| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 13, Number 1, March 2024, pages 12-17
Spontaneous Hemoperitoneum in Pregnancy Due to Endometriosis in the Second Trimester
Shota Ikagawaa, d , Mieko Inagakia, Tetsuo Maedab, Reina Mikic, Shigeki Yoshidaa
aDepartment of obstetrics and gynecology, Chibune General Hospital, 555-0034, 3-2-39, Fuku-machi, Nishiyodogawa-ku, Osaka-shi, Osaka-fu, Japan
bDepartment of radiology, Chibune General Hospital, 555-0034, 3-2-39, Fuku-machi, Nishiyodogawa-ku, Osaka-shi, Osaka-fu, Japan
cDepartment of obstetrics and gynecology, Akashi Medical Center, 674-0063, 743-33, Yagi, Okubo-cho, Akashi-shi, Hyogo-ken, Japan
dCorresponding Author: Shota Ikagawa, Department of obstetrics and gynecology, Chibune General Hospital, 555-0034, 3-2-39, Fuku-machi, Nishiyodogawa-ku, Osaka-shi, Osaka-fu, Japan
Manuscript submitted October 19, 2023, accepted December 29, 2023, published online March 31, 2024
Short title: SHiP Due to Endometriosis
doi: https://doi.org/10.14740/jcgo926
| Abstract | ▴Top |
Spontaneous hemoperitoneum in pregnancy (SHiP) is defined as unprovoked intraperitoneal bleeding during pregnancy or up to 42 days postpartum. A 31-year-old primigravid woman presented to our hospital at 19 weeks of gestation with acute left lower abdominal pain. Upon several examinations, she was found to have intraabdominal bleeding. However, pelvic magnetic resonance imaging (MRI) failed to yield specific findings. The following day, owing to increased pain and progressive anemia, she underwent an exploratory laparotomy. Preoperative ultrasound imaging revealed intrauterine fetal death. Laparotomy revealed oozing-type active bleeding from three delicate tissues related to endometriosis: the myometrium at the uterine anterior and posterior surfaces and a portion of the peritoneum in the vesicouterine excavation. A cesarean section was performed, successfully halting the bleeding and obviating the need for a hysterectomy. She was ultimately diagnosed with SHiP. SHiP in the second trimester is relatively uncommon, and the subacute progression of symptoms made early diagnosis challenging. For a correct diagnosis and appropriate treatments, full consideration of the clinical circumstance is needed without excessive reliance on imaging findings, including MRI, and an early exploratory laparotomy may be the best approach in cases of an unclear source of bleeding.
Keywords: Spontaneous hemoperitoneum in pregnancy; Magnetic resonance imaging; Exploratory laparotomy; Second trimester; Intraabdominal bleeding
| Introduction | ▴Top |
Spontaneous hemoperitoneum in pregnancy (SHiP) is defined as sudden non-traumatic intraperitoneal bleeding in pregnancy and up to 42 days postpartum [1]. This condition is associated with deleterious pregnancy outcomes for both mother and fetus. SHiP mostly occurs in the third trimester of pregnancy, with an incidence of 27% in the second trimester. Predicting the development of SHiP in advance presents a challenge. Furthermore, diagnosing the condition is also difficult [2]. Imaging methods to detect intraabdominal hemorrhage have been documented in one report [3]. Although ultrasound and computed tomography (CT) scan have been found to identify free peritoneal fluid in 62.7% of cases [4], the diagnosis of SHiP is difficult to establish before laparotomy [2]. In some cases, histopathology was required to confirm the decidualized endometriosis [5]. To our knowledge, there are no references regarding diagnostic imaging of SHiP. Herein, we present a case of this rare condition, which was difficult to diagnose using preoperative tests such as magnetic resonance imaging (MRI). The patient provided written informed consent for publication of this report and accompanying images.
| Case Report | ▴Top |
Investigations
A 31-year-old primigravid woman presented to our hospital by herself at 19 weeks of gestation with acute pain in the left lower abdomen. She experienced the pain for 45 min before visiting the hospital with nausea, stomach pain and bloating. She felt the pain as if being pushed and it was constant at one location and did not radiate. The supine position was an exacerbating factor, while the left lateral position was a limiting factor. She was unable to move without assistance. She had achieved a spontaneous pregnancy, and it was her first pregnancy. Although she had previously been diagnosed with focal adenomyosis diagnosed by ultrasound, she had not undergone any specific treatment. No endometriosis was noted in the ovaries or other organs. She had no history of abdominal surgery. Prior to this event, there were no abnormal findings during her pregnancy checkups.
Upon admission, her vital signs were stable, with a blood pressure of 80/49 mm Hg, heart rate of 60 bpm, and body temperature of 36.2 °C. Her physical examination revealed signs of peritoneal irritation. Abdominal ultrasonography revealed a fetus of appropriate size for gestational age with a normal heart rate. Transvaginal ultrasonography detected hyper-echoic lesions in the Douglas fossa (Fig. 1). There were no findings of ovarian swelling, placental thickening, or a retroplacental hematoma. Her white blood cell count was 17,800/µL and hemoglobin level was 11.9 g/dL. Once her vital signs stabilized and the pain subsided a little with the administration of analgesics, MRI of the lower abdomen was performed using a 3-T Magnetom Skyra (Siemens Healthcare, Erlangen, Germany). It identified a hematoma and hemorrhagic ascites in the abdominal cavity. However, the source of bleeding remained unclear (Fig. 2).
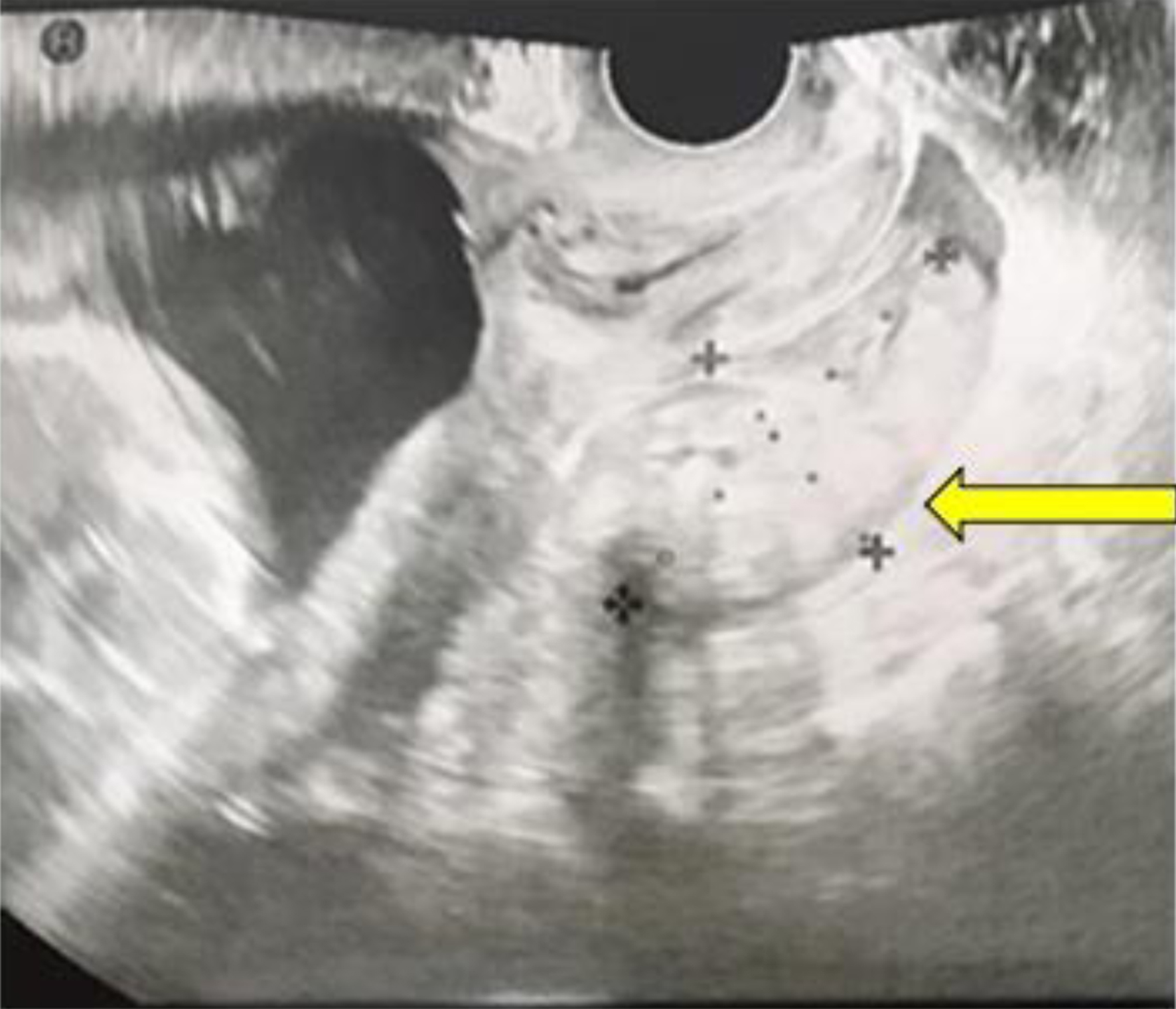 Click for large image | Figure 1. Transvaginal sonography reveals a hyper-echoic lesion measuring 32 × 57 mm located in the Douglas fossa at the time of admission (yellow arrow). |
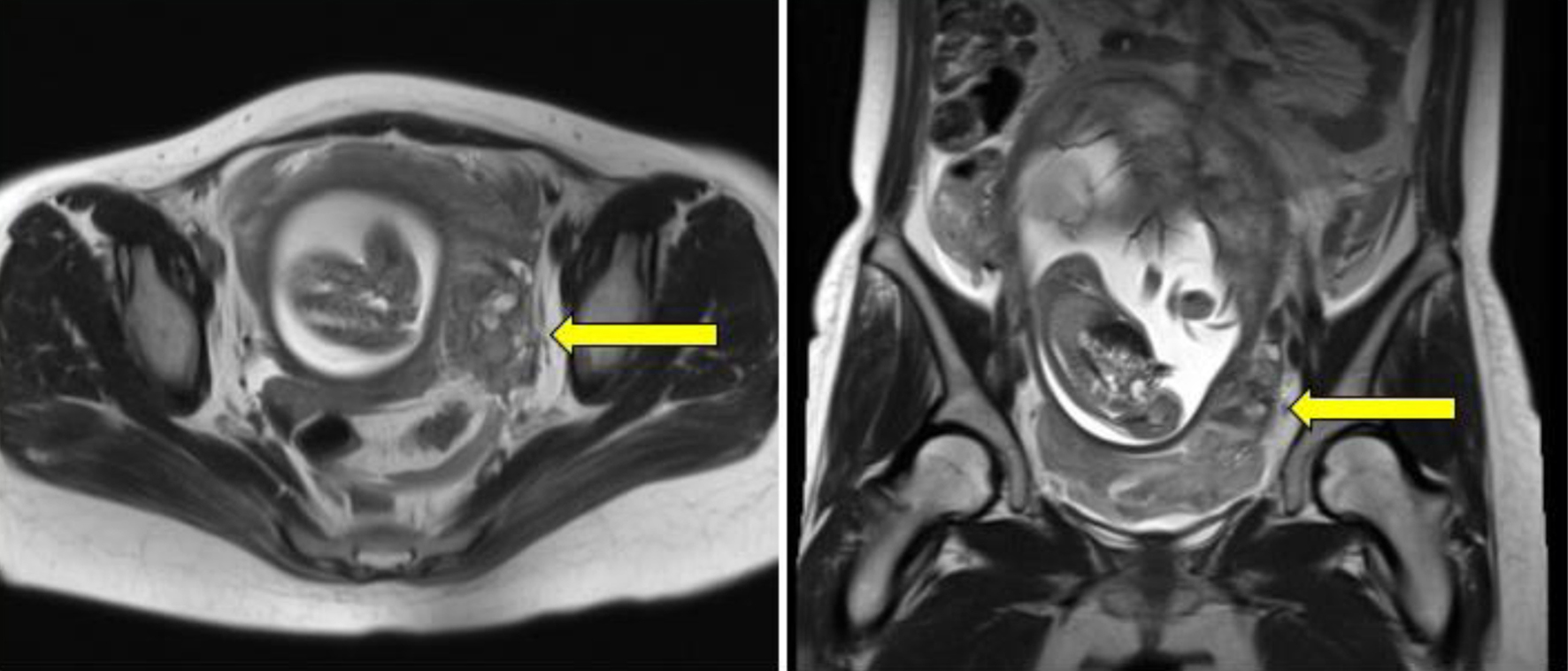 Click for large image | Figure 2. A mass-like lesion is identified in close proximity to the left uterine wall (yellow arrows), exhibiting a heterogeneous signal on both T1- and T2-weighted magnetic resonance images, suggesting a hematoma. |
Diagnosis
The patient did not have a history of a cesarean section or a uterine surgery. Moreover, her fetus was in the uterus, and MRI found no evidence of rupture in the entire myometrium. She had no vaginal bleeding, the fetal heart rate was normal, and there was no evidence of placental thickening or hematoma. On the basis of these findings, we considered the likelihood of uterine rupture and placental abruption to be low. Even if placental abruption had occurred, it was still too early in the gestation period to hope for fetal salvage. Moreover, there were no coagulation abnormalities. Accordingly, we chose a conservative treatment plan. Even though there were no abnormal ovarian findings in the first trimester of her pregnancy, the possibility of ovarian hemorrhage or ovarian tumor rupture remained.
Treatment
Upon the patient’s admission, the patient’s abdominal pain persisted despite the administration of acetaminophen and pentazocine. The following day, her pain worsened; she experienced tachycardia, and her hemoglobin level dropped to 8.8 g/dL (Fig. 3). An emergency exploratory laparotomy was performed owing to her worsening pain and progressive anemia. Prior to the operation, an ultrasound revealed the loss of fetal heart movement, indicating intrauterine fetal death.
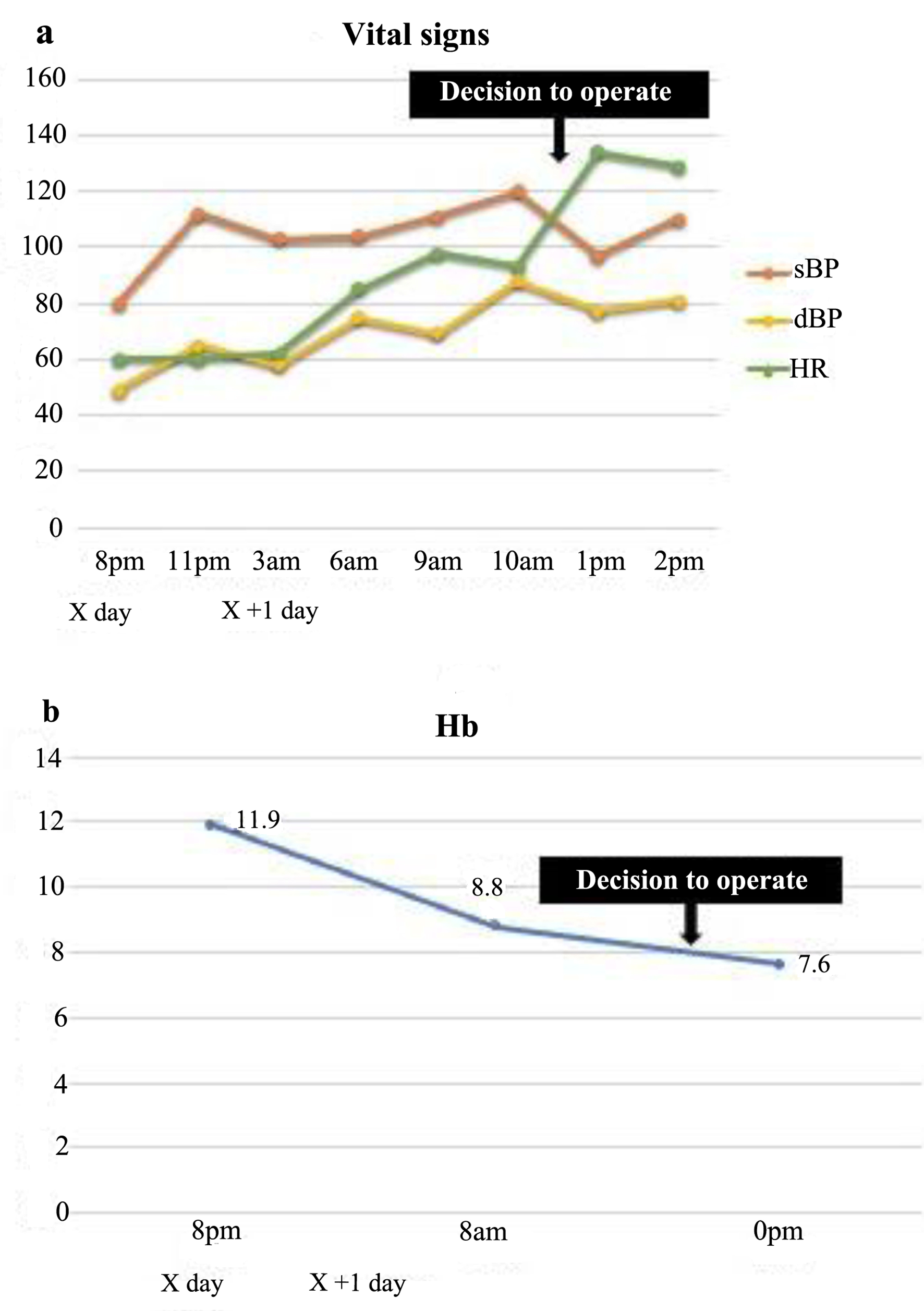 Click for large image | Figure 3. The patient’s vital signs were stable at the time of admission; however, her heart rate increased with time (a), and her hemoglobin levels dropped markedly (b). sBP: systolic blood pressure; dBP: diastolic blood pressure; HR: heart rate; Hb: hemoglobin. |
Under laparotomy, oozing-type active bleeding was found in three delicate tissues: the myometrium at the uterine anterior and posterior surfaces and a portion of the peritoneum in the vesicouterine excavation (Fig. 4). Because the space between the pregnant uterus and the pelvic wall was very small, adequate observation from the posterior to the left lateral walls of the uterus, which is the most area for evaluation, was difficult. On initiation of surgery, other delicate tissues were identified, and observation of the entire abdominal cavity was deemed necessary. For this observation, we had to reduce the size of the gestational uterus; therefore, an emergency cesarean section was performed, avoiding the need for a hysterectomy. The fetus was delivered through a U-shaped incision in the inferior segment of the uterus. Intraoperative findings revealed the presence of endometriosis. A total of 6,000 mL of intraabdominal blood was lost. She was transfused with 16 units of red blood cells, 18 units of fresh frozen plasma, and 20 units of platelets during the surgery. Based on these findings, the patient was diagnosed with SHiP caused by endometriosis. Placental histopathology revealed ischemic changes in the placenta with no adherence of hematoma.
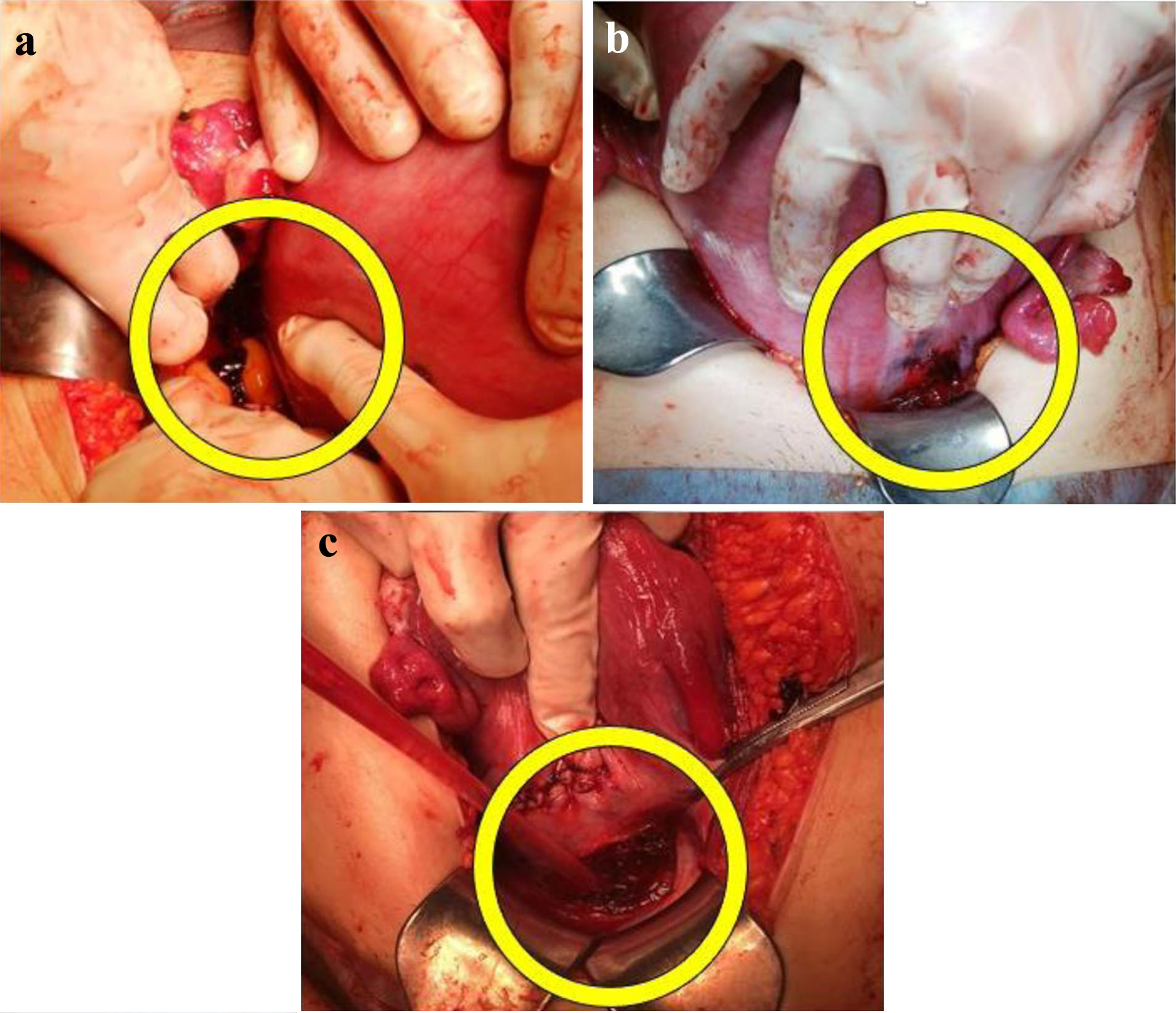 Click for large image | Figure 4. Intraabdominal examination reveals lacerations (yellow circles) on the posterior surface of the lower uterus (a), the anterior surface of the lower uterus (b), and in the vesicouterine pouch peritoneum (c) of the left uterine myometrium. |
Follow-up and outcomes
The patient’s recovery was uneventful. Her hemoglobin level was 9.3 g/dL 2 h after the surgery and did not decrease thereafter. She was discharged from the hospital 7 days post-surgery without additional transfusions. One month following the operation, she underwent a pelvic MRI, which demonstrated the persistence of endometriosis (Fig. 5).
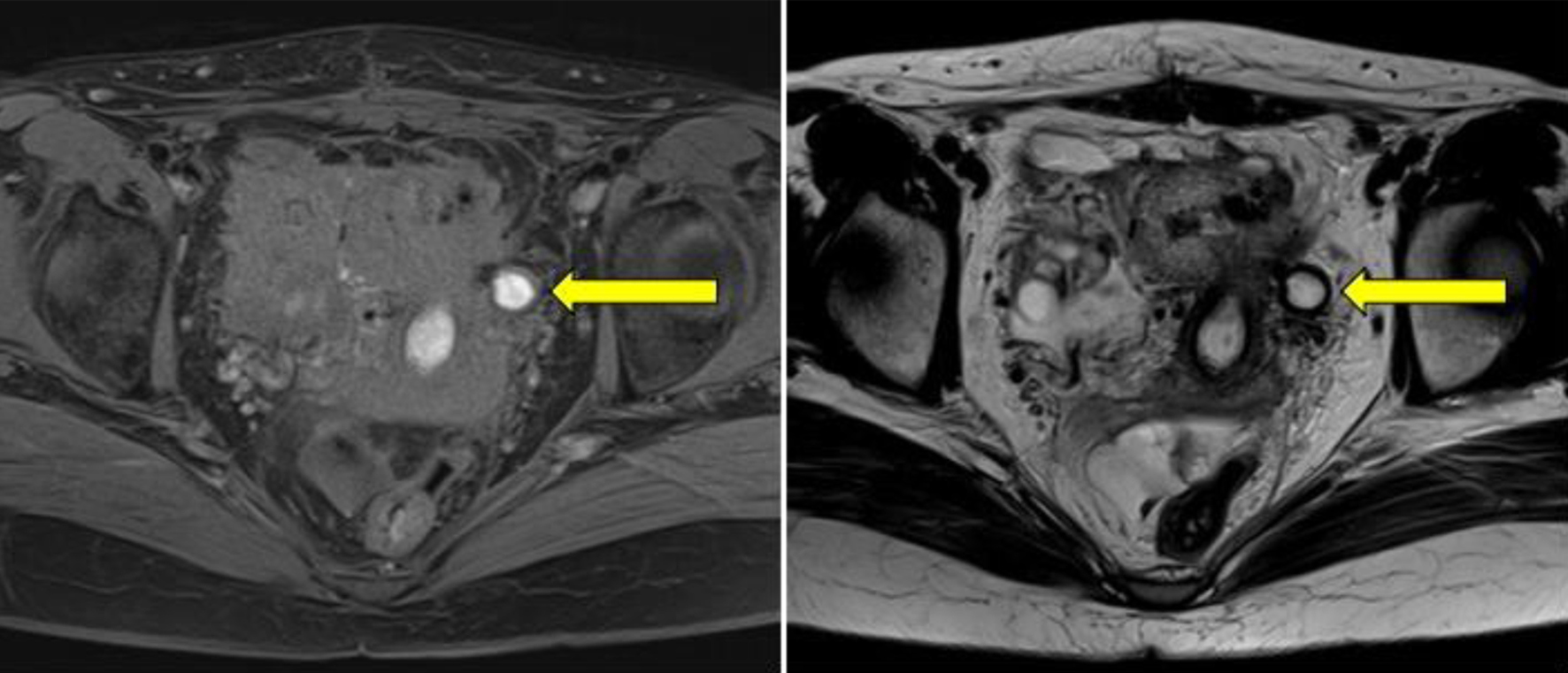 Click for large image | Figure 5. A pelvic magnetic resonance image taken 1 month post-operatively illustrates a cystic lesion (yellow arrows) in close proximity to the left uterine wall, displaying a high signal on both T1- and T2-weighted images, suggesting an endometrial cyst. |
| Discussion | ▴Top |
SHiP is a rare but potentially fatal obstetric complication. According to a systematic review by Lier et al in 2017, 50% of cases occurred in the third trimester of pregnancy, and 27% occurred in the second trimester [2]. Primary symptoms are typically characterized by sudden onset abdominal pain, hypovolemic shock, or fetal distress. Despite a decrease in maternal mortality rate in recent years, fetal mortality rate remains high [6]. While the underlying etiology of the disease remains undetermined, two risk factors have been identified: the use of assisted reproductive technologies and presence of endometriosis. The development of SHiP is difficult to predict as endometriotic lesions may only be observed during surgery. Furthermore, untreated endometriosis may be a risk factor [6, 7].
Huang et al reported a case of endometriosis-related SHiP occurring in the second trimester, and the woman presented with lower abdominal pain and a slow progression of anemia. In their case, eventually, an exploratory laparotomy was employed under the suspicion of a uterine rupture, and the above diagnosis was made [8]. The time of onset was the second trimester and the clinical course of the case resembled that of our case. Although understandable, any pregnant or postpartum woman with severe abdominal pain, progressive anemia, and hypovolemic shock should be evaluated with care, and consideration should be given to the potential diagnosis of idiopathic spontaneous intraperitoneal hemorrhage [9, 10]. Typically, cardiotocographic monitoring is not initiated until 22 weeks of gestation. In this case, the delay in performing immediate surgical intervention was due to the subacute progression of the patient’s symptoms, unclear source of bleeding, and absence of means to detect fetal distress. Consequently, the patient underwent a cesarean delivery of a stillborn fetus and required a blood transfusion because of heavy bleeding. The delay in performing an exploratory laparotomy may have contributed to these outcomes. Thus, an early exploratory laparotomy may result in a better prognosis for both mother and fetus in instances of intraabdominal bleeding with an undetected source, even in the presence of stable vital signs and normal blood results.
Typically, an exploratory laparotomy provides an opportunity to diagnose SHiP [2]. There are a limited number of reported cases using abdominal ultrasound imaging or MRI as diagnostic tools of SHiP, and their utility has not been considered to be high [9]. Although preoperative MRI did not identify endometriotic lesions in the present case, intraoperative findings revealed the presence of endometriosis as the bleeding point. Moreover, a postoperative MRI performed 1 month after confirmed the presence of endometriosis and adenomyosis. It is unclear why no endometriotic lesions could be detected in preoperative images. However, it can be assumed that endometriotic lesions become obscured in the pregnant uterus of pregnant women because of physical factors such as elongation and thinning of the muscle layer and physiological factors such as the decidualization of those lesions. In addition, when active bleeding occurs, as in this case, the blood that accumulates within the endometriosis lesion may leak out and become undetectable on MRI. Endometriosis is most observed in the ovaries and some pelvic sites such as broad ligaments, uterosacral ligaments, pelvic peritoneum, and Douglas pouch with minor lesions without a detectable mass [11]. Therefore, when encountering unexplained hemoperitoneum during pregnancy, the possibility of SHiP caused by endometriosis should be considered, even if the hematoma is not on the uterine surface.
We did not perform contrast-enhanced CT to search for the source of bleeding, avoiding radiation exposure to the fetus and some adverse effects of the contrast agent. Some investigators have reported that, if clinical status permits, CT angiogram scans can serve as a sensitive and specific tool for diagnosing and identifying sites of hemorrhage or pseudoaneurysm in the case of idiopathic spontaneous intraabdominal hemorrhage [10, 12]. However, venous bleeding from the uterine surface, as in this case, may not have been detected using dynamic CT.
According to the study by Brosen et al, the degree of endometriosis may not be a contributing factor in the development of SHiP. However, it may play a role in its severity [2, 6]. Even though the current patient had previously been diagnosed with endometriosis at another hospital, the details of the condition are unknown, and no treatment has been implemented. Therefore, the correlation between the severity of this case and the degree of endometriosis cannot be evaluated.
The main strength of our approach is it minimizes the effects of drugs and radiation exposure on the fetus. On the contrary, the main limitation is that not all hospitals have the facility to perform MRIs early because of the cost or shortage of skilled engineers. Another limitation is that MRI cannot be employed in case of sudden deterioration of the patient’s vital signs. Therefore, early laparotomy can lead to better outcomes, especially, in resource-limited situations.
Learning points
The diagnosis of SHiP during the second trimester poses a major challenge, and excessive reliance on findings of imaging, including MRI, is also problematic. In recent decades, the rate of maternal mortality associated with SHiP has been decreasing; however, fetal mortality rate remains high. For these reasons, both gynecologists and radiologists need to be aware that imaging-invisible endometriosis may cause SHiP, even in the second trimester and in cases where symptoms are subacute. Furthermore, undertaking an exploratory laparotomy at an earlier stage may result in improved maternal and fetal prognoses.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Written informed consent was obtained from the patient for publishing the case.
Author Contributions
SI, MI and RM observed the patient and performed medical treatment. SI, MI and TM drafted the original manuscript. SY supervised the preparation of the manuscript. All authors reviewed the manuscript draft and revised it critically for intellectual consent. All authors approved the final version of the manuscript to be published.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
| References | ▴Top |
- Mazzocco MI, Donati S, Maraschini A, Corsi E, Colciago E, Guelfi F, Cetin I. Spontaneous hemoperitoneum in pregnancy: Italian prospective population-based cohort study. Acta Obstet Gynecol Scand. 2022;101(11):1220-1226.
doi pubmed pmc - Lier MCI, Malik RF, Ket JCF, Lambalk CB, Brosens IA, Mijatovic V. Spontaneous hemoperitoneum in pregnancy (SHiP) and endometriosis - a systematic review of the recent literature. Eur J Obstet Gynecol Reprod Biol. 2017;219:57-65.
doi pubmed - Lier M, Malik RF, van Waesberghe J, Maas JW, van Rumpt-van de Geest DA, Coppus SF, Berger JP, et al. Spontaneous haemoperitoneum in pregnancy and endometriosis: a case series. BJOG. 2017;124(2):306-312.
doi pubmed - Edral A, Costa Gomes C, Martins R, Ferreira A. A rare cause of hemoperitoneum in pregnancy. Acta Med Port. 2022;35(1):59-62.
doi pubmed - Lier MCI, Brosens IA, Mijatovic V, Habiba M, Benagiano G. Decidual bleeding as a cause of spontaneous hemoperitoneum in pregnancy and risk of preterm birth. Gynecol Obstet Invest. 2017;82(4):313-321.
doi pubmed - Brosens IA, Fusi L, Brosens JJ. Endometriosis is a risk factor for spontaneous hemoperitoneum during pregnancy. Fertil Steril. 2009;92(4):1243-1245.
doi pubmed - Brosens IA, Lier MC, Mijatovic V, Habiba M, Benagiano G. Severe spontaneous hemoperitoneum in pregnancy may be linked to in vitro fertilization in patients with endometriosis: a systematic review. Fertil Steril. 2016;106(3):692-703.
doi pubmed - Huang LY, Hsu PY, Chiang CT, Chen HW, Wu MH. Endometriosis-related spontaneous hemoperitoneum in the early second trimester: a case report. Taiwan J Obstet Gynecol. 2021;60(2):328-330.
doi pubmed - Pezzuto A, Pomini P, Steinkasserer M, Nardelli GB, Minelli L. Successful laparoscopic management of spontaneous hemoperitoneum at 15 weeks of pregnancy: case report and review of literature. J Minim Invasive Gynecol. 2009;16(6):792-794.
doi pubmed - Badri D, Killoran C, Aseervatham R. Idiopathic spontaneous intraperitoneal haemorrhage: a near fatal presentation of acute abdomen requiring prompt diagnosis. Int J Surg Case Rep. 2023;110:108650.
doi pubmed pmc - Rei C, Williams T, Feloney M. Endometriosis in a man as a rare source of abdominal pain: a case report and review of the literature. Case Rep Obstet Gynecol. 2018;2018:2083121.
doi pubmed pmc - Law EK, Lee RK, Hung EH, Ng AW. Radiological diagnosis and management of idiopathic spontaneous intra-abdominal haemorrhage (abdominal apoplexy): a case series. Abdom Imaging. 2015;40(2):343-351.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
