| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Original Article
Volume 13, Number 3, December 2024, pages 59-74
Preterm Birth in Black Birthing People: A Systematic Literature Review and Meta-Analysis
Etoroabasi Ekpea, Kimberly Thompsonb, Bethel Samsonc, Majid Nabipoord, Cynthia Maxwelle, f
aDepartment of Obstetrics and Gynecology, University of Toronto, Toronto, ON, Canada
bDepartment of Family Medicine, Dalhousie University, Halifax, NS, Canada
cUniversity of Toronto, Toronto, ON, Canada
dBiostatistics Department, University Health Network, University of Toronto, Toronto, ON, Canada
eDepartment of Obstetrics and Gynecology, Women’s College Hospital and Mount Sinai Hospital, University of Toronto, Toronto, ON, Canada
fCorresponding Author: Cynthia Maxwell, Department of Obstetrics and Gynecology, Women’s College Hospital and Mount Sinai Hospital, University of Toronto, Toronto, ON M5G 1Z5, Canada
Manuscript submitted July 10, 2024, accepted August 23, 2024, published online September 16, 2024
Short title: Preterm Birth in Black People
doi: https://doi.org/10.14740/jcgo985
| Abstract | ▴Top |
Background: Preterm birth (PTB) is a global health challenge with significant morbidity and mortality rates among affected infants driven by various factors. In Canada, approximately 8% of babies are born prematurely, contributing to infant mortality, and imposing social and financial burdens on families and society. Racial disparities in PTB are evident, particularly within Black populations in the United States, with limited data available in Canada. Our objective was to understand the epidemiology, risk factors, and racial disparities related to PTB in this population.
Methods: This review was conducted in English using a combination of search terms “preterm birth”, “Black women” and “Canada” in various online databases. Included and excluded articles were confirmed by each reviewer and organized using The Preferred Reporting Items for Systematic Reviews and Meta-Analyses. The quality assessment was conducted using the Newcastle-Ottawa Scale. A multi-level meta-analysis was performed to account for inter-study correlations, focusing on three primary birth outcomes: PTB, low birth weight (LBW), and small for gestational age (SGA).
Results: Over 1,000 studies were screened, 10 articles in the review, and nine in the meta-analysis. The articles discussed how the Black population experienced higher rates of PTB and other adverse pregnancy outcomes including LBW, SGA, and stillbirth. The articles also addressed the relationship between PTB and socioeconomic risk factors such as level of education, income, poverty, and social isolation. The results of the multi-level meta-analysis indicated that the associations between maternal race/ethnicity and PTB (and between maternal race/ethnicity and SGA) were the only associations found to be significant across the studies.
Conclusions: Despite Canada’s universal healthcare system, Black birthing people face significant disparities in maternal health, underscored by negative healthcare experiences and systemic racism within the healthcare system. Addressing these disparities requires comprehensive approaches including race-based data collection, policy advocacy, and healthcare system reforms to ensure equitable access to care and improve maternal and neonatal health outcomes.
Keywords: Preterm birth; Black population in Canada; Racial disparities; Pregnancy outcomes
| Introduction | ▴Top |
Preterm birth (PTB)
PTB, defined as a live birth before 37 weeks of gestation, remains a significant global health challenge, affecting millions of infants each year [1]. According to the World Health Organization (WHO), PTB complications are the leading cause of death among children under 5, with an estimated 13.4 million babies born prematurely in 2020 [1]. In Canada, approximately 390,000 babies are born each year, with nearly 8% of them arriving prematurely, often without a known cause [2]. The epidemiology of PTB varies widely across regions, with higher rates observed in low and middle-income settings [1]. PTB is multifactorial in nature with risk factors including prior PTB, maternal age, and co-morbidities such as diabetes or hypertension, short cervical length, infections, multiple gestations, genetics, and low socioeconomic status [1, 3-5].
Premature birth poses serious health risks for infants, increasing their chances of developing chronic conditions later in life and contributing to a significant portion of infant mortality [2]. Morbidity and mortality rates are notably higher among preterm infants, especially those born before 32 weeks [6]. They have increased risks of long-term health complications including retinopathy of prematurity (eye disease that can lead to blindness), respiratory distress syndrome (due to poorly developed lungs), and neurodevelopmental disabilities (including learning and behavioral disorders) [1, 6, 7]. Beyond the immediate health concerns, premature birth also places social and financial burdens on families and imposes additional costs on society through healthcare and educational expenses [2].
Efforts to address PTB require a multifaceted approach encompassing medical interventions, public health initiatives, and social determinants of health strategies to mitigate risks and improve outcomes for both mothers and infants globally.
PTB in the Black population
PTB significantly impacts Black reproductive health, representing a critical issue within this demographic group. In the United States and the United Kingdom, PTB rates are 16-18% among Black women in comparison to 5-9% among White women [8]. Black women are also three to four times more likely to have a very early PTB than women from other racial groups [8]. The WHO and Goldenberg et al provide valuable insights into the risk factors for PTB among Black individuals, highlighting both medical and social determinants of health [1, 8]. However, the reason for such disparities is not fully understood even after controlling for socioeconomic factors and maternal comorbidities: Kistka et al demonstrated a three-fold higher rate of PTB (20 - 34 weeks of gestation) among Black birthing people and a five times more likely rate of recurrent PTB even after adjusting for additional risk factors or limiting outcomes to spontaneous PTBs alone [5, 9]. More recently, Black birthing people in the United States experience PTB at a rate of 14.6% which is 1.5 times higher compared to other races (9.0% among Asian and 9.4% among White birthing people) [10].
There are major PTB rate disparities in racial and ethnic minorities as shown from data in the United States and United Kingdom [5, 8-10]. To our knowledge, there are limited data on PTB in the Black population in Canada. Our objective is to understand the epidemiology, risk factors, and racial disparities related to PTB in this population. In this review, we will delve into the epidemiological landscape surrounding PTBs within the Black population of Canada. By examining patterns and trends, we seek to uncover the underlying risk factors that contribute to the occurrence of premature births in this demographic. Additionally, our investigation will specifically address racial disparities, shedding light on gaps in healthcare access, socio-economic factors, and systemic inequalities that may disproportionately affect the Black community in Canada. Through a comprehensive analysis of these factors, we aspire to understand the root causes of PTBs and to propose targeted interventions and policy reforms to alleviate these disparities and improve the overall health outcomes for Black birthing people and infants across the country.
Black population in Canada
To comprehensively understand the disparities in PTB rates among the Black population in Canada, it is necessary to delve into the historical context of Black people within the country, and the specific makeup of the Black Canadian population. Canada (British North America) did engage in the slave trade, particularly in Nova Scotia and Quebec [11]. It is estimated that thousands of Africans were brought to these provinces between the 17th and 19th centuries. For example, after the American Revolution, hundreds of Black people emigrated to Nova Scotia, some freed, others enslaved [11]. The slave trade ended in 1807 and was outlawed with the Slavery Abolition Act in 1834. British North America also became one of the final destinations of the Underground Railroad after 1850 [11].
The Black population in Canada has witnessed substantial growth over the past two decades, with numbers nearly doubling from approximately 574,000 individuals in 1996 to 1.2 million in 2016 [12]. Currently, 4.3% of Canadians identify as Black [13]. In the same 2021 Census, 7.1% identified as South Asian and 4.7% as Chinese, while the top “ethnic/cultural origins” were reported to be 5.7 million Canadian, 5.3 million English, 4.4 million Irish, and 4.0 million French people [13]. Notably, Canada stands out as the primary place of birth for the Black demographic, with over 40% of Black individuals born within the country [14]. While long-established Black immigrants primarily hailed from the Caribbean, recent trends show a significant influx from African nations [14].
Nevertheless, it is difficult to determine what percentage of the current population descends from slavery [11]. This diversity within the Black population is further underscored by linguistic diversity, with a higher percentage speaking French at home compared to the national average [12]. Quebec emerges as a significant hub, hosting the second-largest concentration of Black Canadians at 26.6% of the total Black population [12]. However, defining the Black population for health research purposes remains a challenge, with terms like “Afro-Canadians” possibly excluding individuals from Caribbean backgrounds [15]. Efforts to maximize inclusivity have led to the adoption of terms like “African, Caribbean, and Black communities”, although further clarity is needed to ensure accurate representation in health studies [15].
Experiences of discrimination persist among Black communities in Canada, with nearly half reporting instances of unfair treatment or discrimination within the past 5 years [16]. Notably, discrimination appears more prevalent in public spaces such as stores, banks, or restaurants [16]. Moreover, there is a concerning uptick in reported discrimination compared to previous years. Despite facing such challenges, the Black communities in Canada remain vibrant and dynamic, with a relatively young population and a significant proportion holding university degrees [17]. However, employment rates among this demographic slightly trail behind the national average and even lower incomes for those who are employed well below the general population [17]. The combination of racial discrimination and limited employment opportunities may contribute to unequal access to quality health care. Understanding the complexities of the Black population’s demographic makeup or experiences of discrimination is vital for addressing health disparities, including those related to PTB and maternal health, and fostering an inclusive and equitable society for all Canadians.
| Materials and Methods | ▴Top |
Search strategy and eligibility criteria
We conducted a systematic review on PTB in the Black birthing people in Canada. We used the following online resources: PubMed, Google Scholar, SCOPUS, and a number of databases provided by Gerstein Library search tools through the University of Toronto. This review was conducted in English using a combination of search terms of “preterm birth”, “Black women” and “Canada”, including phrases such as “preterm birth in Canada”, “preterm birth in Black women”, and “preterm birth in Black women in Canada”. Studies were included if they discussed PTB in Canada in the context of adverse pregnancy outcomes within different populations, specifically Black populations. Studies were excluded if they focused on PTB in the Black population but did NOT include Canada or did not include Canada as a primary contributor to data. Articles were excluded if the primary or secondary interventions were not pertaining to the topic of PTB in the Black population in Canada. All study types were included such as prospective and retrospective cohort studies and case-control studies as long as they met the above criteria. There were no minimum study quality inclusion or exclusion criteria; however, all included studies underwent quality assessment which is described later. This review did not have a specific date range and articles were not excluded based on any particular time frame.
The study team consisted of two reviewers (EE and KT) who reviewed the articles by title, abstract, and then by manuscript. Articles that were eventually included or excluded were confirmed and verified by each reviewer until a consensus was reached. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to organize the review process from search results to the final included items. We are reviewing information in the published literature and not individual-level patient data. Therefore, this review was conducted in accordance with prevailing ethical principles and did not require informed consent or Institutional Review Board approval.
Study quality assessment
The quality assessment was conducted by two study team members (EE and MC) using the Newcastle-Ottawa Scale (NOS) which is a validated tool used to assess the methodological quality of non-randomized studies including cohort and case-control studies [18]. The tool consists of a series of questions on the representativeness and selection of the study population and assessment and comparability of exposure and outcome data. Results were achieved through a consensus by the two reviewers.
Data analysis
The primary analysis focused on three primary birth outcomes - specifically PTB, low birth weight (LBW), and small for gestational age (SGA) - within maternal disparities related to race/ethnicity, immigration status, education, income, and vitamin D levels. PTB was defined as birth less than 37 weeks; LBW was defined as birth weight less than 2,500 g; and SGA was defined as birth weight less than the 10th percentile for gestational age. Due to limited data, stillbirth and other outcomes were excluded from the meta-analysis. Some studies provided multiple estimates for PTB with few studies including “extreme”, “very”, or “moderate” PTB with varying parameters for gestational age ranges. This violates the assumption of independence; therefore, we employed a multi-level meta-analysis to address inter-study correlation, as outlined by Cheung [19].
Data that initially presented as adjusted risk ratios (aRRs) and adjusted hazard ratios (aHRs) were converted to adjusted odds ratios (aORs) and standard deviations using validated methodology [20-22]. The final effect size, expressed as aORs, was collected from nine independent population and experimental studies. One study was excluded from the meta-analysis [23], given that it provided multiple PTB estimates in different time periods, mainly focusing on trends in PTB over time rather than on PTB itself. The analyses were conducted based on the log-transformation of aORs, with the final results back-transformed to actual values using R version 4.2.2 and the metafor package, specifically the rma.mv function. A P-value of 0.05 or less was considered significant. The results were presented in the form of forest plots.
| Results | ▴Top |
Description of studies
Over 1,000 studies were imported for screening via multiple databases using our key search terms. Three hundred and nineteen articles were selected by title and abstracts reviewed. Thirty-eight articles were then selected, and the full text was assessed for further eligibility. Excluded studies were those with unrelated titles or abstracts, from countries other than Canada, or with exposures or outcomes not pertaining to our objective. Ten studies were included in the final review and nine in the meta-analysis (Fig. 1).
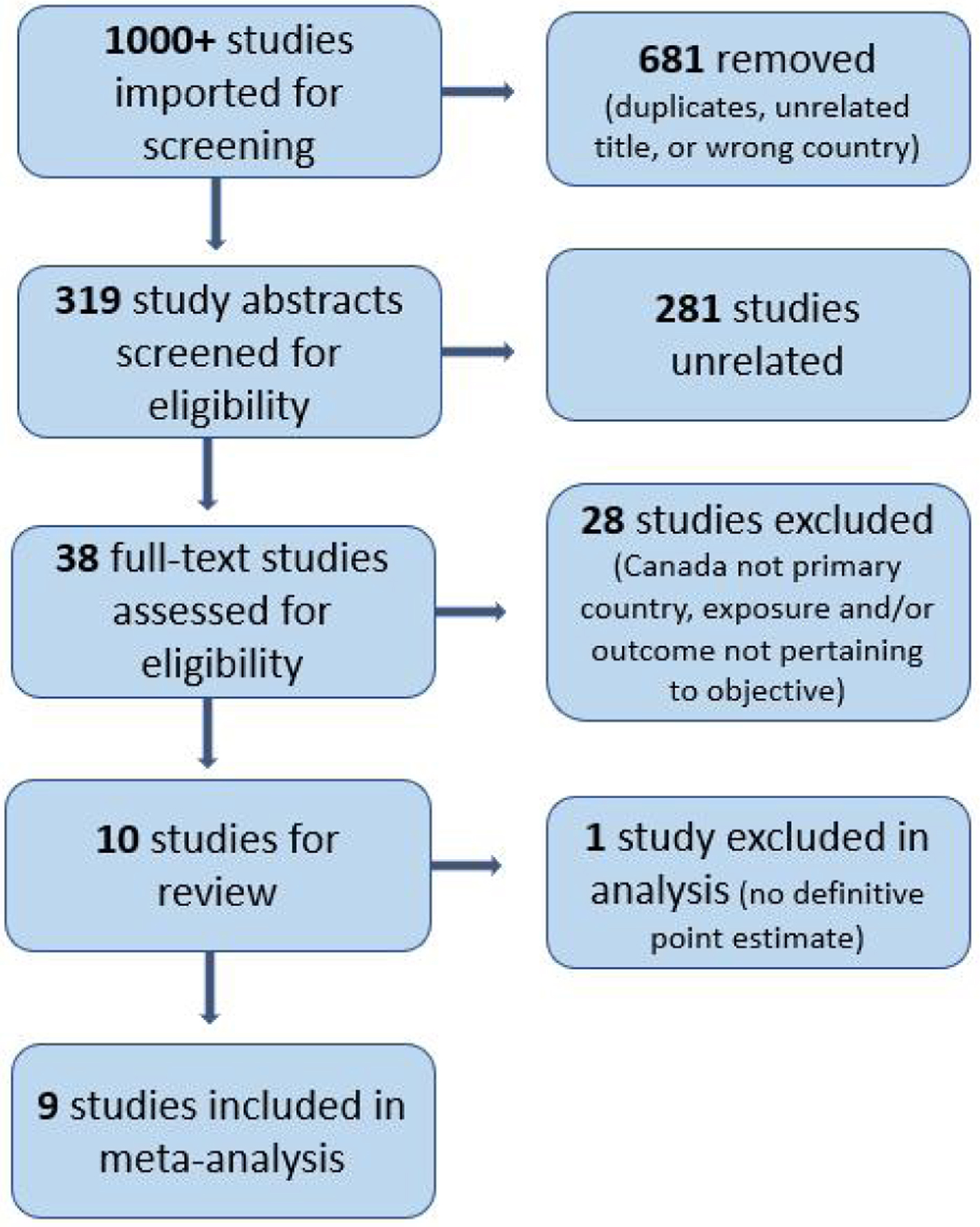 Click for large image | Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart. |
All studies were either from Canada or used data from Canada [23-32]. One study included data from the United States and Canada [28]. The majority of studies were retrospective cohort designs (n = 8); two were case-control studies. Five studies used ethnicity or maternal origin (immigration status or nationality) as the primary exposure [24, 26, 29, 31, 32]; four studies focused on maternal race (Black vs. White) as exposure [28-31]. Variations of socioeconomic status which describes a combination of an individual’s or group’s social standing or class were also used as exposures: maternal educational level in two studies [23, 27] and either income or material vs. social factors in two studies [25, 27]. The last study used levels of vitamin D as an exposure [32]. All studies included PTB as a primary outcome; other outcomes included LBW in two studies [24, 29], SGA in four studies [24, 27, 29, 31], gestational diabetes in two studies [29, 30], pre-eclampsia in two studies [30, 31], and stillbirth or perinatal mortality in two studies [27, 31]. Only three articles specifically addressed and discussed possible reasons for racial or ethnic disparities in their results [24, 28, 30]. A detailed description of the studies can be found in Table 1 [23-32].
 Click to view | Table 1. Summary of Studies Evaluating Preterm Birth in Canada and Preterm Birth in Black Population in Canada |
Studies based on maternal origin
Auger et al (2012) reported that Haitian-born mothers were more likely to have PTBs compared to Canadian-born mothers [24]. The differences reached so far as Haitian-born mothers having four times, two times, and 1.25 times the odds of “extreme” PTBs (< 27 weeks gestation), “very” PTB (28 - 31 weeks), and “moderate” PTB (32 - 36 weeks), respectively [24]. These worsening trends for Haitian-born mothers were similar to other outcomes such as SGA and severe LBW [24]. Similar trends were seen in data from 1987 to 1991: among birthing people with chronic hypertension in Canada, Black women (born in Haiti) had higher rates of perinatal mortality (9.5% vs. 2.9%; P < 0.05) and prematurity (32.4% vs. 19.7%; P < 0.05) when compared to White women (born in Canada) [31].
Lee (2022) compared PTB rates (between 22 and 36 weeks gestation) between foreign-born and Canadian-born mothers across provinces in Canada between 2000 and 2016 [26]. Results showed that PTB rates were lower among Asian, African, and Western immigrant mothers but higher among Bangladeshi, Filipino, and Caribbean mothers [26].
Studies based on race
When comparing perinatal outcomes between Canada’s Black and White populations living in Ontario, Miao et al (2022) outlined how Black women (who made up 10.1% of the eligible pregnant people in the study) experienced more adverse outcomes compared to White women. These outcomes included increased risk of stillbirth, gestational diabetes mellitus, preeclampsia, placental abruption, PTB, cesarean delivery, LBW, low APGAR scores, and neonatal intensive care unit admissions [30]. The greatest disparity was found in rates of very preterm (< 32 weeks gestation) and moderate PTBs (< 34 weeks) [30]. Menard et al (2020) evaluated the rate of adverse pregnancy outcomes in low-income women and described how Black women have higher rates of anemia (aOR: 1.74; 95% confidence interval (CI): 1.29 - 2.35), hypertension (aOR: 2.23; 95% CI: 1.18 - 4.21) and PTBs (aOR: 1.79; 95% CI: 1.01 - 3.19) when compared to their White counterparts [29].
Moreover, McKinnon et al (2015) analyzed PTB rates in White and Black populations in Canada and the United States and compared the disparities between the two countries [28]. Five million non-Hispanic Black and White women in the United States and 91,000 non-Hispanic Black and White women in Canada were included in the study [28]. The Black population in both countries indeed had higher rates of PTB than their White counterparts (in Canada 8.9% vs. 5.9%; in the United States 12.7% vs. 8.0%, respectively) [28]. After adjusted analyses, the relative disparities in PTB were similar between the two countries, although the absolute rates were smaller in Canada for both Black and White populations [28].
The studies that addressed racial and ethnic inequalities argued that health disparities were an indirect result of perceived racial discrimination, unemployment and poverty, food insecurity, and higher rates of comorbidities including obesity, hypertension, diabetes, depression, post-traumatic stress disorder, and domestic violence [24, 28, 30]. This describes systemic inequalities - gaps in wealth, opportunities, and resources due to existing institutional structures in society.
Studies based on maternal risk factors
As for socioeconomic risk factors, Auger et al (2011) found that educational inequalities between the least and most educated have increased over time in those who had extreme and moderate PTBs (< 27 weeks and between 32 and 36 weeks gestation, respectively) [23]. Other risk factors include material deprivation which was defined as poverty and poor living conditions and social deprivation which was defined as isolation and low levels of social support [25]. Auger et al (2012) found that PTB rates were increased in areas with higher material deprivation (7.1% vs. 5.5%) and higher social deprivation (6.8% vs. 5.9%) than their lower counterparts and that material deprivation had a slightly stronger association with PTB than social deprivation (aHR: 1.43, 95% CI: 1.38 - 1.48 vs. aHR: 1.18, 95% CI: 1.14 - 1.22, respectively) [25]. Using maternal education and neighborhood income as socioeconomic status indicators, Luo et al (2006) detailed how lower levels of education (less than high school) and less income (lowest quintile) were associated with higher rates of PTB, SGA, and stillbirth [27].
In Quebec, Canada, Tabatabaei et al (2017) observed that vitamin D deficiency was associated with greater rates of PTBs in ethnic minorities living in Canada [32]. This trend was seen especially among sub-Saharan Africans where lower vitamin D levels were significantly associated with higher rates of PTB in an inverse dose-response relationship (P-trend 0.030) [32]. There was no other study in this review that addressed specific nutritional deficiencies as a cause for PTBs in the Black Canadian population.
Quality assessment results
Of the retrospective cohort studies (n = 8), the majority (n = 6) were categorized as “good” quality based on the modified NOS. The remaining two were characterized as “fair” and “poor” (Table 2) [23-30]. The two case-control studies were considered as “poor” and “good” quality, respectively (Table 3) [31, 32]. The studies that were considered “poor” were due to the uncertainty of representativeness of the cohort or ascertainment of the exposure from secure records and in the case of the case-control study, due to the uncertainty of an adequate case definition, ascertainment of exposure from secure records, and non-response rate for cases and controls. Nevertheless, the studies were included in the final review for the purpose of comprehensive assessment of PTB rates and other outcomes in Black vs. White populations given the limited number of articles that met prior inclusion criteria.
 Click to view | Table 2. Quality Assessment of the Retrospective Cohort Studies (n = 8) Examining Preterm Birth in the Black Population in Canada Using the Modified Newcastle-Ottawa Scale |
 Click to view | Table 3. Quality Assessment of the Case-Control Studies (n = 2) Examining Preterm Birth in the Black Population in Canada Using the Modified Newcastle-Ottawa Scale |
Association of PTB, LBW, and SGA with maternal disparities
The majority of the studies that compared maternal outcomes for Black vs. White or immigrant vs. Canadian-born people found that PTB rates and other adverse perinatal outcomes were higher among racial/ethnic minorities, particularly Black populations [24, 26, 28-32]. Studies that focused on maternal risk factors such as socioeconomic status, mainly educational or income level, found that the lowest tiers also had poorer perinatal outcomes [23, 25, 27]. A multi-level meta-analysis was performed to further understand the relationship between PTB, LBW, and SGA based on maternal disparities (Figs. 2-5).
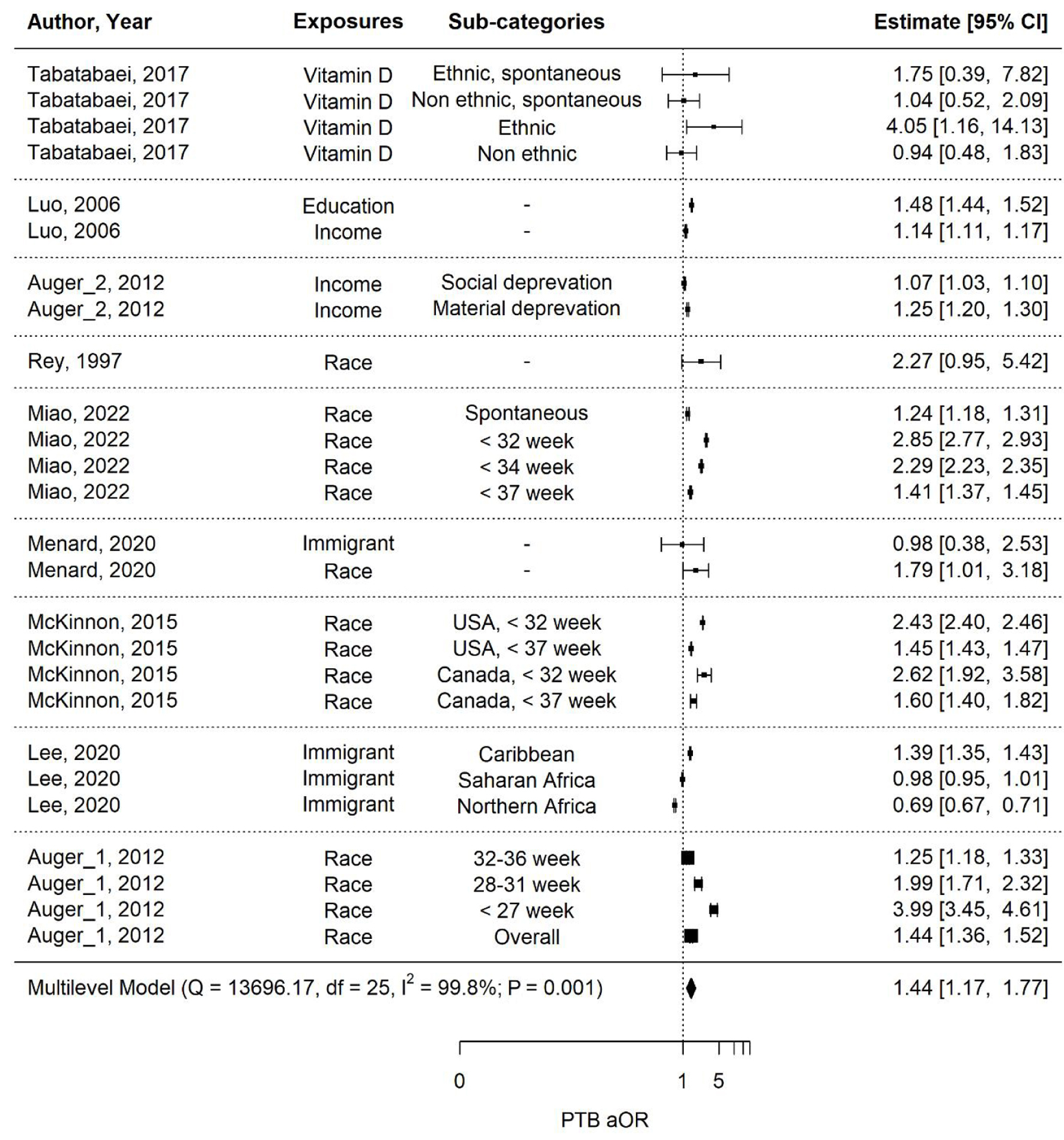 Click for large image | Figure 2. Multi-level meta-analysis and pooled estimate of adjusted odds ratios (aORs) in nine studies for preterm birth (PTB). |
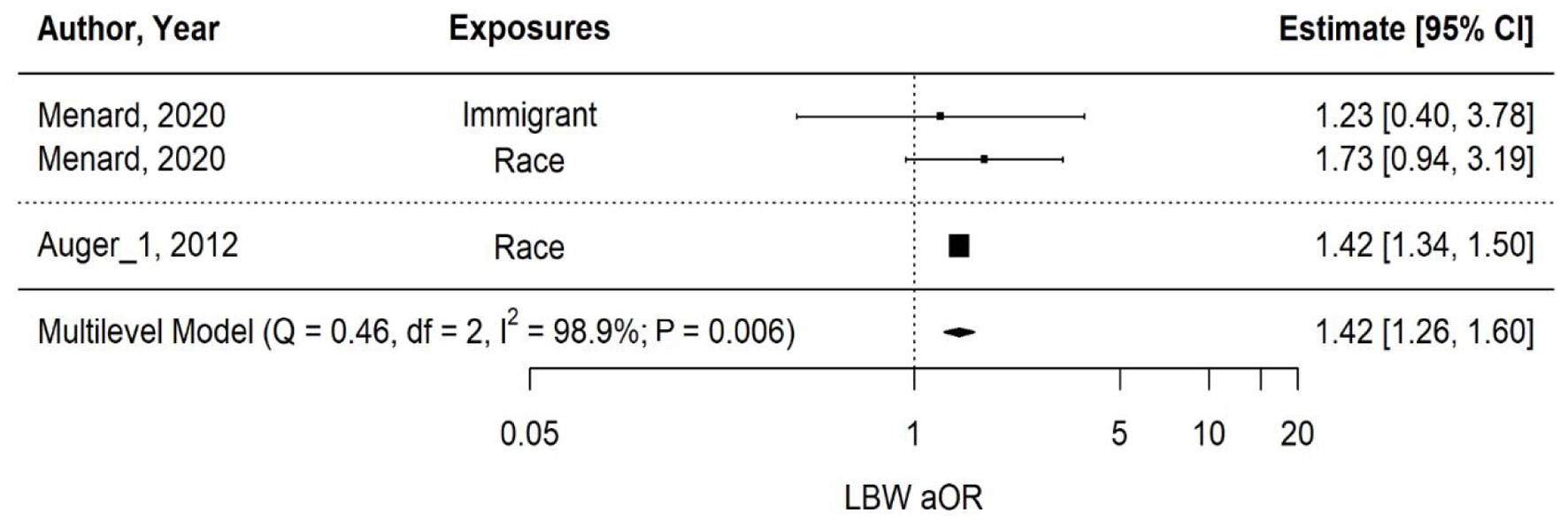 Click for large image | Figure 3. Multi-level meta-analysis and pooled estimate of adjusted odds ratios (aORs) in two studies for low birth weight (LBW). |
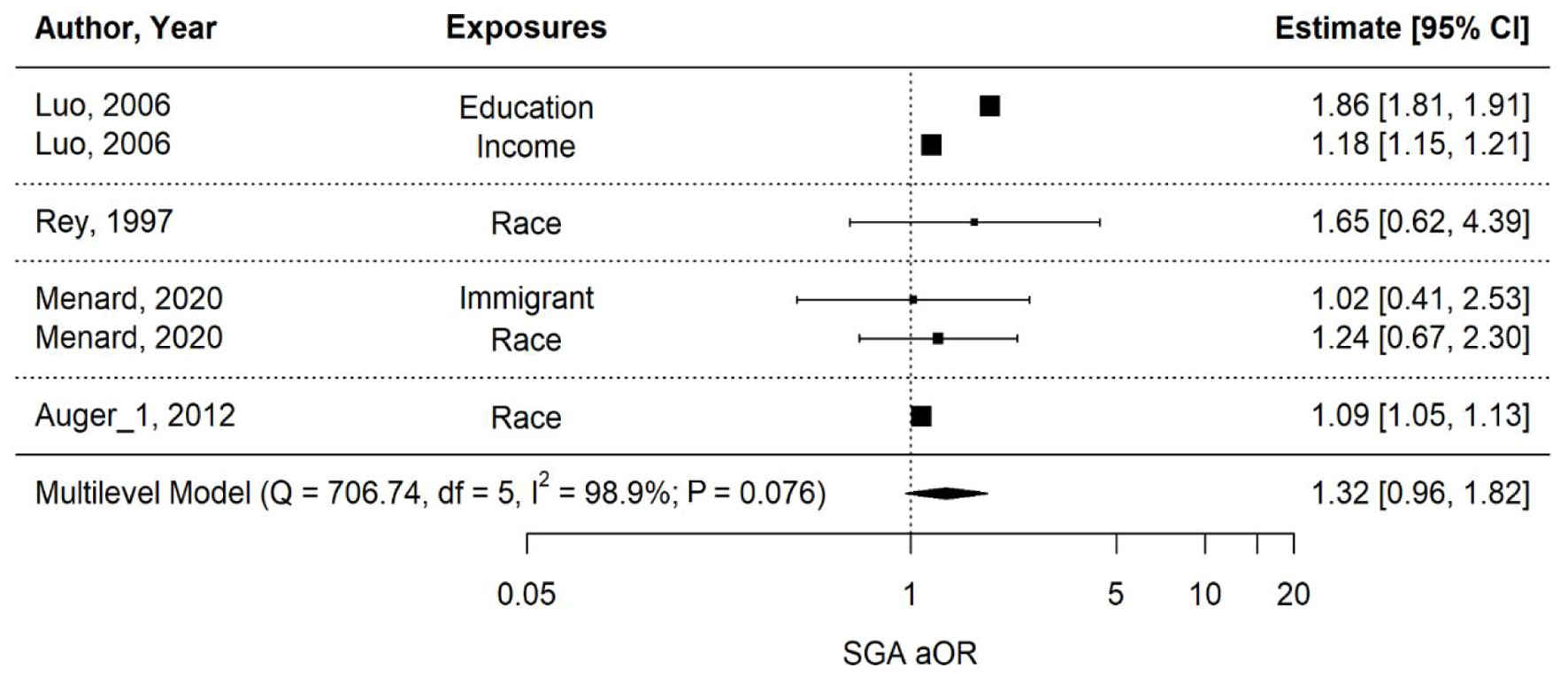 Click for large image | Figure 4. Multi-level meta-analysis and pooled estimate of adjusted odds ratios (aORs) in four studies for small for gestational age (SGA). |
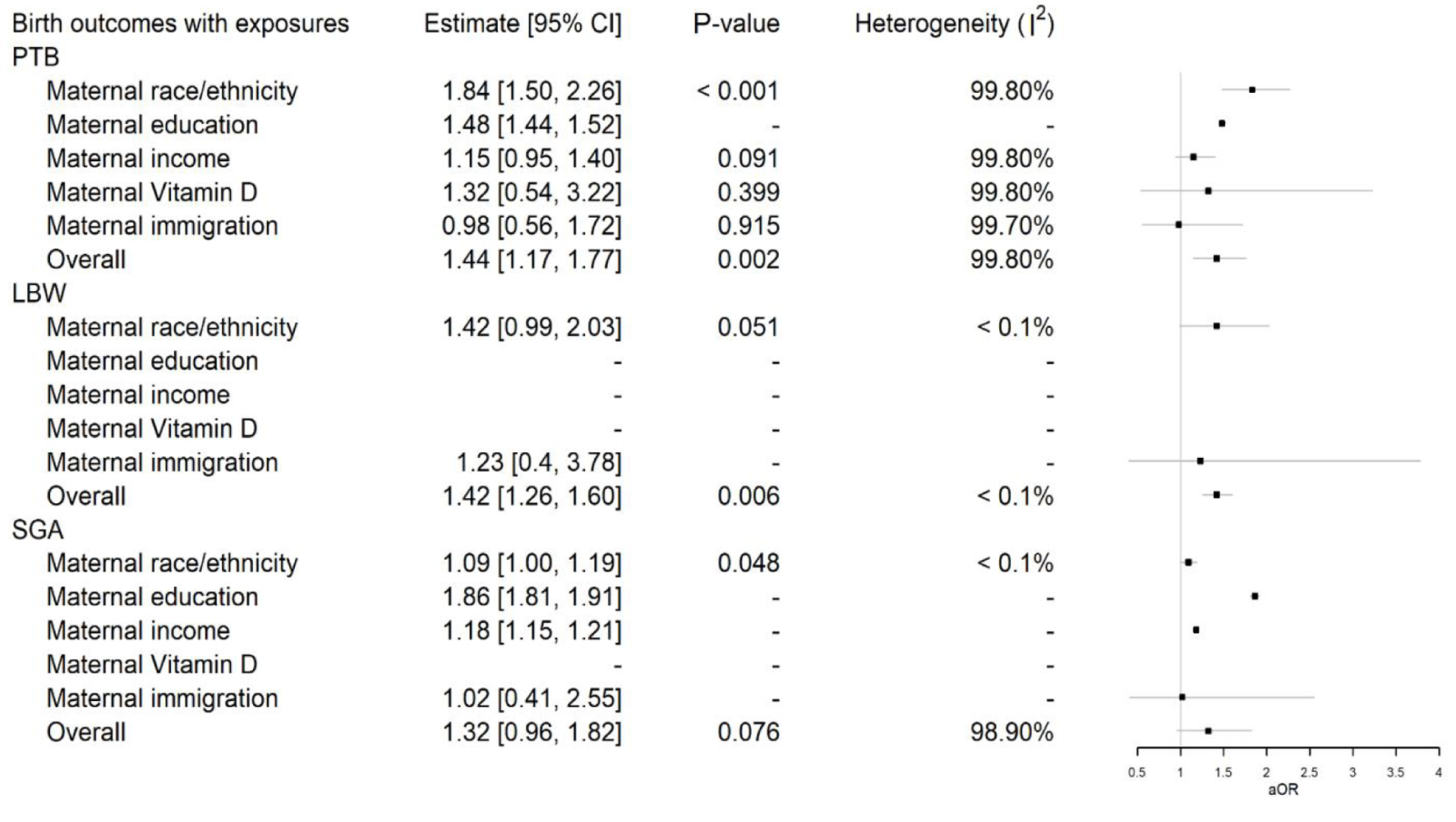 Click for large image | Figure 5. Multi-level meta-analysis and pooled adjusted odds ratios (aORs) for birth outcomes preterm birth (PTB), low birth weight (LBW), and small for gestational age (SGA) across different exposures. |
PTB
The overall estimate for PTB across nine studies with various disparities is 1.44 (1.17 - 1.77), indicating a significant (P-value = 0.001) increase in the aOR associated with the exposures (Fig. 2). However, the heterogeneity of this estimate is extremely high (I2 = 99.8%) due to the diverse nature of the studies, which include both population-based and cohort studies. For instance, Lee (2020) had a very large sample size of over 5 million, while Tabatabaei et al (2017) had a sample size of just 472. This substantial difference in sample sizes contributes to the high heterogeneity observed.
Figure 5 presents the PTB estimates with different exposures and their associated pooled aORs where applicable. The pooled aOR for PTB associated with maternal race/ethnicity is significantly high at 1.84 (1.50 - 2.26) (P-value < 0.001). For maternal income, the association is moderate at 1.15 (0.95 - 1.40) (P-value = 0.091), but non-significant. Significant associations to PTB were not observed with vitamin D levels or maternal immigration status. Level of education was significantly associated to PTB in the one relative article; however, there were not enough data across studies for a pooled estimate to determine overall significance.
LBW
The overall pooled aOR for LBW across two studies examining race and immigration shows a significantly high estimate in favor of exposures at 1.42 (1.26 - 1.60) (P-value = 0.006), as shown in Figure 3. Heterogeneity is very low (I2 < 0.01%) due to the involvement of only two studies with fairly similar estimates. As detailed in Figure 5, the estimate for maternal race/ethnicity is moderately high at 1.42 (0.99 - 2.03) (P-value = 0.051), but non-significant. No significant association was observed between immigration alone and LBW.
SGA
The overall pooled aOR for SGA across four studies examining race, immigration, education, and income shows a moderately high estimate in favor of exposures at 1.32 (0.96 - 1.82) (P-value = 0.076), but non-significant, as depicted in Figure 4. Heterogeneity is high (I2 = 98.9%). Regarding the estimate for maternal race/ethnicity and SGA in Figure 5, it is significantly high at 1.09 (1.00 - 1.19) (P-value = 0.048), while no pooled association could be derived for education, income, and immigration alone, given that only one study was involved with each exposure respectively.
| Discussion | ▴Top |
Main findings
The purpose of this review was to assess and understand the disparities in PTB in Black birthing people in Canada. Our initial literature review included 10 articles, the majority of which were of retrospective cohort design and considered “good” quality. Most studies discussed how ethnic minorities, especially the Black population in Canada, experienced higher rates of PTB and other adverse pregnancy outcomes like LBW, SGA, and stillbirth while also addressing the relationship between PTB and socioeconomic risk factors such as level of education, income, poverty, and social isolation. In this meta-analysis, only the associations between maternal race/ethnicity and PTB (and between maternal race/ethnicity and SGA) were found to be significant across the studies.
Racial disparities in health and PTB
It is not unknown that African Americans in the United States experience worse perinatal outcomes due to its history deeply rooted in slavery, segregation, and ongoing racial prejudice leading to socioeconomic disadvantages in education, housing, and the workforce [33, 34]. In Canada, the Black population also experiences lower education levels, higher rates of unemployment, and poorer living conditions [34]. However, there is speculation that there may be a different experience related to the Black population in Canada, the majority of whom are immigrants who migrated after 1960 as opposed to descendants of slaves subjected to multigenerational discrimination as in the United States [34, 35]. Given Canada’s different historical upbringings and universal healthcare, McKinnon et al predicted that the Black population in Canada would experience less racial disparities in PTB rates than the Black population in the United States when comparing data from 2004 to 2006 [28]. Canada was depicted as the “less extreme” version of the United States, yet relative racial disparities in PTB rates were similar between Canada and the United States, suggesting that racial discrimination and systematic racism may also play a role in Canada [28].
In Canada, Black birthing people encounter significant disparities in maternal health outcomes compared to their White counterparts despite the nation’s universal healthcare system, findings consistent with the results of this review. For Black birthing people in Canada, this leads to poor healthcare experiences, with some Black women sharing that their prenatal care was dehumanizing and lacked quality [36]. These negative experiences, which were highlighted in an Ontario study on prenatal care in Toronto, can lead to delayed or inadequate care and a lack of trust in healthcare providers [36].
Systemic racism within the healthcare system plays a pivotal role in perpetuating these disparities, and racism as a social determinant of health adversely affects Black women’s health outcomes [36]. For example, wealth, education, and employment opportunities are controlled through existing institutions, and combined with historical injustices, one group is allowed advantages over another. Thus, Black women, who experience discrimination from both their gender and race [37, 38], are increasingly at a disadvantage. Studies have shown that even with higher education and economic advancement, Black women still experience higher morbidity and mortality rates: Black women were found to have maternal mortality rates of 40.8 deaths per 100,000 pregnancies compared to 12.7 in their White counterparts in 2018, and in 2019, the CDC reported that college-educated Black women still had higher rates of morbidity and mortality compared to White women who have only obtained a high school diploma [38-40].
By recognizing and addressing the intersecting factors that Black women face in America, we can also understand what contributes to the higher rates of PTB among Black birthing people in Canada and work towards achieving equitable maternal health outcomes.
Research implications and current guidelines
Part of achieving this is by engaging in more Canadian race-based studies to further illuminate the racial disparities regarding PTB among Black Canadians [36, 41]. It requires a multifaceted approach that addresses systemic racism within the healthcare system. The United Kingdom and the United States have adopted approaches to reducing racial inequities regarding reproductive and birthing care by collecting race-based data [42]. The United States is unique in that most of its studies are categorized by race and ethnicity instead of by maternal nationality. Canada has been lagging in the collection of race-based maternal-newborn data that could facilitate the study of racial inequity in maternal morbidity and mortality at the population level [42]. Advocacy for policies that address systemic racism and promote equitable access to healthcare is also necessary to reduce these disparities. It necessitates a comprehensive approach that acknowledges and confronts systemic racism within the healthcare system. This includes implementing cultural competency training for healthcare providers, enhancing access to healthcare services in marginalized communities, advocating for policy changes to address socioeconomic disparities, and amplifying the voices of Black women in healthcare decision-making processes [43].
Other countries have researched several aspects of PTB among Black birthing mothers including the treatment and prevention of PTB and its effects on infant morbidity and mortality rates. Studies in the United States demonstrate that Black mothers were less likely to receive antenatal corticosteroids (ACS) [44, 45]. Another United States study highlighted how Black infants who were born very prematurely were more likely to be born at hospitals with higher morbidity and mortality rates, which contributes to excess morbidity and mortality among Black infants [46]. The most up-to-date clinical practice guidelines by the Society of Obstetricians and Gynecologists of Canada (SOGC) state that the use of ACS in late PTB decreases the risk of respiratory morbidity in neonates; however, the effects on long-term neurodevelopment are unknown [47]. The SOGC also recommends the use of vaginal progesterone to aid in preventing PTB in pregnant people with a history of spontaneous PTB and/or short cervical lengths [48]. The SOGC has developed comprehensive guidelines for managing PTB, reflecting extensive research and clinical expertise. However, the absence of race-based guidelines and their implementation regarding PTB highlights a critical gap in addressing disparities among Black birthing people in Canada. The need for more data collection on Black Canadians regarding PTB is paramount to addressing significant health disparities not only in the epidemiology but also in the management and prevention of PTB. The data are essential for informing targeted interventions, policy development, and resource allocation aimed at reducing disparities ultimately ensuring equitable access to healthcare and improving maternal and neonatal health outcomes.
Strengths and limitations
Our review provides an examination of PTB in the Black population in Canada, and there are a number of strengths and limitations. One strength is that the authors are healthcare providers and part of the community of the study population. Other strengths include the use of multiple databases through University library search tools in order to have access to a broad range of articles that were reviewed extensively by our study team. Our meta-analysis was able to focus on three outcomes (PTB, LBW, and SGA) given that these data points were provided across the majority of the studies. Our meta-analysis was confined to nine articles and the population sizes varied leading to high heterogeneity - population studies involving over 5 million participants and cohort studies involving less than 1,500 participants. To correct this, our meta-analysis combines results from different studies by weighting them based on the inverse variance. This means that more precise studies or those with larger sample sizes received a higher weight in the pooled estimate, ensuring that the results are properly integrated.
Not all studies included data on race. There was also a need to reference data already presented by other countries like the United States and United Kingdom given that our review was limited by the small population size of Black people in Canada and our little understanding of the historical context and racial complexity of this population. This all emphasizes the need for additional research on racial disparities in adverse pregnancy and perinatal outcomes in Canada in general.
Conclusion
Addressing PTB within the context of Black reproductive health in Canada requires comprehensive strategies that prioritize equity, cultural competence, and community engagement. This article offers an overview of the epidemiology, risk factors, and racial disparities in PTB rates among the Black population in Canada. Future research should specifically focus on maternal risk factors, including racial and social factors that influence maternal health outcomes, chronic diseases that disproportionately affect Black populations, and current PTB interventions and their implementation in Black birthing mothers and their infants. By centering Black reproductive health within broader public health initiatives, engaging with and hiring more Black representatives in leadership positions, including raced-based data in Canadian research, and advocating for policies that focus on advancing black maternal health, there are plenty of opportunities to mitigate the adverse impact of PTB and improve maternal and neonatal outcomes within this marginalized community.
Acknowledgments
We would like to acknowledge that Dr. Cynthia Maxwell is a member of the Black Reproductive Health Working Group in Ontario, Canada, and we appreciate her contributions to this review.
Financial Disclosure
The authors report no financial disclosures.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Study conception and design: Ekpe E, Thompson K, Samson B, and Maxwell C. Data collection: Ekpe E, Thompson K, and Samson B. Data analysis: Nabipoor M. Data interpretation: Ekpe E and Nabipoor M. Draft manuscript preparation: Ekpe E, Thompson K, Samson B, and Nabipoor M. Final manuscript preparation: Ekpe E, Thompson K, Samson B, Nabipoor M, and Maxwell C.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACS: antenatal corticosteroids; NOS: Newcastle-Ottawa Scale; SOGC: Society of Obstetricians and Gynecologists of Canada; WHO: World Health Organization
| References | ▴Top |
- World Health Organization. Preterm birth fact sheet. 2023. Accessed April 2024 from: https://www.who.int/news-room/fact-sheets/detail/preterm-birth#:∼:text=Key%20facts,deaths%20in%202019%20(2).
- Canadian Institutes of Health Research. Preterm birth research initiative. Canada.ca 2017. Accessed April 2024 from: https://www.canada.ca/en/institutes-health-research/news/2017/05/preterm_birth_researchinitiative.html.
- Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3-12.
doi pubmed - Raab R, Hoffmann J, Spies M, Geyer K, Meyer D, Gunther J, Hauner H. Are pre- and early pregnancy lifestyle factors associated with the risk of preterm birth? A secondary cohort analysis of the cluster-randomised GeliS trial. BMC Pregnancy Childbirth. 2022;22(1):230.
doi pubmed pmc - Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41(7):387-391.
doi pubmed - Center for Disease Control and Prevention. Preterm Birth. Reproductive Health 2023. Accessed April 2024 from: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pretermbirth.htm.
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health. 2013;10 Suppl (Suppl 1):S2.
doi pubmed pmc - Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75-84.
doi pubmed pmc - Kistka ZA, Palomar L, Lee KA, Boslaugh SE, Wangler MF, Cole FS, DeBaun MR, et al. Racial disparity in the frequency of recurrence of preterm birth. Am J Obstet Gynecol. 2007;196(2):131.e131-136.
doi pubmed - National Center for Health Statistics, 2020-2022 Natality Data. Accessed August 2024 from: https://www.marchofdimes.org/peristats/reports/united-states/report-card.
- Black Enslavement in Canada (Plain-Language Summary). The Canadian Encyclopedia 2020. Accessed April 2024 from: https://www.thecanadianencyclopedia.ca/en.
- Maheux H, Do D. Diversity of the black population in canada: an overview. ethnicity, language, and immigration thematic series. Statistics Canada. 2019.
- The Canadian census: A rich portrait of the country's religious and ethnocultural diversity. Statistics Canada 2022. Accessed April 2024 from: https://www150.statcan.gc.ca/n1/daily-quotidien/221026/dq221026b-eng.htm.
- Cenat JM. Who is Black? The urgency of accurately defining the black population when conducting health research in Canada. Canadian Medical Association Journal. 2022;194(27):948-949.
- Cenat JM. Who is Black? There is an urgent need to clarify the definition of the black population in the conduct of health research in Canada. Canadian Medical Association Journal. 2022;194(36):1271-1273.
- Cotter A. Experiences of discrimination among the Black and Indigenous populations in Canada, 2019. Juristat. 2022:1-14.
- Lindsay C. The African community in Canada. Ottawa: Statistics Canada, Social and Aboriginal Statistics Division; 2001.
- Wells GA, O’Connell BS, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. The Ottawa Hospital Research Institute. 2020. Accessed April 2024 at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Cheung MW. Modeling dependent effect sizes with three-level meta-analyses: a structural equation modeling approach. Psychol Methods. 2014;19(2):211-229.
doi pubmed - Higgins JPT, Green S. (Eds.). Cochrane handbook for systematic reviews of interventions. 2011 (Version 5.1.0).
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691.
doi pubmed - Shor E, Roelfs D, Vang ZM. The "Hispanic mortality paradox" revisited: Meta-analysis and meta-regression of life-course differentials in Latin American and Caribbean immigrants' mortality. Soc Sci Med. 2017;186:20-33.
doi pubmed - Auger N, Roncarolo F, Harper S. Increasing educational inequality in preterm birth in Quebec, Canada, 1981-2006. J Epidemiol Community Health. 2011;65(12):1091-1096.
doi pubmed - Auger N, Chery M, Daniel M. Rising disparities in severe adverse birth outcomes among Haitians in Quebec, Canada, 1981-2006. J Immigr Minor Health. 2012;14(2):198-208.
doi pubmed - Auger N, Park AL, Gamache P, Pampalon R, Daniel M. Weighing the contributions of material and social area deprivation to preterm birth. Soc Sci Med. 2012;75(6):1032-1037.
doi pubmed - Boram Lee J, Hinds A, Urquia ML. Provincial variations in birth outcomes according to maternal country of birth, 2000 to 2016. Health Rep. 2020;31(4):13-21.
doi pubmed - Luo ZC, Wilkins R, Kramer MS, Fetal, Infant Health Study Group of the Canadian Perinatal Surveillance. Effect of neighbourhood income and maternal education on birth outcomes: a population-based study. CMAJ. 2006;174(10):1415-1420.
doi pubmed pmc - McKinnon B, Yang S, Kramer MS, Bushnik T, Sheppard AJ, Kaufman JS. Comparison of black-white disparities in preterm birth between Canada and the United States. CMAJ. 2016;188(1):E19-E26.
doi pubmed pmc - Menard V, Sotunde OF, Weiler HA. Ethnicity and immigration status as risk factors for gestational diabetes mellitus, anemia and pregnancy outcomes among food insecure women attending the Montreal Diet Dispensary Program. Can J Diabetes. 2020;44(2):139-145.e131.
doi pubmed - Miao Q, Guo Y, Erwin E, Sharif F, Berhe M, Wen SW, Walker M. Racial variations of adverse perinatal outcomes: A population-based retrospective cohort study in Ontario, Canada. PLoS One. 2022;17(6):e0269158.
doi pubmed pmc - Rey E. Preeclampsia and neonatal outcomes in chronic hypertension: comparison between white and black women. Ethn Dis. 1997;7(1):5-11.
pubmed - Tabatabaei N, Auger N, Herba CM, Wei S, Allard C, Fink GD, Fraser WD. Maternal vitamin D insufficiency early in pregnancy is associated with increased risk of preterm birth in ethnic minority women in Canada. J Nutr. 2017;147(6):1145-1151.
doi pubmed - Brown HL, Small MJ, Clare CA, Hill WC. Black women health inequity: the origin of perinatal health disparity. J Natl Med Assoc. 2021;113(1):105-113.
doi pubmed - Frazier JW, Darden JT, Henry NF. The African diaspora in the United States and Canada at the dawn of the 21st century. Albany (NY): State University of New York Press. 2010.
- Attewell P, Kasinitz P, Dunn K. Black Canadians and black Americans: racial income inequality in comparative perspective. Ethn Racial Stud. 2010;33:473-495.
- Boakye PN, Prendergast N, Bandari B, Anane Brown E, Odutayo AA, Salami S. Obstetric racism and perceived quality of maternity care in Canada: Voices of Black women. Womens Health (Lond). 2023;19:17455057231199651.
doi pubmed pmc - Crenshaw 2023 Crenshaw, KW. On Intersectionality: Essential Writings, 2017. Faculty Books. 255. https://scholarship.law.columbia.edu/books/255.
- James-Conterelli S, Dunkley D, McIntosh JT, Julien T, Nelson MD, Richard-Eaglin A. The impact of systemic racism on health outcomes among Black women: Recommendations for change. Nurse Pract. 2023;48(2):23-32.
doi pubmed - Petersen EE, Davis NL, Goodman D, Cox S, Syverson C, Seed K, Shapiro-Mendoza C, et al. Racial/ethnic disparities in pregnancy-related deaths - United States, 2007-2016. MMWR Morb Mortal Wkly Rep. 2019;68(35):762-765.
doi pubmed pmc - Declercq E, Zephyrin L. Maternal mortality in the United States: a primer. Commonwealth Fund, 2020.
doi - Dayo E, Christy K, Habte R. Health in colour: black women, racism, and maternal health. Lancet Reg Health Am. 2023;17:100408.
doi pubmed pmc - Maxwell C, Tunde-Byass M, Wilson-Mitchell K. Achieving equity in reproductive care and birth outcomes for Black people in Canada. CMAJ. 2024;196(10):E343-E345.
doi pubmed pmc - Castle B, Wendel M, Kerr J, Brooms D, Rollins A. Public Health's approach to systemic racism: a systematic literature review. J Racial Ethn Health Disparities. 2019;6(1):27-36.
doi pubmed - Ondusko DS, Garg B, Caughey AB, Pilliod RA, Carter EH. Is appropriate administration of antenatal corticosteroids associated with maternal race? Am J Perinatol. 2022;39(11):1204-1211.
doi pubmed - Gulersen M, Grunebaum A, Lenchner E, Chervenak FA, Bornstein E. Racial disparities in the administration of antenatal corticosteroids in women with preterm birth. Am J Obstet Gynecol. 2020;223(6):933-934.
doi pubmed - Howell EA, Janevic T, Hebert PL, Egorova NN, Balbierz A, Zeitlin J. Differences in morbidity and mortality rates in black, white, and Hispanic very preterm infants among New York City hospitals. JAMA Pediatr. 2018;172(3):269-277.
doi pubmed pmc - Liauw J, Foggin H, Socha P, Crane J, Joseph KS, Burrows J, Lacaze-Masmonteil T, et al. Erratum to technical update No. 438: antenatal corticosteroids at late preterm gestation. Journal of Obstetrics and Gynaecology Canada 2023;45(6):445-457.E2. J Obstet Gynaecol Can. 2023;45(12):102270.
doi pubmed - Jain V, McDonald SD, Mundle WR, Farine D. Guideline No. 398: progesterone for prevention of spontaneous preterm birth. J Obstet Gynaecol Can. 2020;42(6):806-812.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
