| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 12, Number 3, December 2023, pages 110-116
What to Expect With Pregnant or Postpartum Prescribing of Extended-Release Buprenorphine (CAM2038)
Michelle R. Lofwalla, l, Jessica L. Youngb, Zachary Hansenc, Elisha M. Wachmand, Christine Wildere, f, Constance Guilleg, Jasmin E. Charlesh, i, Lawrence Leemanj, Jessica R. Grayk, T. John Winhusene, f
aDepartments of Behavioral Science and Psychiatry, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, Lexington, KY, USA
bDepartment of Obstetrics and Gynecology, Vanderbilt University Medical Center, Nashville, TN, USA
cDepartment of Family Medicine, Division of Addiction Science, Marshall University, Huntington, WV, USA
dDepartment of Pediatrics, Boston Medical Center, Boston, MA, USA
eDepartment of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, Cincinnati, OH, USA
fCenter for Addiction Research, University of Cincinnati College of Medicine, Cincinnati, OH, USA
gDepartment of Psychiatry and Behavioral Science, Medical University of South Carolina, Charleston, SC 29425, USA
hDepartment of Obstetrics & Gynecology, University of Utah Health, Salt Lake City, UT, USA
iDepartment of Internal Medicine, Program for Addiction Research, Clinical Care, Knowledge and Advocacy (PARCKA), Division of Epidemiology, University of Utah School of Medicine, Salt Lake City, UT, USA
jDepartment of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque, NM, USA
kSubstance Use Disorder Initiative, Department of Psychiatry, and Departments of Medicine and Pediatrics, Massachusetts General Hospital, Boston, MA, USA
lCorresponding Author: Michelle Lofwall, Departments of Behavioral Science and Psychiatry, Center on Drug and Alcohol Research, University of Kentucky College of Medicine, Lexington, KY 40508, USA
Manuscript submitted October 5, 2023, accepted November 22, 2023, published online December 28, 2023
Short title: XR bup (CAM2038) in Pregnancy and Postpartum
doi: https://doi.org/10.14740/jcgo919
| Abstract | ▴Top |
Weekly and monthly CAM2038 (Brixadi®) extended-release subcutaneous buprenorphine (XR bup) has been available in Europe and Australia for several years and was approved by the Food and Drug Administration in May 2023. Little is known about the clinical experience of patients and providers using this new medication during prenatal care. Two cases of pregnant persons with opioid use disorder receiving weekly XR bup in an ongoing randomized multi-site outpatient clinical trial are presented along with a brief review of the pharmacology and literature on XR bup formulations. The cases in pregnancy illustrate how treatment with the weekly formulation is initiated including how to make dose adjustments, which may be necessary given the longer half-life; it takes 1 month to achieve steady state. Injection site pain with medication administration was time limited and managed readily. Other injection site reactions experienced included subcutaneous erythema and induration that was delayed in onset and typically mild, resolving with minimal intervention. Delivery management and breastfeeding recommendations while on weekly XR bup were not different compared to sublingual buprenorphine (SL bup). Weekly XR bup is a new treatment for opioid use disorder that may be used in the obstetric population. Obstetric and addiction medicine clinicians should be aware of this new formulation as its use is expected to increase.
Keywords: Opioid use disorder; Extended-release buprenorphine; Pregnancy; Postpartum
| Introduction | ▴Top |
In the United States, drug overdose is a leading cause of maternal mortality with drug-related maternal deaths increasing by 190% between 2010 and 2019 [1, 2]. To date, sublingual buprenorphine (SL bup) and oral methadone are considered standards of care for perinatal opioid use disorder (OUD); however, there is a lower risk of adverse neonatal outcomes for people using buprenorphine compared to methadone during pregnancy [3]. There are a myriad of barriers to accessing and continuing these morbidity and mortality reducing treatments. Some barriers include stigma, transportation, concerns for diversion/misuse and unintended pediatric exposures, incarceration, legal repercussions and inadequate pharmacy and health care system medication access, and problems tolerating sublingual medications that can worsen pregnancy-associated nausea and olfactory sensitivity [4-6]. Additional treatment options are imperative for pregnant and parenting persons with OUD, especially options that do not require daily dosing because missed doses can result in subtherapeutic medication concentrations that increase risk for craving, withdrawal and return to use. This is particularly important during pregnancy when physiological changes can further decrease medication blood levels [6-8]. Extended-release subcutaneous buprenorphine (XR bup) injections that provide steady continuous buprenorphine exposure may help address some of these challenges and improve maternal and neonatal outcomes among those interested in injectable medications.
In May 2023, the Food and Drug Administration (FDA) approved weekly and monthly XR bup CAM2038 (trade name in USA is Brixadi® and outside the USA is Buvidal®) for OUD treatment. This medication has been available in Europe and Australia for several years but was delayed in the USA due in part to another XR monthly buprenorphine formulation (RBP-6000, Sublocade®) receiving approval first and having market exclusivity. Both monthly formulations and the weekly formulation are allowed to be prescribed in pregnancy and during breastfeeding although the FDA labeling does not provide a specific indication for use in pregnant persons and during breastfeeding.
The FDA determined CAM2038 was safe and efficacious based upon phase 2 and phase 3 studies in non-pregnant adults [9, 10]. A subsequent open-label outpatient observational multi-site study demonstrated high retention at 48 weeks (73.6%) with 68% of participants rating XR bup as better than their previous experience with SL bup [11]. A recent randomized controlled trial (RCT) in emergency departments demonstrated that the weekly formulation as well as SL bup could be started among non-pregnant persons using fentanyl with infrequent reports of precipitated withdrawal [12].
With CAM2038 now available for use in the USA since September 2023, it is expected that obstetric populations may be interested in this treatment. Thus, this paper: 1) reviews the indications, dose options, relevant solvents, general use and pharmacology of CAM2038; 2) briefly reviews RBP-6000 pregnancy studies; and 3) provides two cases of the use of weekly CAM2038 in pregnancy with a subsequent discussion to help providers learn about this new medication option.
CAM2038 comes in two different subcutaneous formulations both indicated for treatment of moderate-severe OUD. Its FDA Risk Evaluation and Mitigation Strategy (REMS) requires the medication never to be in the hands of the patient because self-administration into a vein or artery may be life-threatening. If medication enters the bloodstream, it could cause thrombosis or an embolism. The medication is stored at room temperature. The weekly formulation has doses of 8, 16, 24 and 32 mg, which approximates ≤ 6, 8 - 10, 12 - 16, and 18 - 24 mg daily doses of SL bup, respectively (for review of plasma level comparisons between SL and all XR bup formulations, see Coe et al [13]). The peak maximum bup concentration occurs within 24 h and slowly declines over the week (Fig. 1a, b). The terminal plasma elimination half-life is 3 - 5 days. Figure 2 shows how the concentration of weekly XR bup accumulates compared to daily SL bup. Weekly XR bup can be initiated on the first day of treatment. A single injection can be administered subcutaneously after a single SL bup dose to ensure tolerability (e.g., no precipitated withdrawal). Injection sites include the upper arm, abdomen, buttock, and thigh, but the upper arm site should be used only after steady-state has been achieved (i.e., 4 weekly doses) because this site is associated with 10% lower plasma levels than the other sites [14]. Low dose ethanol is the solvent in weekly CAM2038. Specifically, ethanol doses are 15, 31, 46 and 61 mg in the 8, 16, 24, and 32 mg XR bup doses, respectively. One standard drink contains 14 g of ethanol. Thus, a pregnant person receiving the highest weekly XR bup dose would be exposed to a fraction of 1 standard drink over the entire course of the pregnancy. While there is no safe amount of ethanol in pregnancy, the Medication treatment for opioid use disorder in expectant mothers (MOMS) study is being conducted under an FDA investigational drug application, and FDA required use of weekly CAM2038 during pregnancy and when breastfeeding rather than the monthly product that contains the solvent, N-methyl-2-pyrrolidone (NMP) [15].
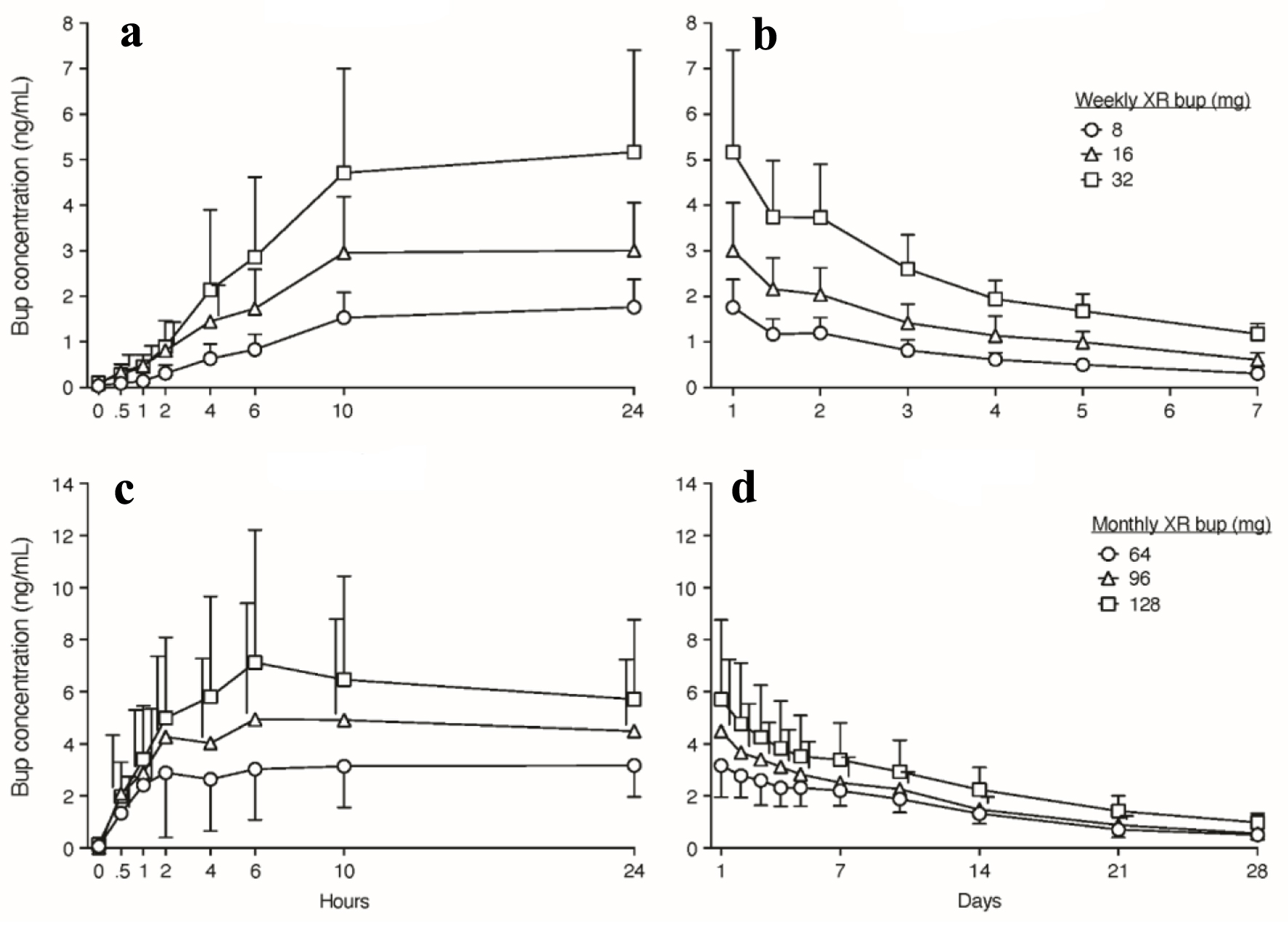 Click for large image | Figure 1. Buprenorphine concentrations over time after weekly and monthly XR-buprenorphine administration. Data displayed are means with standard deviations (SDs) in one direction and staggered to the side of the mean value when needed to preserve figure clarity. (a, b) Buprenorphine (Bup) concentrations (ng/mL) over the first 24 h and 7 days, respectively, after administration of weekly XR-bup. (c, d) Bup concentrations over the first 24 h and 28 days, respectively, after administration of monthly XR-bup. CAM2038 Investigators Brochure. Braeburn Inc. Edition 17. April 5, 2023. |
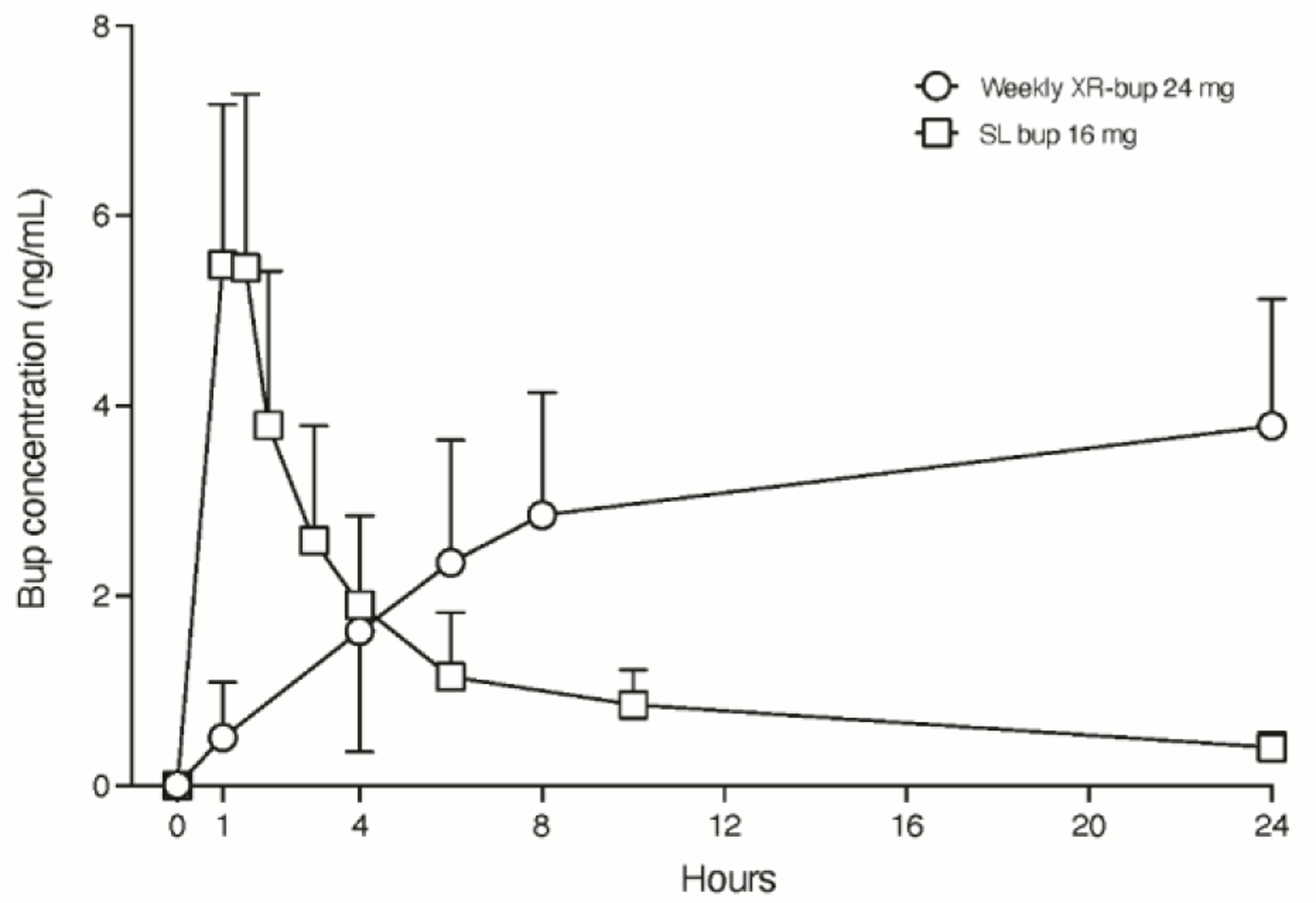 Click for large image | Figure 2. Linear plasma buprenorphine (bup) concentration time curve over 24 h after single dose administration of weekly XR-bup 24 mg and sublingual bup 16 mg. Data displayed are means and standard deviations in one direction to preserve figure clarity. CAM2038 Investigators Brochure. Braeburn Inc. Edition 17. April 5, 2023. |
The monthly formulation of CAM2038 comes in 64, 96, and 128 mg doses approximating 8 - 10, 12 - 16, and 18 - 24 mg of daily SL bup, respectively and is intended for those already stable on buprenorphine. Peak concentration occurs within 6 - 8 h and slowly declines over the month (Fig. 1c, d). The apparent terminal plasma half-life is 19 - 26 days. Steady state is achieved after 4 months of treatment. Monthly XR CAM2038 doses contain NMP, which is also in monthly RBP-6000, and many other pharmaceuticals [16]. RBP-6000 FDA labeling states that 100 mg and 300 mg doses contain 278 and 833 mg of NMP, respectively, and that in preclinical studies, NMP can produce preimplantation losses, delayed ossification, developmental delays and reduced cognitive function such that pregnant persons should be advised of the potential risk to a fetus. Monthly CAM2038 contains NMP doses ranging from 57 to 115 mg per injection with lower amounts in the lower doses. Neither monthly (nor weekly) XR formulation is contraindicated in pregnancy or while breastfeeding. We are not aware of teratogenic effects caused by XR bup formulations in humans although there are limited data. Thus, clinicians are advised to use shared decision making and balance the relative risks and benefits of treatment with the various XR and SL formulations and alternatives (e.g., methadone, no medication, etc.) based on each patients’ history and treatment needs.
The use of RBP-6000 in pregnancy was investigated in an open-label trial that stopped early after the first three patients reported feeling euphoric over days 1 - 3 followed by reports of withdrawal from days 6 - 10 and requests for supplemental buprenorphine over days 7 - 28 [17]. A case report also describes two individuals with unplanned pregnancies who experienced first trimester RBP-6000 exposure after which they declined further XR bup and SL bup [18]. Both individuals had buprenorphine and/or norbuprenorphine detectable in their urine at delivery highlighting its long apparent terminal plasma half-life of 43 - 60 days per FDA labeling.
We now report on two cases from an ongoing multi-site pragmatic open-label two-arm clinical trial titled MOMS that enrolled pregnant persons aged 18 - 41 years old with opioid use disorder and a single fetus pregnancy at an estimated gestation age of 6 - 30 weeks at time of randomization [15]. Participants were randomized to either SL bup with/without naloxone or to XR bup throughout pregnancy and 12 months postpartum. For individuals not already on bup, the protocol required a single dose of 4 mg SL bup followed by a single weekly 16 mg XR bup injection. Due in part to time required for screening and informed consent, most pregnant persons were receiving SL bup at the time of randomization. Their starting XR bup dose approximated their current total daily SL dose. The 8 mg weekly injection was used between the scheduled weekly doses to increase the dose if needed (e.g., to mitigate opioid craving and/or withdrawal symptoms). After delivery, postpartum individuals receiving XR bup who were not breastfeeding could elect to transfer to monthly CAM2038. These cases were chosen to highlight common topics discussed during study implementation.
| Case Reports | ▴Top |
Case 1
A 30-year-old gravida 3 para 1011 with OUD (severe, heroin, IV route), stimulant use disorder (severe, methamphetamine, IV route, in remission), major depressive disorder and sleep difficulties presented at 25 weeks of gestation maintained on SL bup 8 mg twice daily with no non-prescribed opioid use for 8 months. Also, she was taking oral quetiapine 100 mg nightly for sleep, venlafaxine XR 150 mg daily for depression, clonidine 0.1 mg thrice daily as needed for opioid withdrawal symptoms, cyclobenzaprine 5 mg thrice daily as needed for opioid withdrawal symptoms, cetirizine 10 mg daily as needed for allergic rhinitis and a prenatal vitamin daily.
She received XR bup 24 mg at her first study visit to approximate her current dose of 16 mg SL bup daily and stopped all SL bup. Three days after this XR dose, the patient reported mild fatigue, body aches, chills, sweating and hot flashes that were treated as opioid withdrawal with a supplemental weekly XR bup 8 mg dose per study protocol. These symptoms decreased in severity after this supplemental dose but reemerged about a day prior to the regularly scheduled weekly dose. The clinician and patient decided to continue the weekly scheduled dose of XR bup 24 mg (alternative option was to increase the weekly scheduled dose to XR bup 32 mg). The clinician explained that the weekly buprenorphine doses “stack up” on each other until steady state buprenorphine levels are achieved after about a month. The patient was provided time to ask questions, was involved in individual and group therapy and received peer recovery support services while living in supportive recovery housing. For the next 3 weeks, an 8 mg weekly XR bup booster was administered, and the patient reported gradual and consistent improvement in her withdrawal symptoms. No further booster doses were needed after the fifth weekly XR bup 24 mg dose. At no point in her care did the participant report relapse, significant cravings, or a desire to increase her standing 24 mg weekly dose. She subsequently delivered a term healthy infant (3,780 g, 1- and 5-min Apgars of 7 and 9, respectively) at 39+1 weeks by vaginal delivery with an epidural with no complications. Infant was formula fed and monitored for 7 days in a specialized unit for neonatal withdrawal. Modified Finnegan scoring showed a peak 24-h average score of 11.1 on day 5 and was 10.1 on day 6, the day prior to discharge. No treatment outside therapeutic handling and provision of a low stimulation environment was initiated to treat the infant’s withdrawal symptoms. Mom elected to continue weekly 24 mg XR bup.
Case 2
A 35-year-old gravida 2 para 0101 pregnant person with severe OUD, chronic hypertension, and history of prior cesarean delivery presented to an affiliated hospital for medically managed withdrawal from intranasal heroin during the early second trimester. Her drug of choice was oxymorphone, but she had primarily been using heroin prior to her admission. Her initial goal was to undergo medication assisted withdrawal but after education on treatment options she opted for agonist treatment and was initiated onto SL bup. She was discharged on 12 mg SL bup and enrolled at 18 weeks into an outpatient-based OUD treatment program for pregnant people. In this program, she obtained both prenatal care and substance use disorder treatment including buprenorphine MOUD, mental health assessments and medication, peer support, as well as group and individual therapy. She was offered enrollment in the MOMS trial and randomized to XR bup. She expressed concern about potential discomfort with the injection but was looking forward to not taking medication daily. She received her first study injection at 24 weeks. At her return visits, she described preferring XR bup to SL bup due to better control of cravings and decreased ability to overuse or misuse the medication. However, she complained of moderate stinging, throbbing pain with administration of each XR injection that lasted for 5 - 10 min during and post injection. Topical lidocaine 4% cream was applied to the injection site 15 min prior to XR bup administration, which helped to diminish the discomfort with injection. Six weeks after the first XR bup dose was given, there were delayed site reactions at seven previous injection sites which consisted of itching, erythema, and up to 2 cm of induration with no evidence of systemic reactions (e.g., no fever, lymphadenopathy). Her site reactions were primarily on her abdomen and thighs despite having received injections in all available sites during the study. These were managed with hydrocortisone cream 1% applied topically to affected areas and resolved completely within 2 - 6 weeks. She delivered at 35.6 weeks gestational age by repeat cesarean section due to severe preeclampsia and breech position. Apgars were 7 and 8 at 1 and 5 min, respectively, and baby weighed 2,175 g. Baby was admitted to the neonatal intensive care unit (NICU) for management of respiratory distress due to prematurity and was transferred to pediatric hospitalist team at day 3. At day of life (DOL) 3, baby was diagnosed with neonatal abstinence syndrome (NAS) for elevated Finnegan scores of 8 (twice) and given one dose of prn morphine. On DOL 4, baby was given additional prn dose of morphine for elevated Finnegan score. On DOL 5, baby was started on scheduled oral morphine for Finnegan scores of 6 - 11 overnight. Baby was maintained on oral morphine of 0.03 mg/kg every 3 h for 24 h and then tapered daily by 10%. Morphine dosing was stopped on DOL 11 and discharged on DOL 12. Our patient’s initial plan was to formula feed baby but after support from the lactation specialist she was able to pump and successfully give baby expressed breast milk as well as formula.
Operative and postoperative pain were managed using standard OUD anesthesia protocols. The last delayed site reaction occurred 2 days after delivery and resolved after 3 weeks. She continued study XR bup until completing the study at 12 months postpartum. The participant had no additional injection site reactions and continued to voice a preference for the injection. She had no return to use of illicit opioids during pregnancy or in the 12 months postpartum. After study completion, she transitioned to RBP-6000, since CAM2038 was not clinically available.
| Discussion | ▴Top |
Our study adds to the growing case reports of XR bup among pregnant and postpartum individuals and aims to educate clinicians who may care for pregnant patients and their infants about the use and impact of CAM2038, particularly the weekly formulation. The MOMS study has completed enrollment and the last participant will finish the study in late November of 2024, with primary outcome data available in 2025. Neurodevelopmental outcomes from the first 2 years of the babies exposed to XR bup will be available later.
We acknowledge the limitations of presenting only two cases from this study who both started treatment after the first trimester. Specifically, we cannot draw broad conclusions about CAM2038 on outcomes such as overall safety, teratogenicity risk, or neonatal opioid withdrawal syndrome; however, there were no planned interim analyses for this ongoing trial. The lessons that we can share so far from our patients, clinicians and study teams include the importance of explaining the pharmacology of the XR medication using words and pictures (Fig. 2) in lay terms, including possible subjective differences that patients who are already familiar with SL bup may experience. Patients should be reassured that doses can still be titrated upwards using additional 8 mg weekly CAM2038 injections and that reaching their optimal dose may take time. We encourage providers to allow patients to choose the injection site but remember to rotate the injection sites and wait 8 weeks before returning to the same site. The gravid abdomen is an acceptable injection site, but injections in the arms should not occur until after a month of weekly dosing. For those with a fear of needles, it is important to explain that the needle is short (1/2 inch), thin (23-gauge) and the amount of fluid (< 1 cc) injected under the skin is small. These strategies have been helpful for educating and alleviating concerns among some patients who may be familiar with other XR OUD medications that are larger volume with larger needles (e.g., depot naltrexone).
Our study sites all provided collaborative multidisciplinary care and involved obstetricians with experience and comfort treating pregnant persons with OUD with buprenorphine [19]. Given the ongoing opioid epidemic, treatment need, and removal of the federal requirement for a waiver to prescribe buprenorphine for OUD, we anticipate that non-addiction medicine boarded obstetricians and other clinicians (e.g., lactation consultants) will be increasingly called upon to provide best practice treatment in this patient population (e.g., become knowledgeable about OUD treatment as well as breastfeeding recommendations in the setting of substance use, substance use disorder, and medication treatments) such that ongoing education is critical [20]. In addition, education on how XR bup is appropriately obtained (e.g., via FDA REMS-certified specialty pharmacy), logged when received and administered (e.g., recording of each dose received, date of receipt and administration, expiration date, lot number) and stored in a DEA schedule 3 compliant manner (e.g., CAM2038 doses stored in a locked room in a locked cabinet at room temperature).
Our clinical teams emphasized the importance of counseling and ancillary services to assist with non-pharmacologic aspects of OUD treatment. Extinction of behaviors such as non-prescribed opioid use and complete suppression of intrusive cravings for opioids cannot always be adequately addressed with medication alone and ongoing non-prescribed use is common in early OUD treatment [21]. This is not an indication to stop medication treatment.
Injection site reactions reported herein were like those reported in the phase 3 RCT of XR bup among non-pregnant adults - generally mild-moderate severity and resolving with minimal medical intervention [9]. The standard protocols for managing pain during and immediately after delivery for patients with OUD on buprenorphine and for managing neonatal opioid withdrawal were employed without needing to change these protocols to accommodate the XR bup dosing. The decision to only use the weekly XR bup during pregnancy and while breastfeeding was because it lacked NMP; however, it is important to note that NMP is rapidly released into the blood stream after injection and may be present in breastmilk for only a limited time. Future research could evaluate the pharmacokinetic profile of NMP in breastmilk for both monthly XR formulations to determine if pumping breastmilk for a day or two after injection and disposing of it may be a feasible solution for breastfeeding individual’s wanting to receive monthly XR bup but not expose their infants to NMP in breastmilk.
Conclusions
With XR bup now available to clinicians since September 2023, it is worth considering how the new medication may affect treatment of pregnant and postpartum individuals with OUD and how it can be incorporated into standard obstetric practice. The case studies presented here are designed to introduce how to approach using XR bup in the pregnant and postpartum individual, anticipate side effects, and provide a framework from which clinicians can feel more comfortable engaging in shared decision making with patients who are pregnant and have OUD.
Learning points
The weekly XR bup formulation has several dose options and dose adjustments can be made using this formulation. When starting XR bup, it is important to understand and manage patient expectations, explaining the pharmacology in terms patients can understand. The lack of daily peak and trough levels experienced with SL bup may create a different subjective experience in patients who have previously taken SL bup.
Acknowledgments
The authors would like to acknowledge the patients and study staff without whom the case report would not be possible.
Financial Disclosure
This work was supported by the National Institute of Health through the NIH HEAL Initiative under award numbers UG1DA013732 to the University of Cincinnati (T. John Winhusen), UG1DA049444 (Adam Gordon and Gerald Cochran), UG1DA015831 (Roger Weiss and Gail D’Onofrio), UG1DA013727 (Kathleen Brady and Matthew Carpenter), and UG1DA049468 (Kimberly Page). The work was also supported by NIDA contracts to the CCC (HHSN271201500065C) and DSC (HHSN271201400028C). The Publications Committee of the National Drug Abuse Treatment Clinical Trials Network reviewed and gave approval for submission of this manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or its NIH HEAL Initiative. The cases reported in this paper are from a clinical trial (NCT03918850) for which CAM2038 was donated by Braeburn. Braeburn gave permission to use Figures 1 and 2 from their Investigator Brochure.
Conflict of Interest
Lofwall: research consultant for treatments in development for substance use disorder for Titan Pharmaceuticals, Journey Colab, Berkshire Biomedical and Braeburn. Wilder: none. Young: none. Gray: none. Guille: Visiting Scientist and Research Consultant for Maven Clinic. Leeman: none. Charles: serves as a medical consultant for Gilead Science Inc for hepatitis C treatment in pregnancy and RH Capital. Winhusen: none. Hansen: none. Wachman: none.
Informed Consent
Written patient consent has been obtained from the two patients.
Author Contributions
Lofwall wrote first draft. Young and Hansen provided patient case descriptions. Winhusen conceived idea for case report. All authors reviewed and provided edits.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Trost SL, Beauregard J, Chandra G, Njie F, Berry J, Harvey A, Goodman DA. Pregnancy-related deaths: data from maternal mortality review committees in 36 US States, 2017-2019. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2022.
- Margerison CE, Roberts MH, Gemmill A, Goldman-Mellor S. Pregnancy-associated deaths due to drugs, suicide, and homicide in the United States, 2010-2019. Obstet Gynecol. 2022;139(2):172-180.
doi pubmed pmc - Suarez EA, Huybrechts KF, Straub L, Hernandez-Diaz S, Jones HE, Connery HS, Davis JM, et al. Buprenorphine versus methadone for opioid use disorder in pregnancy. N Engl J Med. 2022;387(22):2033-2044.
doi pubmed pmc - Committee opinion No. 711: opioid use and opioid use disorder in pregnancy. Obstet Gynecol. 2017;130(2):e81-e94.
doi pubmed - Cooper HLF, Cloud DH, Young AM, Freeman PR. When prescribing isn't enough - pharmacy-level barriers to buprenorphine access. N Engl J Med. 2020;383(8):703-705.
doi pubmed - Substance Abuse and Mental Health Services Administration (SAMHSA) The management of pregnant people who have opioid use disorder. Advisory. Publication No. REP23-02-01-002. Rockville, MD. SAMHSA. 2023.
- Bastian JR, Chen H, Zhang H, Rothenberger S, Tarter R, English D, Venkataramanan R, et al. Dose-adjusted plasma concentrations of sublingual buprenorphine are lower during than after pregnancy. Am J Obstet Gynecol. 2017;216(1):64.e1-e7.
doi pubmed pmc - Caritis SN, Bastian JR, Zhang H, Kalluri H, English D, England M, Bobby S, et al. An evidence-based recommendation to increase the dosing frequency of buprenorphine during pregnancy. Am J Obstet Gynecol. 2017;217(4):459.e1-e6.
doi pubmed pmc - Walsh SL, Comer SD, Lofwall MR, Vince B, Levy-Cooperman N, Kelsh D, Coe MA, et al. Effect of buprenorphine weekly depot (CAM2038) and hydromorphone blockade in individuals with opioid use disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):894-902.
doi pubmed pmc - Lofwall MR, Walsh SL, Nunes EV, Bailey GL, Sigmon SC, Kampman KM, Frost M, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178(6):764-773.
doi pubmed pmc - Frost M, Bailey GL, Lintzeris N, Strang J, Dunlop A, Nunes EV, Jansen JB, et al. Long-term safety of a weekly and monthly subcutaneous buprenorphine depot (CAM2038) in the treatment of adult out-patients with opioid use disorder. Addiction. 2019;114(8):1416-1426.
doi pubmed pmc - D'Onofrio G, Hawk KF, Perrone J, Walsh SL, Lofwall MR, Fiellin DA, Herring A. Incidence of precipitated withdrawal during a multisite emergency department-initiated buprenorphine clinical trial in the era of fentanyl. JAMA Netw Open. 2023;6(3):e236108.
doi pubmed pmc - Coe MA, Lofwall MR, Walsh SL. Buprenorphine pharmacology review: update on transmucosal and long-acting formulations. J Addict Med. 2019;13(2):93-103.
doi pubmed pmc - FDA product label. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/210136Orig1s000lbl.pdf.
- Winhusen T, Lofwall M, Jones HE, Wilder C, Lindblad R, Schiff DM, Wexelblatt S, et al. Medication treatment for opioid use disorder in expectant mothers (MOMs): Design considerations for a pragmatic randomized trial comparing extended-release and daily buprenorphine formulations. Contemp Clin Trials. 2020;93:106014.
doi pubmed pmc - Jouyban A, Fakhree MA, Shayanfar A. Review of pharmaceutical applications of N-methyl-2-pyrrolidone. J Pharm Pharm Sci. 2010;13(4):524-535.
doi pubmed - Cleary EM, Byron RK, Hinely KA, Talley AW, Costantine MM, Rood KM. Subcutaneous buprenorphine extended-release use among pregnant and postpartum women. Obstet Gynecol. 2020;136(5):902-903.
doi pubmed pmc - Towers CV, Deisher H. Subcutaneous Extended-Release Buprenorphine Use in Pregnancy. Case Rep Obstet Gynecol. 2020;2020:3127676.
doi pubmed pmc - Kropp FB, Smid MC, Lofwall MR, Wachman EM, Martin PR, Murphy SM, Wilder CM, et al. Collaborative care programs for pregnant and postpartum individuals with opioid use disorder: Organizational characteristics of sites participating in the NIDA CTN0080 MOMs study. J Subst Use Addict Treat. 2023;149:209030.
doi pubmed pmc - Harris M, Schiff DM, Saia K, Muftu S, Standish KR, Wachman EM. Academy of Breastfeeding Medicine Clinical Protocol #21: Breastfeeding in the Setting of Substance Use and Substance Use Disorder (Revised 2023). Breastfeed Med. 2023;18(10):715-733.
doi pubmed - Hser YI, Evans E, Huang D, Weiss R, Saxon A, Carroll KM, Woody G, et al. Long-term outcomes after randomization to buprenorphine/naloxone versus methadone in a multi-site trial. Addiction. 2016;111(4):695-705.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
