| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 12, Number 3, December 2023, pages 88-92
Obstetric Management of a Novel Superimposition of Facial Onset Sensory and Motor Neuronopathy on Parsonage Turner Syndrome
Oluwatomisin Bello Adejugbaa, b, Tanya Cupinoa, Courtney Martina, Ruofan Yaoa
aDepartment of Gynecology and Obstetrics, Loma Linda University Medical Center, Loma Linda, CA 92354, USA
bCorresponding Author: Oluwatomisin Bello Adejugba, Department of Gynecology and Obstetrics, Loma Linda University Medical Center, Loma Linda, CA 92354, USA
Manuscript submitted September 8, 2023, accepted November 1, 2023, published online December 28, 2023
Short title: Obstetric Management of FOSMN on PTS
doi: https://doi.org/10.14740/jcgo909
| Abstract | ▴Top |
Parsonage-Turner syndrome (PTS) is a rare neurologic disorder characterized by pain and weakness in the distribution of the brachial plexus. In severe cases, the phrenic nerve may be involved resulting in hemidiaphragm paralysis. Facial onset sensory and motor neuronopathy (FOSMN) is another rare disease that typically progresses slowly, with facial paresthesias preceding the onset of bulbar symptoms. A lack of corneal reflex is pathognomonic for FOSMN. This case is regarding a 35-year-old G7P1051 with known multi-episodic PTS involving the phrenic nerve that had been successfully managed for several years with intravenous immunoglobulin (IVIG) therapy. She presented at 28 weeks gestational age for an acute PTS flare with left upper extremity pain and weakness, and dyspnea. She received IVIG therapy with improvement of her symptoms and was discharged. She returned the day after discharge with left upper extremity paralysis, dysarthria, facial allodynia, and worsening dyspnea and dysphagia. After further evaluation by the Neurology, she received an additional diagnosis of FOSMN, a condition with limited treatment options. Despite aggressive immunotherapy, her dyspnea worsened, and she developed anarthria, trismus, and aphagia. She was delivered at 31 weeks gestational age due to continued clinical deterioration. By postpartum day 4, her dyspnea and dysphagia were resolved, and her speech was markedly improved. This is the first report of FOSMN onset in pregnancy, superimposition on PTS, and symptom abatement after delivery. This report is unique because it discusses management for conditions for which there are no formal neurologic or obstetric society guidelines on management in pregnancy. Our report highlights that in gravid individuals with maternal disease processes managed with immunotherapy, consideration should be given to the possibility that placental physiology may render previously effective immunotherapy less effective or ineffective during pregnancy.
Keywords: Parsonage-Turner syndrome; Facial onset sensory and motor neuronopathy; Pregnancy; Intravenous immunoglobulin therapy; Neurologic disorders in pregnancy; Autoimmune diseases in pregnancy
| Introduction | ▴Top |
Parsonage-Turner syndrome (PTS), also known as neuralgic amyotrophy or idiopathic brachial plexopathy, is a rare neurologic disorder that is classically characterized by neuropathic pain followed by weakness in the distribution of the brachial plexus secondary to denervation of the affected muscle [1]. Non-classical symptoms may include speech difficulties and respiratory impairment due to ipsilateral phrenic nerve involvement and are seen in a minority of cases [2]. PTS can either be provoked or idiopathic in etiology with provoked cases being associated with abnormal immune responses after a tissue-damaging insult such as surgery or infection [3]. PTS has an estimated incidence of up to 1 per 1,000 per year [4]. There is no definitive treatment for PTS [1]. Management of acute flares primarily focuses on pharmacological or electrostimulation for relief of neurogenic pain paired with physical and occupational therapy to counteract loss of function. There is limited and mixed evidence for the efficacy of steroids or intravenous immunoglobulin (IVIG) therapy for shortening the duration of symptoms but with few other treatment options available, these therapies are often used during PTS flares [5].
An even rarer condition, facial onset sensory and motor neuronopathy (FOSMN), is characterized by progressive allodynia of the face that spreads up toward the scalp and down to the neck and upper trunk followed by facial and bulbar motor nerve dysfunction. The pathognomonic finding of FOSMN is a diminished or absent corneal reflex [6]. A total of 71 cases have been reported ranging from ages 7 to 75, and with variable courses ranging from a few years to several decades [7]. The true incidence is unknown due to limited reports in literature. The leading theory on the pathogenesis of FOSMN is a degenerative process in the nervous system, similar to that seen in amyotrophic lateral sclerosis [7, 8]. However, a variety of studies suggest an underlying autoimmune inflammatory process [7, 9, 10]. The disease course is more benign in some patients, while in others, it is deadly with respiratory insufficiency being the leading cause of death [7]. Furthermore, like PTS, treatment options are limited. Some individuals experience temporary relief of symptoms following IVIG or plasmapheresis; most cases do not respond to these immunomodulatory therapies [6, 7, 11, 12].
In this report we present the case of a 35-year-old gravid woman with idiopathic PTS that had been successfully managed for 14 years with IVIG. She presented at 28 weeks gestational age (WGA) with a PTS flare involving non-classical respiratory symptoms as well as dysphonia that partially responded to IVIG therapy. Further neurologic evaluation revealed loss of her left corneal reflex in addition to bulbar symptoms and new facial allodynia, causing her to be diagnosed with FOSMN. Worsening of some symptoms, and development of new symptoms ultimately necessitated preterm delivery, after which her symptoms rapidly improved. This report is the first known instance in which onset of FOSMN occurred during pregnancy and the first in which FOSMN was diagnosed in a patient with known PTS. It is also the first report of FOSMN symptom abatement after delivery. This case report highlights the challenges faced in management of two concurrent rare neurologic disorders with limited efficacious therapy during pregnancy. It further highlights the necessity of multidisciplinary management of novel patient presentations.
| Case Report | ▴Top |
Investigations
The patient was a 35-year-old G7P1051 who presented to our maternal-fetal medicine (MFM) clinic at 19 WGA as a transfer for higher level of obstetric care for PTS. Her PTS history was notable for diagnosis at age 21 after an episode of left upper extremity (LUE) pain followed by weakness. Review of her external records revealed that two electromyographies had been completed in the years following her initial diagnosis, and both showed “mild left brachial plexopathy”. Over the next 6 years she experienced eleven additional PTS flares (Fig. 1). Her specific disease characteristics included rapid symptom progression from pain to paralysis in as little as 4 h, and response to one to two rounds of 1 g/kg/day of intravenous IVIG therapy. There were no clear precipitating events prior to her flares. After her flare at age 27, she received prophylactic IVIG every 3 - 6 months. She went into full remission for 3 years until her first pregnancy at age 30, where she had a PTS flare at 14 WGA that responded to IVIG. She developed gestational hypertension at 35 WGA, and for that reason, underwent an induction of labor of 37 WGA at which time a second PTS flare occurred. Due to the arrest of dilation and non-reassuring fetal heart tracing, she delivered by primary cesarean section. Her subsequent obstetric history is significant for five spontaneous abortions, one of which was in the context of an active PTS flare at 9 WGA, and after which her PTS symptoms improved.
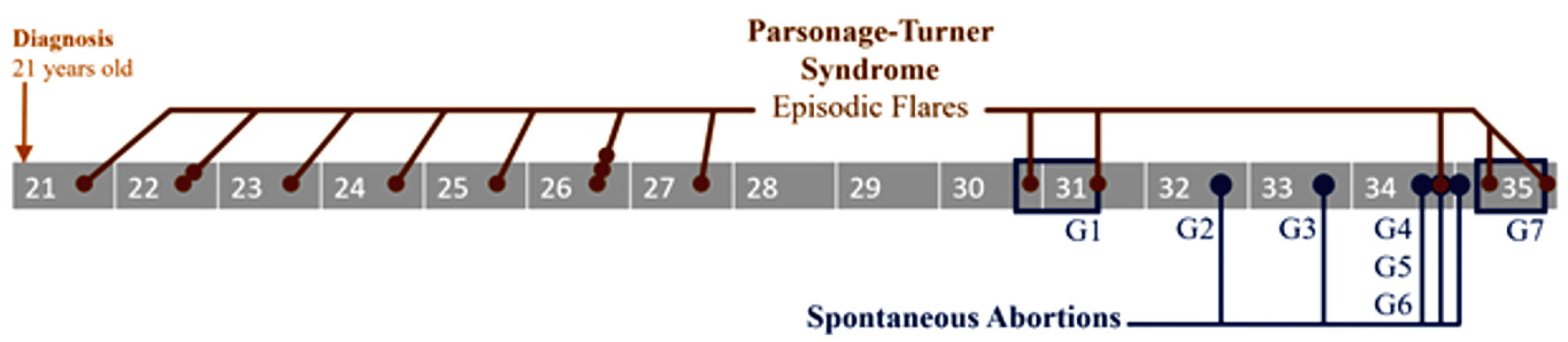 Click for large image | Figure 1. Timeline illustrating episodic Parsonage-Turner syndrome flares (brown dots), from diagnosis at 21 years old to most recent pregnancy (blue box, G7) at 35 years old. Miscarriages following initial pregnancy (blue box, G1) are also shown (blue dots). |
Prior to the current pregnancy, her residual symptoms since diagnosis included minor left arm weakness and minimal dyspnea secondary to left sided diaphragmatic weakness. She had experienced intermittent dysphagia and dysphonia but was asymptomatic at the onset of this pregnancy. She had her first PTS flare of this pregnancy at about 15 WGA that involved progressive left arm weakness and new left facial palsy. That was her first PTS flare with facial involvement. She received a course of IVIG therapy and reported significantly less-than-usual benefit. Upon presenting to our institution for evaluation by MFM at 19 WGA, her history of rapid symptom progression raised concern for unanticipated complications during pregnancy, especially given her phrenic nerve involvement which could lead to respiratory distress that could compromise both the patient and the fetus. The patient was referred by MFM to our Internal Neurology Department to facilitate multidisciplinary management of her PTS in this pregnancy. She was evaluated by the Neurology at 25 WGA and baseline shortness of breath, as well as sensory and motor deficits in the face and LUE were documented. Following discussions between MFM and Neurology, a plan was made for treatment with her usual course of two doses of 1 g/kg/day of IVIG followed by weekly prophylactic IVIG infusions until delivery if she had another flare.
Diagnosis
At 28 WGA, she presented to the Obstetric Emergency Room with acute LUE pain and weakness, dysphonia, and dyspnea that did not require supplemental oxygen. She was admitted to the antepartum service for further monitoring as well as initiation of her usual two doses of 1 g/kg/day IVIG. The patient endorsed new chest heaviness with the onset of the infusion of IVIG on formulary, and this resolved after the infusion rate was slowed down. Consequently, she received her standard two doses over the course of a few days. This slowed infusion rate was not a novel event in her treatment history as she had received slower infusions of certain formulations of IVIG in the past in lieu of her usual regimen due to tolerance considerations with good response. Her symptoms improved but did not resolve over the course of treatment. This was the second episode of her experiencing significantly less-than-usual benefit from IVIG therapy. During this admission, she received a course of betamethasone to accelerate fetal lung maturity in preparation for possible preterm delivery. She was discharged home after a week of admission with a plan to return in a week for prophylactic IVIG infusion.
She re-presented within 24 h, now at 29 WGA, with worsened symptoms. Motor strength in her LUE had diminished to the point of complete paralysis. Motor function testing by the Neurology revealed almost or completely diminished finger, wrist and elbow extension, flexion of the metacarpophalangeal joints and extension of the interphalangeal joints, wrist and elbow flexion, and shoulder abduction, all actions involving nerve roots C5 - T1. She also had new dysarthria and new dysphagia. Sensory testing revealed decreased sensation in the distributions of the ulnar and median nerves. Additionally, the left hemifacial paralysis that initially occurred at 15 WGA had returned and was accompanied by allodynia extending from her scalp to her neck. Her dyspnea was stable from prior admission. She was readmitted and immediately started on a slowed infusion of IVIG for tolerance considerations. The next morning, her dysarthria had worsened to anarthria, and she was completely reliant on written text for communication. Over the course of a few days, she developed progressive left eye pain followed by weakness of the surrounding extraocular muscles. She was re-examined by the Neurology in light of her new symptoms, including significant bulbar symptoms, deviant from classical PTS syndrome. She was diagnosed with FOSMN at 30 WGA after being found to have absent left corneal reflexes in addition to her prominent bulbar symptoms and allodynia consistent with the syndrome.
Treatment
Given the new diagnosis of FOSMN, Neurology recommended IVIG of 0.4 g/kg/day for 5 days and a 9-day 60 - 40 - 20 mg/day oral prednisone taper. The dose of IVIG was changed from 1 g/kg/day to 0.4 g/kg/day, given the need to slow down the infusion rate over the last several days for patient tolerance. The patient did note a trial of steroids, route, and duration uncertain, 2 years after her initial diagnosis of PTS with no benefit. After 5 days of dual therapy, her symptoms did not improve, and her previously stable dyspnea began to worsen, raising concern for impending respiratory compromise. This, in turn, raised concern for preterm delivery in the event of further deterioration. A rescue course of betamethasone was given in lieu of her ongoing prednisone taper once it had been 14 days since the initial betamethasone course. Fetal monitoring via nonstress tests was performed at least twice daily for the entirety of her admission. On the day after completion of the steroid taper, the patient had several episodes of trismus and aphagia which required her to be made non per os. After further discussions between MFM and Neurology, it was decided that her continuing deterioration despite prolonged and aggressive treatment necessitated delivery. She underwent an uncomplicated repeat cesarean section at 31 weeks 5 days.
Follow-up and outcomes
By postpartum day 2, the patient demonstrated symptomatic improvement. She was able to speak and swallow 4 days after delivery. Per recommendations from Occupational and Physical Therapy, she was discharged to an acute inpatient rehabilitation facility and made rapid progress. By her 6-week postpartum visit she was nearly back to her pre-pregnancy baseline for motor strength and sensory function. Her infant did well and was discharged from the neonatal intensive care unit 6 weeks after birth.
| Discussion | ▴Top |
A noteworthy question that arose during our evaluation of this patient was whether her initial PTS diagnosis was accurate given some overlapping symptomatology with other entities such as FOSMN. It is our belief that this patient had both disease processes going on concurrently as opposed to an initial wrong diagnosis. However, the true time of onset of FOSMN is unclear. The PTS component was not in question given her longstanding weakness in the brachial plexus distribution that fit the diagnostic criteria for PTS. The new diagnosis of FOSMN was based on her new allodynia, prominent bulbar symptoms, new facial palsy in this pregnancy and the ultimate pathognomonic finding of absent left corneal reflexes. Per external records, she had experienced dysphagia and dysphonia in the past. These bulbar symptoms and her ipsilateral phrenic nerve weakness were previously thought to be further presentations of a non-classical PTS; however, our team remains curious as to whether these were early presenting symptoms of FOSMN in this patient.
The primary take-away from this case is that in gravid individuals with maternal disease processes managed with immunotherapy, consideration should be given to the possibility that placental physiology may render previously effective immunotherapy less effective or ineffective during pregnancy. Immunoglobulin G (IgG) is known to cross the placenta from maternal circulation into fetal circulation, and IVIG is used in pregnancy for conditions such as fetal-neonatal alloimmune thrombocytopenia, and prevention of hemolytic disease of the newborn [13-15]. There is evidence of IgG placental crossing occurring down a concentration gradient which leads to high enough concentrations of IVIG in the fetal compartment and thus efficacy for fetal disease processes [13]. However, in patients like ours where IVIG is being administered for a maternal disease process, transfer of IVIG down the placental concentration gradient may render IVIG levels in the maternal compartment subtherapeutic. We posit that this process may have contributed to our patient’s PTS symptoms being unresponsive to immunotherapy during pregnancy when they were known to be responsive to immunotherapy prior to pregnancy. Furthermore, if her FOSMN was of the variety that should have responded to immunotherapy, her gravid states may have prevented a response due to the aforementioned process. In the future, consideration should be given to higher dose or higher frequency of dosage of IVIG for gravid patients receiving immunotherapy for maternal indications.
An additional take-away from this case is that in a gravid individual with a rare or complex illness, early involvement of a multidisciplinary team is critical in identifying and managing changes in status, including diagnosis of new-onset disorders. The limited information about the pathogeneses and definitive treatments of PTS and FOSMN contributed to the novelty of managing this patient. Our approach to managing this patient included early involvement of MFM and Neurology after her initial referral from an outside community hospital to our generalist obstetrics clinic. This allowed for early formulation of a multidisciplinary management plan in the event of future PTS flares and through to delivery. Thorough documentation of baseline neurologic exam also allowed for accurate detection of new exam findings and facilitated the diagnosis of a concurrent rare neurologic disorder.
Furthermore, this case highlights the need for continual weighing of the risks of worsening maternal debilitation in the setting of continued pregnancy against the risks of neonatal prematurity in patients with rare conditions, for which no definitive efficacious treatment exists. In this case, early awareness of the possibility of preterm delivery and subsequent administration of initial and rescue courses of betamethasone allowed the neonate to acquire the many benefits of antenatal corticosteroid therapy prior to its preterm delivery.
It is noteworthy that both her PTS and FOSMN symptoms improved rapidly shortly after she delivered. This was the second instance in this patient in which her symptoms resolved after pregnancy, the first being after completion of a spontaneous abortion at 9 WGA. In this patient, this pattern raises the possibility of a pregnancy-related immunologic dysregulation triggering her symptoms, with resolution of dysregulation, and thus symptoms, upon completion of pregnancy. Additionally, while there have publications on viruses such as hepatitis E and cytomegalovirus acting as infectious triggers for the immunologic dysregulation causing PTS, no such relationships have been identified in FOSMN and this would be a meaningful area for future exploration [16, 17].
Learning points
In gravid individuals with maternal disease processes managed with immunotherapy, consideration should be given to the possibility that placental physiology may render previously effective immunotherapy less effective or ineffective during pregnancy. Furthermore, in gravid individuals with a rare or complex illness, early involvement of a multidisciplinary team is critical in identifying and managing changes in status, including diagnosis of new-onset disorders. Lastly, there is a need for continual weighing of the risks of worsening maternal debilitation in the setting of continued pregnancy against the risks of neonatal prematurity in patients with rare conditions for which no definitive efficacious treatment exist.
Acknowledgments
We acknowledge our colleagues from the Department of Neurology, Loma Linda University Medical Center, Loma Linda, CA, USA.
Financial Disclosure
All authors have no financial disclosures. All authors have no financial and non-financial competing interests. This case report required no funding.
Conflict of Interest
None to declare.
Informed Consent
The patient gave written informed consent for the publication of this case report.
Author Contributions
Oluwatomisin Bello Adejugba and Tanya Cupino contributed to the literature review and writing of the manuscript. Ruofan Yao and Courtney Martin contributed to the study design as well as revision of final manuscript.
Data Availability
Data generated during the current case are available from the corresponding author upon reasonable request.
Abbreviations
PTS: Parsonage-Turner syndrome; FOSMN: facial onset sensory and motor neuronopathy; LUE: left upper extremity; IVIG: intravenous immunoglobulin; MFM: maternal-fetal medicine; WGA: weeks gestational age
| References | ▴Top |
- Feinberg JH, Radecki J. Parsonage-turner syndrome. HSS J. 2010;6(2):199-205.
doi pubmed pmc - van Alfen N, van Engelen BG. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(Pt 2):438-450.
doi pubmed - Minas V, Aust T. Idiopathic brachial plexus neuritis after laparoscopic treatment of endometriosis: a complication that may mimic position-related brachial plexus injury. J Minim Invasive Gynecol. 2013;20(6):891-893.
doi pubmed - van Alfen N, van Eijk JJ, Ennik T, Flynn SO, Nobacht IE, Groothuis JT, Pillen S, et al. Incidence of neuralgic amyotrophy (Parsonage Turner syndrome) in a primary care setting—a prospective cohort study. PLoS One. 2015;10(5):e0128361.
doi pubmed pmc - Naito KS, Fukushima K, Suzuki S, Kuwahara M, Morita H, Kusunoki S, Ikeda S. Intravenous immunoglobulin (IVIg) with methylprednisolone pulse therapy for motor impairment of neuralgic amyotrophy: clinical observations in 10 cases. Intern Med. 2012;51(12):1493-1500.
doi pubmed - Zheng Q, Chu L, Tan L, Zhang H. Facial onset sensory and motor neuronopathy. Neurol Sci. 2016;37(12):1905-1909.
doi pubmed - Hu N, Zhang L, Yang X, Fu H, Cui L, Liu M. Facial onset sensory and motor neuronopathy (FOSMN syndrome): Cases series and systematic review. Neurol Sci. 2023;44(6):1969-1978.
doi pubmed - de Boer EMJ, Barritt AW, Elamin M, Anderson SJ, Broad R, Nisbet A, Goedee HS, et al. Facial onset sensory and motor neuronopathy: new cases, cognitive changes, and pathophysiology. Neurol Clin Pract. 2021;11(2):147-157.
doi pubmed pmc - Vucic S, Tian D, Chong PS, Cudkowicz ME, Hedley-Whyte ET, Cros D. Facial onset sensory and motor neuronopathy (FOSMN syndrome): a novel syndrome in neurology. Brain. 2006;129(Pt 12):3384-3390.
doi pubmed - Vucic S, Stein TD, Hedley-Whyte ET, Reddel SR, Tisch S, Kotschet K, Cros D, et al. FOSMN syndrome: novel insight into disease pathophysiology. Neurology. 2012;79(1):73-79.
doi pubmed - Hokonohara T, Shigeto H, Kawano Y, Ohyagi Y, Uehara M, Kira J. Facial onset sensory and motor neuronopathy (FOSMN) syndrome responding to immunotherapies. J Neurol Sci. 2008;275(1-2):157-158.
doi pubmed - Knopp M, Vaghela NN, Shanmugam SV, Rajabally YA. Facial onset sensory motor neuronopathy: an immunoglobulin-responsive case. J Clin Neuromuscul Dis. 2013;14(4):176-179.
doi pubmed - Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646.
doi pubmed pmc - Mayer B, Hinkson L, Hillebrand W, Henrich W, Salama A. Efficacy of antenatal intravenous immunoglobulin treatment in pregnancies at high risk due to alloimmunization to red blood cells. Transfus Med Hemother. 2018;45(6):429-436.
doi pubmed pmc - Winkelhorst D, Murphy MF, Greinacher A, Shehata N, Bakchoul T, Massey E, Baker J, et al. Antenatal management in fetal and neonatal alloimmune thrombocytopenia: a systematic review. Blood. 2017;129(11):1538-1547.
doi pubmed - Dartevel A, Colombe B, Bosseray A, Larrat S, Sarrot-Reynauld F, Belbezier A, Lagrange E, et al. Hepatitis E and neuralgic amyotrophy: Five cases and review of literature. J Clin Virol. 2015;69:156-164.
doi pubmed - Mastroianni A, Mauro MV. Parsonage-Turner syndrome and cytomegalovirus disease. Clin Neuropathol. 2022;41(3):135-144.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
