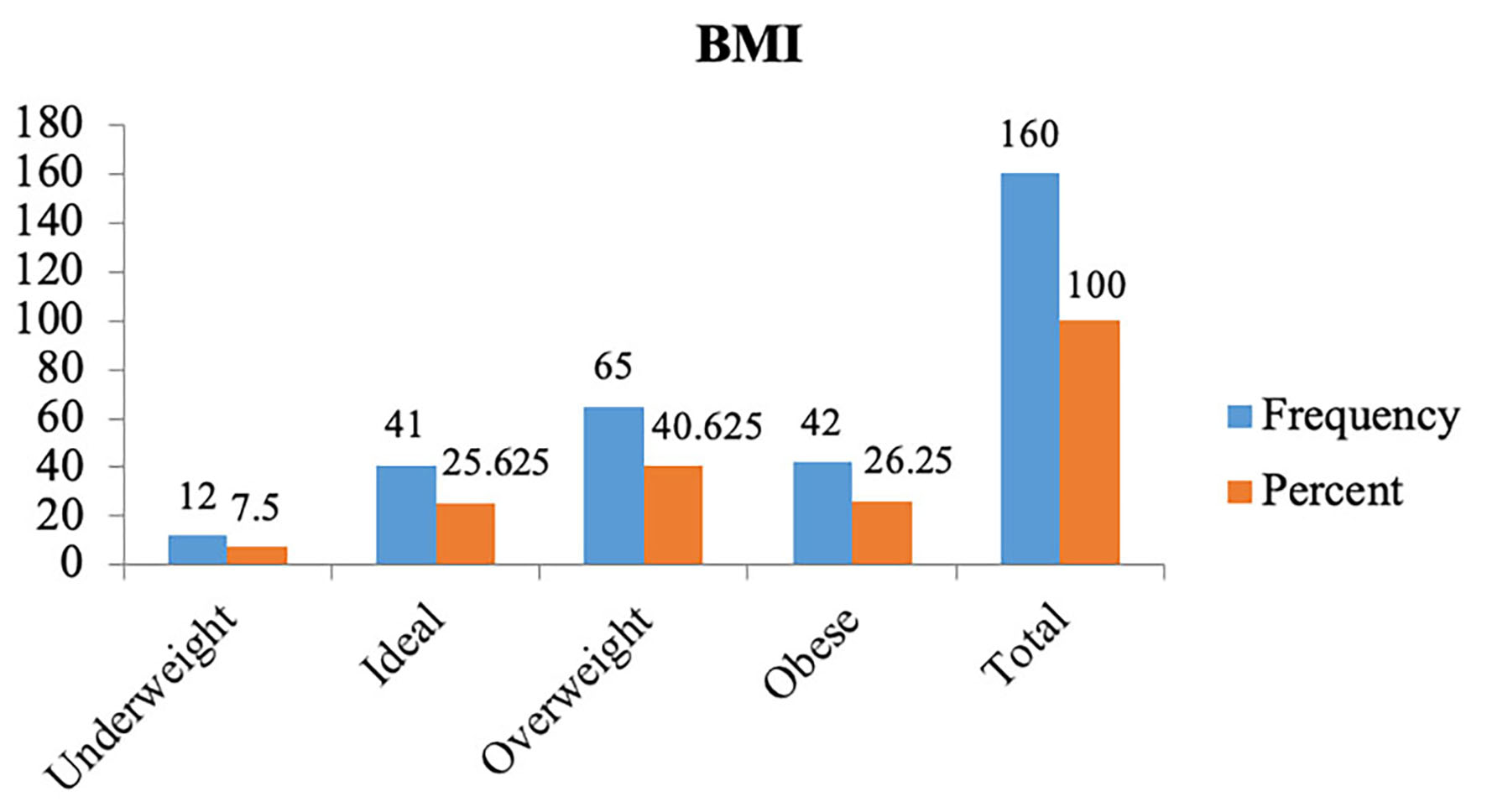 Click for large image | Figure 1. BMI status. BMI: body mass index. |
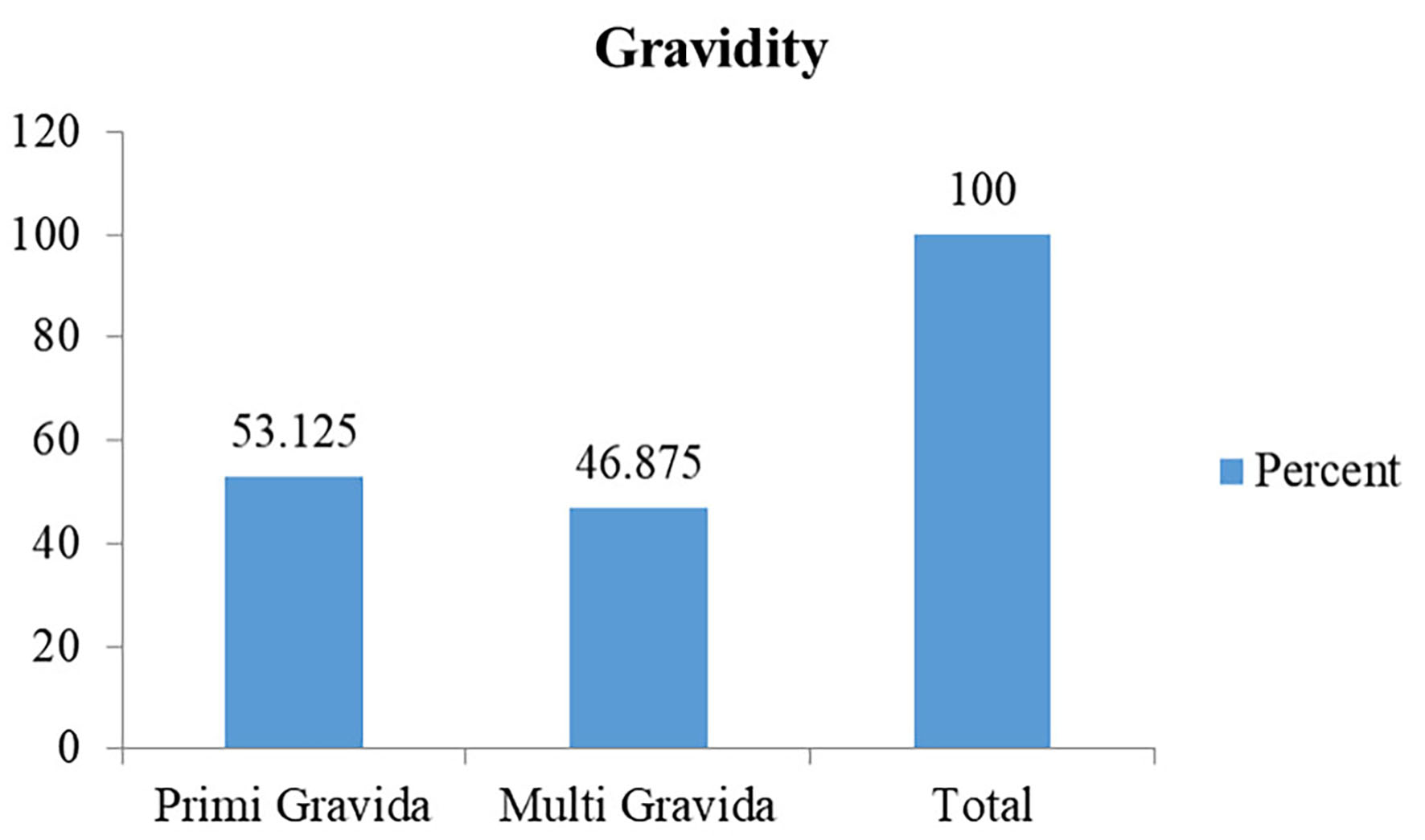 Click for large image | Figure 2. Distribution based on gravidity. |
Distribution based on symptoms of thyroid disorders
None of the pregnant females enrolled in the study were found to have symptoms of either hyperthyroidism or hypothyroidism. The prevalence of hyperthyroidism among them was 3.125% while that of hypothyroidism was 4.375% (Fig. 3). Similarly, 3.125% had subclinical hypothyroidism, 2.5% had overt hypothyroidism, 1.875% had subclinical hyperthyroidism and 1.25 % had overt hyperthyroidism. Among the five hyperthyroid patients, subclinical hyperthyroidism was seen in three (60%) patients; meanwhile two (40%) patients had features suggestive of overt hyperthyroidism (Fig. 4). Among the nine hypothyroid patients, subclinical hypothyroidism was seen in five (55.6%) patients and overt hypothyroidism was found in four (44.4%) of them.
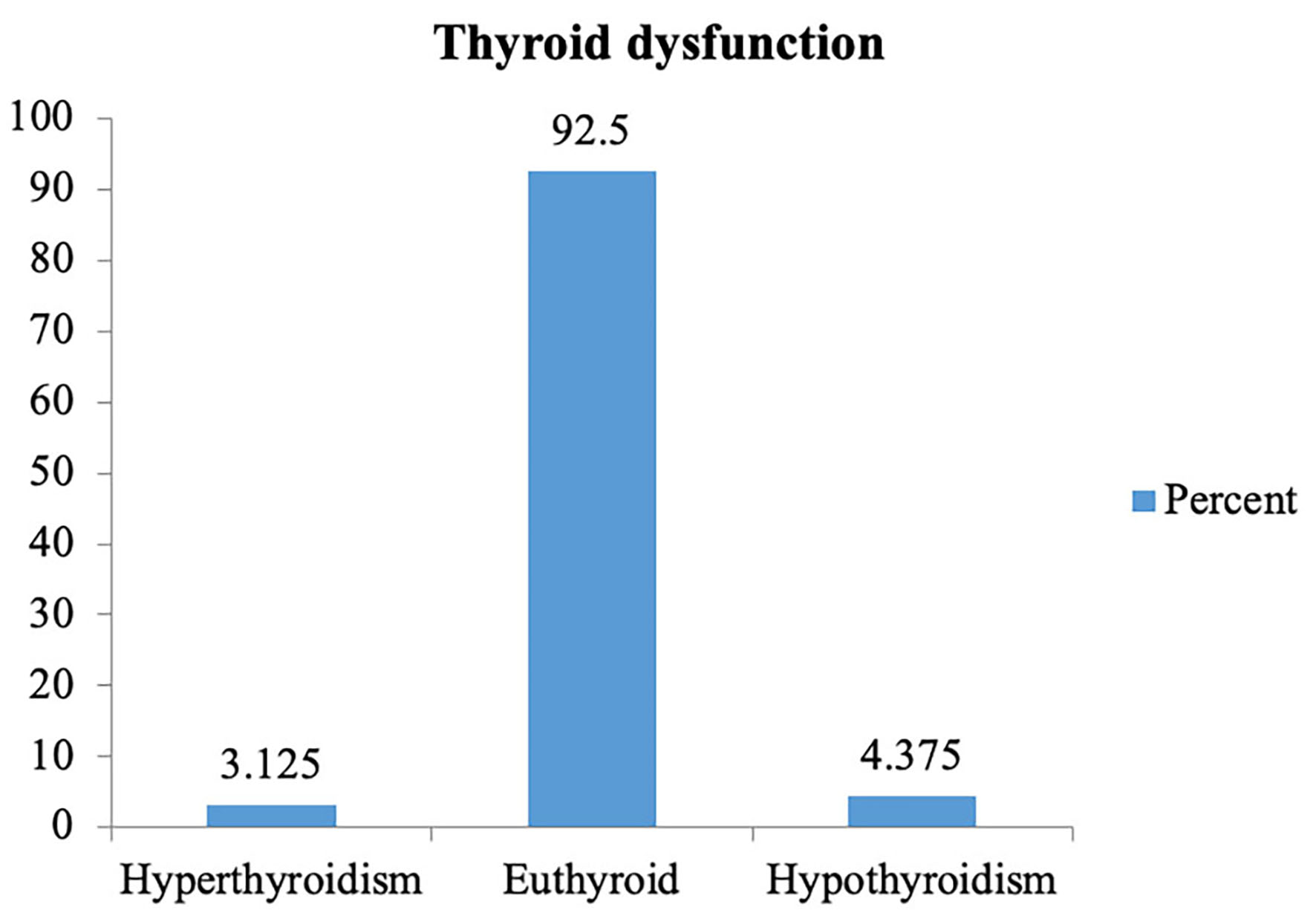 Click for large image | Figure 3. Distribution according to thyroid dysfunction. |
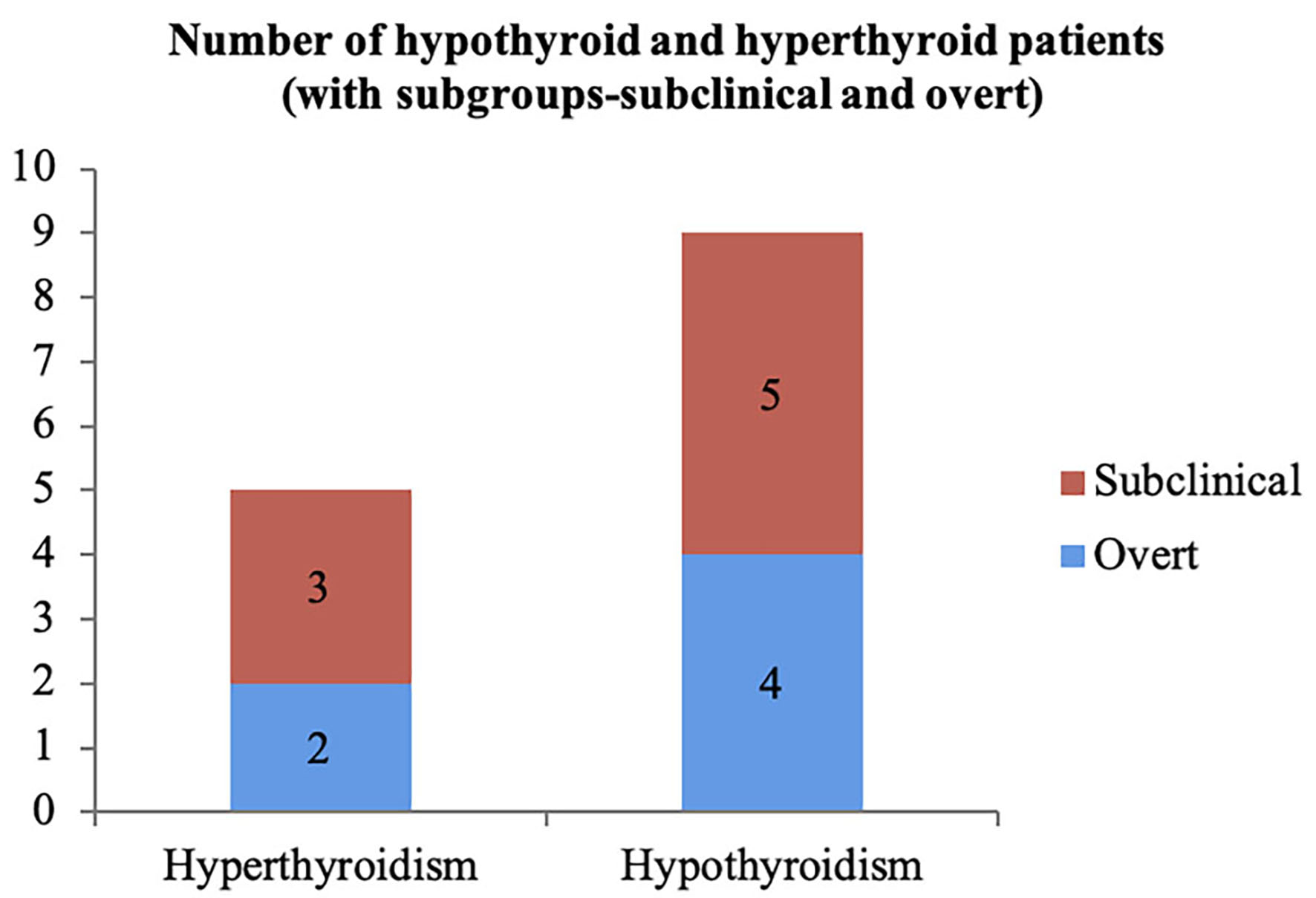 Click for large image | Figure 4. Number of hypothyroid and hyperthyroid cases. |
Karl Pearson correlation coefficient was to estimate the correlation between BMI and TSH which showed statistically significant correlation (P = 0.038).
| Discussion | ▴Top |
Change and/or alteration of thyroid functions during pregnancy can affect the health of the mother as well as the child and have been known to have significant maternal and fetal morbidity and mortality. The effects can extend even beyond pregnancy in newborns affecting their neuro-intellectual development [18]. Therefore, the burden of thyroid disorders in pregnant females should be evaluated. In this study, the total 160 pregnant women in first trimester were included.
The mean age of patients, among 160 pregnant females, was 25.83 years, which was comparable to the study done by Altomare et al [19]. Similar findings were seen in studies by Krishnamma et al [20] and Pahwa et al [21]. However, the mean age was 31.6 years in a study done by Al Shanqeeti et al [22]. BMI of the patients included in our study population was comparable to studies done by Tania et al [18].
Most of the pregnant females included in the study were primigravida (53.125%) and 46.87% of them were multigravida. However, in a study done in 2014 by Tania et al [18] in Bangladesh, 56% of the pregnant females in the first trimester were multigravida (56%). In contrast to our study, another study by Murty et al [23] had multigravida females more than primigravida (53% and 47% respectively).
The overall prevalence of thyroid disorders in first trimester of pregnancy in our study was 8.75% among which 3.125% of the females had subclinical hypothyroidism, 2.5% had overt hypothyroidism, 1.875% had subclinical hyperthyroidism and 1.25% had overt hyperthyroidism. A similar study done by Saraladevi et al [17] in India reported prevalence of 11.6% among which 6.4% had subclinical hypothyroidism and 2.8% had overt hypothyroidism which was higher than our study. Similarly, prevalence of subclinical hyperthyroidism and overt hyperthyroidism was 1.8% and 0.6% respectively which was comparable to our study. One of the studies done by Thanuja et al [24] reported prevalence of thyroid disorders as 5% where the occurrence of subclinical and overt hyperthyroidism in our study was 1.3% of the females with subclinical and 2% with overt hyperthyroidism respectively while 0.7% with subclinical and 1% of the females with overt hypothyroidism respectively. The overall prevalence of thyroid disorder along with prevalence of subclinical and overt hypothyroidism was higher in our study as compared to the above study, meanwhile the prevalence of overt and subclinical hyperthyroidism (1.25% and 1.875%) was comparable. Similar findings for hypothyroidism were reported by Dieguez et al [25] in their study (overt hypothyroidism (1.9%), subclinical hypothyroidism (3.6%)); meanwhile prevalence of overt (0.9%) and subclinical (0.8%) hyperthyroidism was not consistent with our study.
Other several studies done around the world showed prevalence of subclinical hypothyroidism in pregnant women ranged from 6.0-16.13% [20-23, 26-28]. Some of the studies showed prevalence of overt hypothyroidism to be 2.0-2.8% [21, 28] which is similar to our study; however, other studies showed prevalence of overt hypothyroidism to be 3.28-3.9% [20, 23] slightly higher than in our study.
In this study, the correlation between BMI and TSH was tested by using Karl Pearson correlation coefficient. There was statistically significant correlation with coefficient of 0.164 (P = 0.038). Similar significant correlation (r = 0.254 and P = 0.034) between BMI and TSH level was reported by a study done by Kumar et al [29] among 210 pregnant females. Similarly, another study also showed high BMI or obesity to be related to thyroid dysfunction [30].
Shrestha et al [31] in their study regarding pregnancy outcomes in patients with hypothyroidism have found recurrent abortions, antepartum hemorrhage, preeclampsia, intrauterine growth restriction (IUGR), and preterm delivery among 239 pregnant females included in their study. Similarly, pregnant females with hyperthyroidism are known to have increased odds of gestational hypertensive disorders, cesarean sections, placental abruptions, preterm births, small-for-gestational-age newborns, and neonatal intensive care unit treatment [32]. Therefore, early diagnosis and initiation of treatment is very essential for the successful pregnancy outcome and reduced morbidity and mortality. Based on the findings of this study, we can conclude that thyroid disorders are very common during pregnancy and early diagnosis and treatment are needed to prevent maternal or fetal morbidity and mortality associated with it.
Conclusion
Based on the findings of our study, one in every 12 pregnant females in the first trimester has thyroid disorder. Early diagnosis and prompt initiation of treatment are very essential for good maternal and fetal outcome in pregnant females diagnosed with thyroid disorders. So, universal screening for thyroid disorders is essential which would play an important role in preventing maternal complications and improving fetal outcome.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
All subjects provided written informed consent.
Author Contributions
SM and SK designed and performed the study. SM and SD drafted the manuscript and did critical editing. SK, PY, SU, BB, UT, SM and SD reviewed and edited the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Baral N, Lamsal M, Koner BC, Koirala S. Thyroid dysfunction in eastern Nepal. Southeast Asian J Trop Med Public Health. 2002;33(3):638-641.
pubmed - World Health Organization. Assessment of the iodine deficiency disorders and monitoring their elimination. A guide for programme managers. WHO, Geneva. 2007
- Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12(10):839-847.
doi pubmed - Wiersinga WM. Subclinical hypothyroidism and hyperthyroidism. I. Prevalence and clinical relevance. Neth J Med. 1995;46(4):197-204.
doi pubmed - Risal P, Maharjan BR, Koju R, Makaju RK, Gautam M. Variation of total serum cholesterol among the patient with thyroid dysfunction. Kathmandu Univ Med J (KUMJ). 2010;8(30):265-268.
doi pubmed - Fung H, Kologlu M, McGregor AM. Thyroid disease and pregnancy. Practitioner. 1986;230(1419):803-804, 806-807.
pubmed - Klein RZ, Haddow JE, Faix JD, Brown RS, Hermos RJ, Pulkkinen A, Mitchell ML. Prevalence of thyroid deficiency in pregnant women. Clin Endocrinol (Oxf). 1991;35(1):41-46.
doi pubmed - Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31(5):702-755.
doi pubmed - Rugge JB, Bougatsos C, Chou R. Screening and treatment of thyroid dysfunction: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(1):35-45.
doi pubmed - Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105(2):239-245.
doi pubmed - Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12(1):63-68.
doi pubmed - Longmore M, Wilkinson I, Turmezei T, Cheung C. Oxford Handbook of Clinical Medicine. 7th ed. Oxford press; 2010. 200.
- Barse SP, Korde VR, Barla JS. Prevalence of thyroid dysfunction in pregnancy and its impact on maternal and fetal outcome: a prospective study in a tertiary care hospital in Maharashtra, India. Int J Reprod Contraception, Obstet Gynecol. 2020;9(5):1938
- LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. 2006;35(1):117-136.
doi pubmed - Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck Keizer-Schrama SM, Hooijkaas H, et al. Maternal thyroid autoimmunity during pregnancy and the risk of attention deficit/hyperactivity problems in children: the Generation R Study. Thyroid. 2012;22(2):178-186.
doi pubmed pmc - Nazarpour S, Amiri M, Bidhendi Yarandi R, Azizi F, Ramezani Tehrani F. Maternal subclinical hyperthyroidism and adverse pregnancy outcomes: a systematic review and meta-analysis of observational studies. Int J Endocrinol Metab. 2022;20(3):e120949.
doi pubmed pmc - Saraladevi R, Nirmala T, Shreen B. Prevalence of thyroid disorder in pegnancy and pregnancy outcome. 2016;1-11.
- Tania S, Islam F. Screening of thyroid function in the first half of pregnancy. Bangladesh J Obstet Gynaecol. 2016;29(1):26-31.
- Altomare M, La Vignera S, Asero P, Recupero D, Condorelli RA, Scollo P, Gulisano A, et al. High prevalence of thyroid dysfunction in pregnant women. J Endocrinol Invest. 2013;36(6):407-411.
doi pubmed - Krishnamma B, Prabhavathi V, Prasad DK. Prevalence of thyroid dysfunction in pregnant women and the need for universal screening: an observational study in Northern Andhra Pradesh population. Int J Reprod Contraception, Obstet Gynecol. 2017;6(6):2536.
- Pahwa S, Mangat S. Prevalence of thyroid disorders in pregnancy. Int J Reprod Contraception, Obstet Gynecol. 2018;7(9):3493.
- Al Shanqeeti SA, Alkhudairy YN, Alabdulwahed AA, Ahmed AE, Al-Adham MS, Mahmood NM. Prevalence of subclinical hypothyroidism in pregnancy in Saudi Arabia. Saudi Med J. 2018;39(3):254-260.
doi pubmed pmc - Murty NVR, Uma B, Rao J, Sampurna K, Vasantha K, Vijayalakshmi G. High prevalence of subclinical hypothyroidism in pregnant women in South India. Int J Reprod Contraception, Obstet Gynecol [Internet]. 2015;4(2):453.
- Thanuja P, Rajgopal K, Sadiqunnisa D. Thyroid dysfunction in pregnancy and its maternal outcome. IOSR J Dent Med Sci. 2014;13(1):11-5.
- Dieguez M, Herrero A, Avello N, Suarez P, Delgado E, Menendez E. Prevalence of thyroid dysfunction in women in early pregnancy: does it increase with maternal age? Clin Endocrinol (Oxf). 2016;84(1):121-126.
doi pubmed - Dhanwal DK, Prasad S, Agarwal AK, Dixit V, Banerjee AK. High prevalence of subclinical hypothyroidism during first trimester of pregnancy in North India. Indian J Endocrinol Metab. 2013;17(2):281-284.
doi pubmed pmc - Mandal RC, Bhar D, Das A, Basunia SR, Kundu SB, Mahapatra C. Subclinical hypothyroidism in pregnancy: An emerging problem in Southern West Bengal: A cross-sectional study. J Nat Sci Biol Med. 2016;7(1):80-84.
doi pubmed pmc - Kumar K. Prevalence of thyroid disorder in pregnancy and pregnancy outcome. 2018;3(3):1-11.
- Kumar S, Chiinngaihlun T, Singh MR, Punyabati O. Correlation of body mass index (BMI) with thyroid function in euthyroid pregnant women in Manipur, India. J Clin Diagn Res. 2017;11(4):CC13-CC15.
doi pubmed pmc - Han C, Li C, Mao J, Wang W, Xie X, Zhou W, Li C, et al. High body mass index is an indicator of maternal hypothyroidism, hypothyroxinemia, and thyroid-peroxidase antibody positivity during early pregnancy. Biomed Res Int. 2015;2015:351831.
doi pubmed pmc - Shrestha A, Tripathi P, Dongol A. Pregnancy outcomes in patients with hypothyroidism. Kathmandu Univ Med J (KUMJ). 2019;17(65):57-60.
pubmed - Nazarpour S, Ramezani Tehrani F, Simbar M, Azizi F. Thyroid dysfunction and pregnancy outcomes. Iran J Reprod Med. 2015;13(7):387-396.
pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
