| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 13, Number 3, December 2024, pages 95-100
A Rare Case of Leiomyosarcoma in a Fourteen-Year-Old Female: Challenges in Treatment and Recurrence Management
Dina Marlinaa, e , Aditya Utomoa, Aditiyono Aditiyonob, Muhammad Alamsyah Azizc, Putri Nadhira Adinda Adriansyahd
aDepartment of Obstetrics and Gynecology, Faculty of Medicine, University of Padjadjaran - Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
bDepartment of Obstetrics and Gynecology, Faculty of Medicine, University of Jendral Soedirman - Prof. Dr. Margono Soekarjo General Hospital, Puwokerto, Indonesia
cFetal and Maternal Division, Faculty of Medicine, University of Padjadjaran - Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
dFaculty of Medicine, University of Padjadjaran - Dr. Hasan Sadikin General Hospital, Bandung, Indonesia
eCorresponding Author: Dina Marlina, Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, Indonesia
Manuscript submitted August 6, 2024, accepted September 30, 2024, published online December 2, 2024
Short title: Rare Case of Endometrial Teenage LMS
doi: https://doi.org/10.14740/jcgo994
| Abstract | ▴Top |
Uterine sarcoma is rare and accounts for 1.7 per 100,000 cases annually. It most often occurs in postmenopausal women aged 44.6 to 58 years and is rarely observed in adolescents. The gold standard therapy is surgery; however, oophorectomy, adjuvant radiotherapy, and chemotherapy remain controversial. A 14-year-old girl presented with intermenstrual bleeding for a year. Ultrasonography revealed a solid tumor, and the biopsy result was endometrial sarcoma. The patient was diagnosed with uterine sarcoma grade I-II. The tumor was inoperable on surgical resection planning, and the patient underwent chemoradiotherapy. An abdominal computed tomography (CT) scan post-chemoradiotherapy showed a solid mass with lymphadenopathy in the uterus. The patient underwent a second course of chemoradiotherapy and showed a good response, although recurrence still occurred. Leiomyosarcoma has a poor prognosis and low survival rate, even in the early stages, despite undergoing surgery with adjuvant chemoradiotherapy. In our case, the patient was an adolescent who presented challenges in management, particularly regarding the necessity of oophorectomy and the role of adjuvant radiotherapy and chemotherapy in young women.
Keywords: Adolescent leiomyosarcoma; Uterine sarcoma; Chemoradiotherapy response; Pelvic mass in adolescents
| Introduction | ▴Top |
Leiomyosarcoma (LMS) is a type of cancer that arises from abnormal development of smooth muscle cells [1]. LMS is an uncommon yet very aggressive tumor that affects the vaginal tract and other organs [2]. Unlike endometrial carcinomas, which mostly metastasize to the lymph nodes, uterine LMS exhibits a significant tendency for hematogenous dissemination, predominantly to the lungs. Uterine LMSs predominantly metastasize to the lungs, peritoneum, bones, and liver. Local recurrence was linked to peritoneal dissemination and pulmonary metastases along with additional sites of hematogenous metastasis [3]. It is associated with adverse clinical outcomes, high recurrence rate (53-71%), and poor prognosis. It constitutes approximately 3-7% of all cancers of the uterus and 1% of all cancers of the female reproductive system [4]. The average occurrence age ranges from 44.6 to 58.1 years [5, 6]. In childhood, they are classified as ultra-rare sarcomas with an annual incidence of less than 1 per 1,000,000 [7].
This tumor is uncommon, and its occurrence in teens is extremely rare [8]. Risk factors contributing to LMS include a history of pelvic radiation, nulliparity, advancing age, and obesity due to exposure to tamoxifen. The extended use of tamoxifen, an estrogen receptor agonist in the uterus, is linked to a triple risk of sarcoma development [9]. Signs and symptoms of LMS include abnormal uterine bleeding, a palpable pelvic mass, pelvic discomfort, and protrusion in the vaginal area [2, 9]. Uterine sarcomas are histologically classified as carcinosarcoma (malignant mesodermal mixed tumor), LMS, endometrial stromal sarcoma, and undifferentiated sarcoma. LMS is one of the most prevalent subtypes of soft tissue sarcoma (STS) in adults, along with carcinosarcoma, constituting 10-20% of newly diagnosed cases [1]. In addition to patient history and physical examination, imaging methods are crucial for diagnosing LMS. Preoperative computed tomography (CT), positron emission tomography, contrast-enhanced magnetic resonance imaging (MRI), and diffusion-weighted MRI are reported to be beneficial in distinguishing between tumor types and aiding in the differential diagnosis [2]. The gold standard therapy for LMS is surgery, specifically total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH-BSO). This surgical procedure ensures complete removal of the uterus and tumor, thus minimizing the risk of tumor rupture or spillage into the peritoneal cavity. However, there is controversy regarding the necessity of oophorectomy as well as the role of adjuvant radiotherapy and chemotherapy, particularly in young women [2]. We report a rare case of LMS in a young woman who underwent chemoradiotherapy due to an inoperable case.
| Case Report | ▴Top |
A 14-year-old girl was referred to Margono Hospital Polyclinic by Primary Health Care (PHC) with the chief complaint of intermenstrual bleeding persisting for the past year. The bleeding was heavy, requiring 5 - 6 pads per day, but was not accompanied by abdominal pain or cramping. The patient had no history of vaginal discharge, weight loss, abnormal urination, or changes in bowel habits. Menarche occurred at age 12, with regular menstrual cycles lasting 5 days, typically requiring five pads per day. She had no history of dysmenorrhea. The patient was unmarried, had no history of sexual activity, and had never used contraceptives or had undergone surgical procedures.
Upon physical examination, the patient was alert with stable vital signs: blood pressure, 105/53 mm Hg; heart rate, 130 bpm; respiratory rate, 20 breaths/min; and oxygen saturation, 98% in room air. Her body weight was 38 kg and her height was 146 cm, placing her in the underweight category based on body mass index (BMI).
Initial diagnostic investigations included ultrasonography (performed before her visit to the hospital), which revealed a uterine mass measuring 7.11 × 6.06 × 5.28 cm. Abdominal palpation revealed a mass, and rectovaginal examination revealed an intact hymen and a mass in the adnexal region. A cervical biopsy was performed on August 15, 2022, which revealed endometrial cartilaginous metaplasia. A follow-up histopathological examination on September 3, 2022 confirmed the diagnosis of endometrial sarcoma. Biopsy revealed necrotic tissue with polymorphic nuclear cells, hyperchromatic features, and mitotic tumor masses (Figs. 1 and 2).
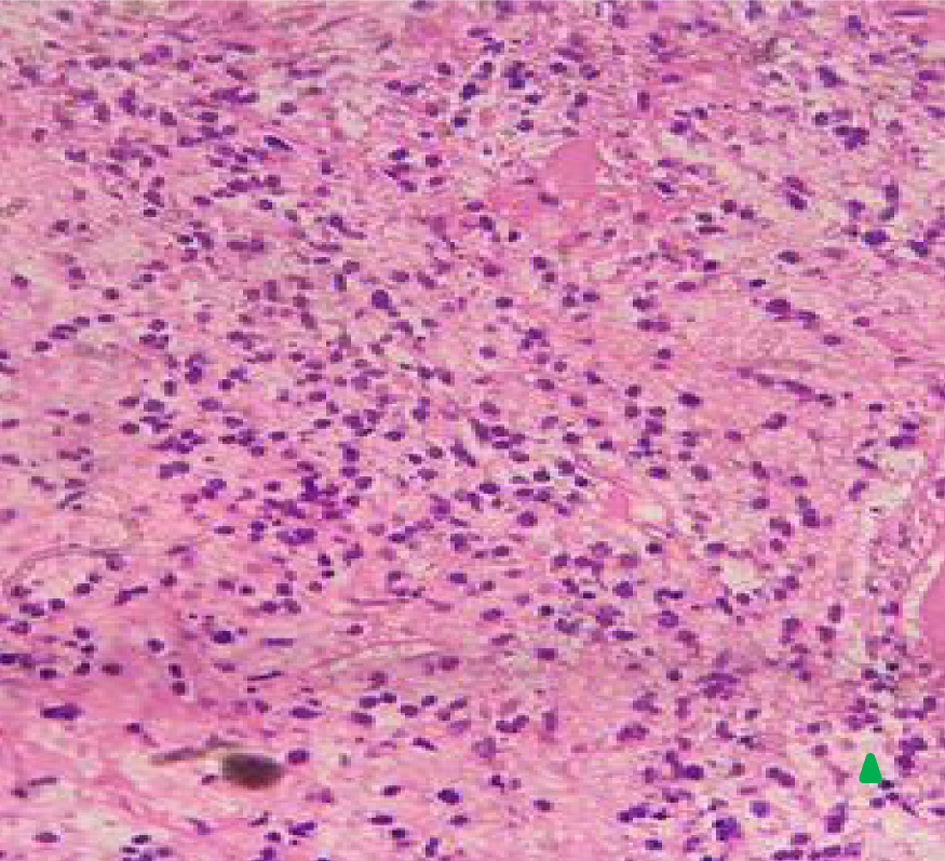 Click for large image | Figure 1. Histopathology of vaginal uterine mass (× 40) showing endometrial cartilaginous metaplasia upon magnification × 40 (arrow). |
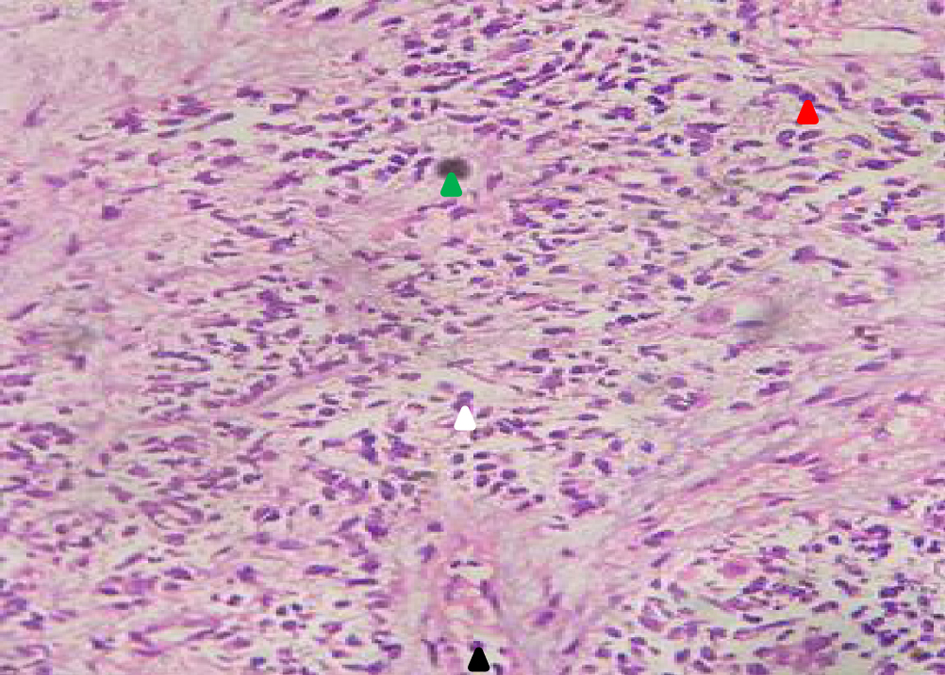 Click for large image | Figure 2. Histopathology of uterine mass (× 40) showing necrotic tissues (green) with rounded, oval, polymorphic nuclear cells (red arrow), hyperchromatic (white arrow), and mitotic tumor mass (black arrow). The presence of endometrial sarcoma was seen in the biopsy result. |
During her initial visit to Margono Hospital on July 7, 2022, the patient was noted to be anemic with a normal neutrophil-lymphocyte ratio (NLR). Subsequently, on September 6, 2022, the patient was admitted for chronic kidney disease (CKD) management, with a urinary protein-to-creatinine ratio (Ur/Cr) of 78.88/6.84 based on tests conducted on September 10, 2022. By September 21, 2022, the patient was diagnosed with stage I-II uterine sarcomas. The highest recorded NLR was 22.35 during this period. Throughout her treatment course, the patient’s thrombocyte levels and NLR initially fluctuated but later stabilized (Figs. 3 and 4).
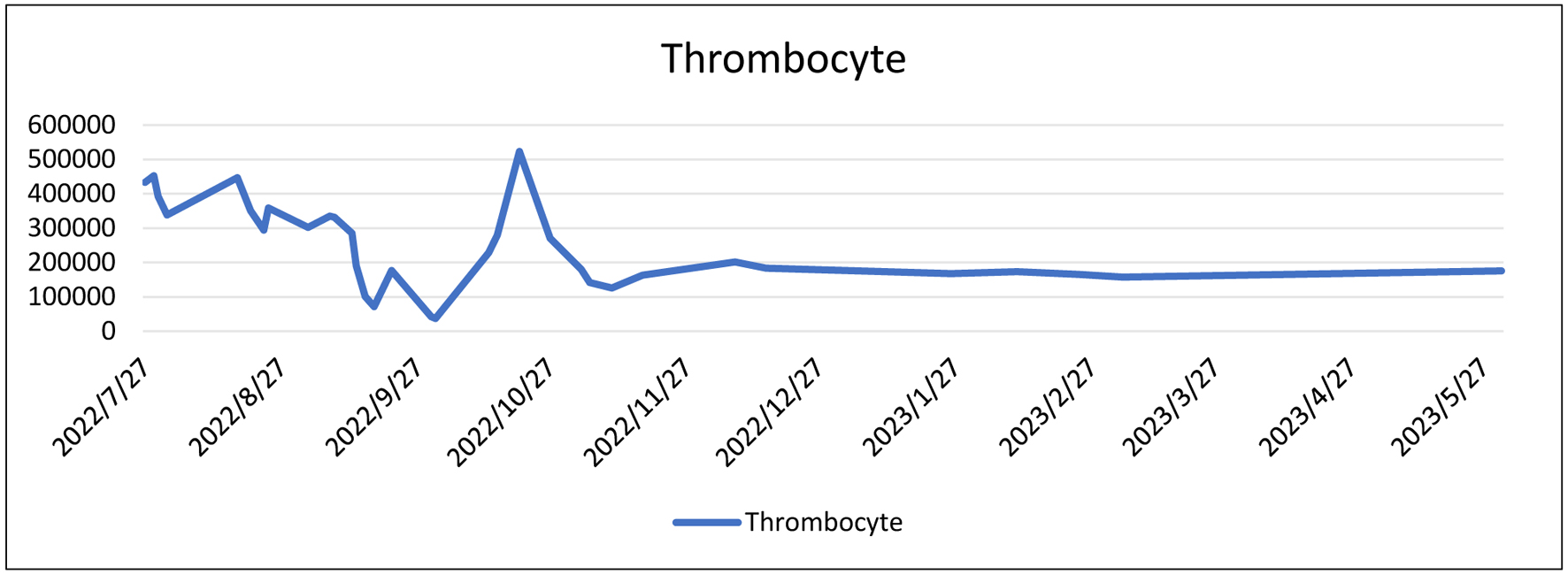 Click for large image | Figure 3. Laboratory trend of thrombocyte level. |
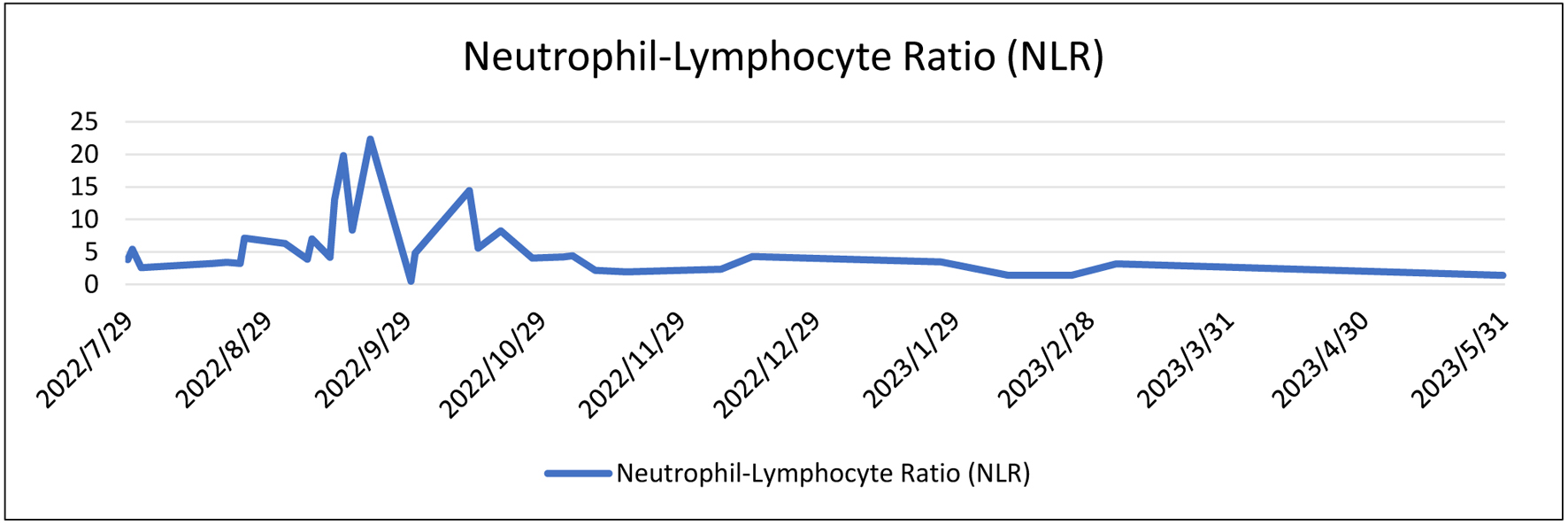 Click for large image | Figure 4. Laboratory trend of neutrophil-lymphocyte level. |
Surgical resection was performed based on the diagnosis. During the procedure, the uterus measured 5 × 5 cm and had a cervical mass compressing the bladder and rectum against the pelvic wall. The mass was immobile and prone to bleeding, which rendered it inoperable. The mass was extracted for biopsy, which confirmed the diagnosis. The patient subsequently underwent chemoradiation therapy.
Following the first cycle of chemoradiotherapy, an abdominal CT scan with contrast was performed (Fig. 5), which showed a persistent lobulated solid mass with calcification in the uterine corpus (measuring 3.05 × 5.13 × 5.05 cm) and surrounding fat stranding, suggestive of LMS. Multiple lymph nodes were enlarged in the parailiac and inguinal regions, with the largest measuring 1.74 × 1.56 cm. A simple cyst was also noted on the upper pole of the right kidney (1.24 × 1.09 cm). Despite this, the patient continued to receive regular chemoradiotherapy, showing gradual improvement over the course of treatment, despite recurrent disease episodes.
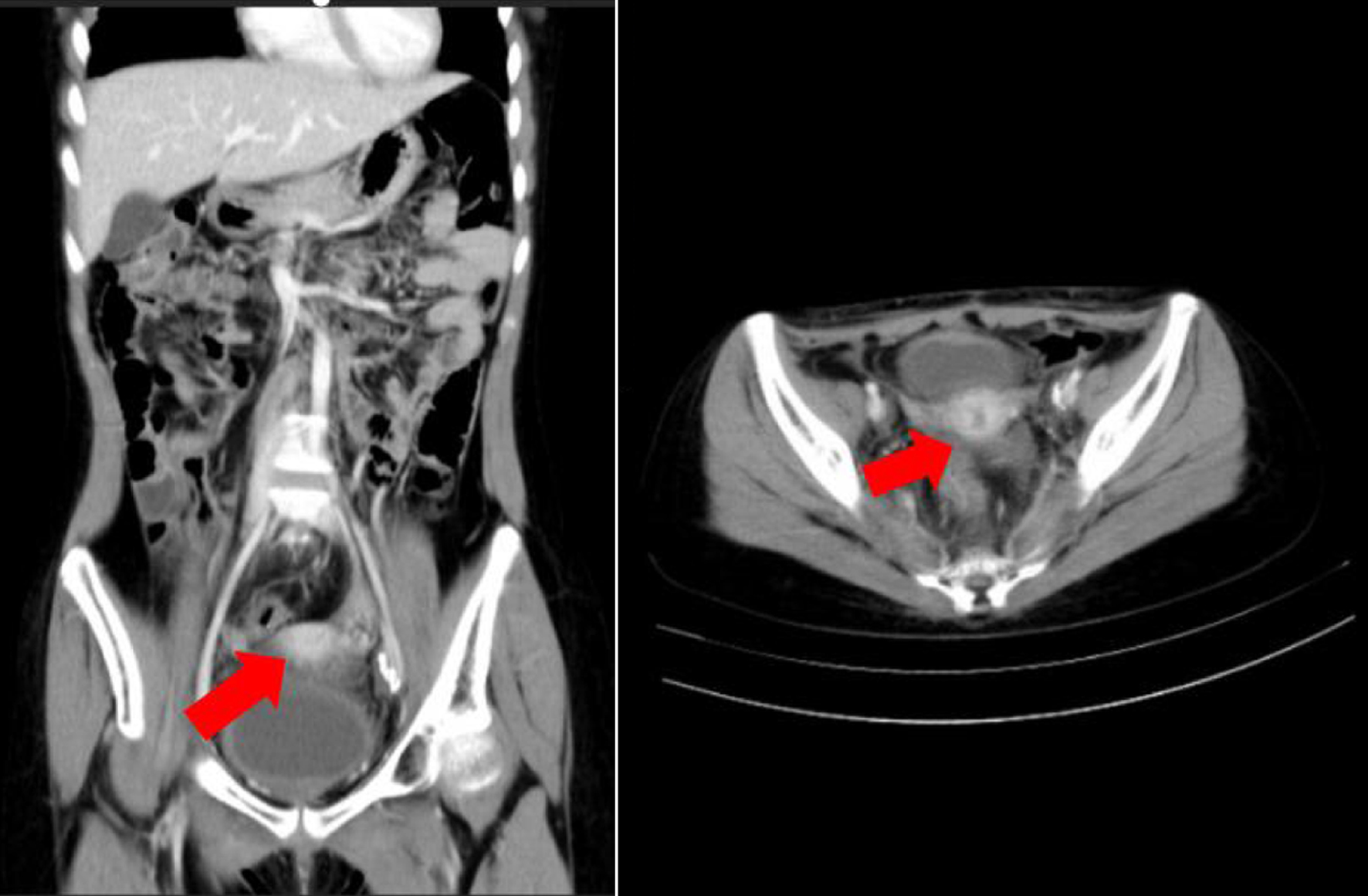 Click for large image | Figure 5. Computed tomography scan of the patient. Red arrow shows lobulated solid mass with calcification at corpus of uterine (dimension 3.05 × 5.13 × 5.05 cm) with fat stranding around, suspected for leiomyosarcoma. |
Chemoradiotherapy extended her survival to 3 years, without requiring surgical intervention, despite several recurrences over the treatment period.
| Discussion | ▴Top |
LMS is a highly aggressive type of mesenchymal cancer and is among the most prevalent forms of STSs. It exhibits heterogeneity in disease presentation, originating from different sites and demonstrating diverse genomic profiles. The annual occurrence rate of uterine sarcoma is roughly 1.7 cases per 100,000 women, classifying it as a rare condition. LMS constitutes over 60% of all STSs and typically appears around the age of 48 years. It predominantly affects postmenopausal women, with an average age ranging from 44.6 to 58.1 years. LMSs are primarily found in the myometrium or subserosal layer of the uterus [5, 6]. The median age at diagnosis of LMS is 4 - 5 decades. Thus, this case could be considered a unique case, as it occurred in a teenage girl and was in the endometrium (not married yet, with nulliparity status P0A0). Several factors have been identified as potential risk factors for LMS development. These include obesity, postmenopausal hormone therapy with estrogen and progesterone, oral contraceptive use, and history of pelvic radiotherapy [5]. According to Felix et al, significant risk factors for uterine sarcoma include obesity (BMI > 30 compared to BMI < 25; odds ratio (OR) 1.73, 95% confidence interval (CI) 1.22 - 2.46, P-value = 0.008) and a history of diabetes (OR 2.33, 95% CI 1.41 - 3.83), while older age at menarche was inversely associated with uterine sarcoma risk (> 15 years vs. < 11 years, OR 3.03, 95% CI 2.82 - 3.26). However, we have not identified any risk factors for LMS in this case [10].
Typical clinical manifestations of LMS include abnormal uterine bleeding, palpable pelvic mass, and pelvic discomfort. These are not only found in LMS, but also in leiomyoma. Kohler’s analysis revealed that none of the measured symptoms, such as intermenstrual bleeding or postmenopausal bleeding, and parameters were specific enough to differentiate LMS from other types of abnormal uterine bleeding [6, 11]. The most conclusive approach for distinguishing between distinct types is by histological analysis of surgical samples to ascertain if the tumor is benign or malignant. Currently, preoperative diagnosis is crucial. However, performing biopsy in patients with LMS has many constraints. Initially, the LMS lesion arises from the innermost layer of the myometrium, making it more difficult to reach. In addition, it is necessary to perform a histological assessment of advanced regions, taking into account three factors: mitotic index, degree of cytological atypia, and the presence or absence of coagulative tumor cell necrosis. However, conducting this evaluation is challenging because of the difficulty in obtaining the tissue samples [10]. Related to our case, the patient presented with the chief complaint of intermenstrual bleeding in the previous year. The bleeding was for 5 - 6 pads per day, with no abdominal pain or cramps felt when the bleeding came out. There had no history of vaginal discharge, weight loss, abnormal micturition, or defecation. The anthropometric body weight was 38 kg, body height was 146 cm, and the BMI was classified as underweight. A mass was observed on abdominal palpation. On rectovaginal examination, the hymen was intact, and a mass was found on the adnexa.
Several scoring systems have been developed to aid in the preoperative diagnosis of LMS, some of which are based on laboratory tests, while others rely on imaging techniques. First, Nagai et al introduced the preoperative sarcoma score (PRESS), which assesses clinical findings, blood tests, imaging (ultrasound and MRI), and endometrial cytology. Among these parameters, age, lactate dehydrogenase (LDH) level, MRI findings, and endometrial biopsy results were identified as the most significant predictors of sarcoma. A total of 7 points were assigned if all parameters were positive. Additionally, 2 points were given for age over 49 years, serum LDH levels exceeding 279, and positive endometrial histopathological test findings. The authors recommended a cut-off score of 3 points, indicating the need for surgical intervention [12]. Second, Kohler et al devised a scale based on bleeding symptoms, such as intermenstrual bleeding, heavy menstrual bleeding, dysmenorrhea, and postmenopausal bleeding, as well as imaging. This scale assesses the likelihood of predicting LMS through methods such as endometrial biopsy, color Doppler sonography, LDH levels, and transcervical biopsy [11].
Zhang et al introduced one of the most intricate and precise scoring systems. This system considers parameters such as age > 49 years, tumor size > 7 cm, NLR > 2.8, platelet count > 298 × 109, and LDH levels > 193 U/L. The total score on this scale is 7 points, with 2 points assigned for LDH and tumor size and 1 point for the remaining parameters. A prediction of LMS was made if the total score exceeded 4 [13]. Based on age stratification data indicating the highest incidence of LMS in individuals over 75 years of age, our case was initially suspected to be cervical carcinoma due to the patient’s young age. Due to the unexpected diagnosis of LMS, we did not perform any laboratory testing to fulfill the score criteria.
According to the histopathological results, lymph node involvement included grade III sarcomas. Surgery remains the primary treatment for uterine sarcoma, irrespective of grade. The gold standard involves the complete removal of the disease without fragmentation, ensuring negative surgical margins. This typically entails a TAH-BSO. Ovarian-conserving surgery may be an option for early-stage LMS in premenopausal individuals without compromising survival. In cases of advanced disease, maximal cytoreduction, when possible, has the potential to substantially enhance survival. Residual after surgery is an indicator of poor prognosis. Adjuvant radiation therapy has not demonstrated efficacy in stage I and II patients, but chemotherapy following complete surgical resection may prolong the disease-free survival time. The management strategy for non-metastatic disease involves a multimodal approach, starting with optimal surgery, followed by chemotherapy and radiotherapy, even in the early stages. Palliative chemotherapy is recommended in metastatic or recurrent settings, with the carboplatin/paclitaxel doublet regimen being the first-line option [14-16]. In this case, the patient underwent surgical resection first, but during the operation, the operator found it inoperable because the mass bled easily and pressed the surrounding organs such as the bladder and rectum. Therefore, the patient underwent chemoradiotherapy. Due to the young age of the patient, the response to the therapy was good, yet it still recurred several times, as evaluated by CT scan imaging.
As LMS is rare, only a limited number of systematic reviews have been conducted [15]. Therefore, it remains inconclusive which therapy is effective for LMS, although Schoffski et al showed that recurrent or metastasized tumors respond more positively to chemotherapy than other types of sarcomas [17]. Currently, our patient is undergoing a second round of chemotherapy as a result of a CT scan conducted after the initial treatment, which revealed the presence of a lump. For high-grade sarcomas, the recommended follow-up regimen includes regular physical examinations and imaging every 3 - 4 months for the first 2 - 3 years, followed by assessments every 6 months for the next 5 years, and annually thereafter. Imaging modalities should include CT scans of the chest, abdomen, and pelvis, optionally supplemented with pelvic MRI [14].
LMS was diagnosed according to World Health Organization (WHO) criteria. Even when confined to the time of diagnosis, LMS is associated with a poor prognosis. In a multicenter study, Brohl et al observed that being diagnosed under the age of 50 years was associated with a more favorable prognosis. They noted the highest risk of sarcoma, at 10 cases per 1,000, among individuals aged 75 - 79 years, whereas those under 30 years had the lowest risk, at 1 case per 500 [14]. The median overall survival was less than 2 years, with a 5-year overall survival rate of less than 30% (approximately 50% and 20% in the early and advanced stages, respectively). Additional prognostic factors include tumor size, age, vascular space involvement, mitotic count, residual disease post-surgery, and adjuvant chemotherapy. An adverse outcome is predicted by a tumor size exceeding 5 cm, infiltration, high-grade cytologic features, a mitotic rate exceeding 50, necrosis, or lymphovascular invasion [2, 6, 16]. Based on the age of the patient in her teenage years, we inferred a favorable prognosis. However, owing to incomplete data regarding other parameters, it remains inconclusive whether this case will have a poor or good prognosis.
Conclusion
Although LMSs typically occur at an advanced age, they can also manifest in adolescents. Therefore, considering LMS as a potential differential diagnosis initially prompts assessors to gather confirming data to fulfill the scoring criteria. LMS has a poor prognosis and low survival rate, even in the early stages, despite undergoing surgery with adjuvant chemoradiotherapy. In our case, the patient was an adolescent who presented challenges in management, particularly regarding the necessity of oophorectomy and the role of adjuvant radiotherapy and chemotherapy in young women.
Acknowledgments
We thank the nurses and midwives of Prof. Dr. Margono Soekarjo (RSMS) General Hospital for their support in this case.
Financial Disclosure
This study did not receive any specific grants from public, commercial, or not-for-profit funding agencies.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Informed Consent
Written informed consent was obtained from the patient for publication of the case report and accompanying images.
Author Contributions
Dina Marlina contributed to drafting the manuscript, undertaking a literature review, and critically revising the article for important intellectual content. Muhammad Alamsyah Aziz and Aditiyono Aditiyono contributed to patient care, conception of the case report, acquisition and interpretation of data, and critical revision of the article for important intellectual content. Putri Nadhira Adinda Adriansyah, Dina Marlina, and Aditya Utomo drafted the manuscript and critically revised the article for important intellectual content. All authors approved the final submitted manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available in the article.
Abbreviations
BMI: body mass index; CKD: chronic kidney disease; CT: commuted tomography; LMS: leiomyosarcoma; NLR: neutrophil-lymphocyte ratio; TAH-BSO: total abdominal hysterectomy with bilateral salpingo-oophorectomy; Ur/Cr: urinary protein to creatinine ratio
| References | ▴Top |
- Lacuna K, Bose S, Ingham M, Schwartz G. Therapeutic advances in leiomyosarcoma. Front Oncol. 2023;13:1149106.
doi pubmed pmc - Adewole AA, Onile TG, Ugiagbe AO, Fadahunsi OO, Awelimobor DI, Akinro O. Cervical leiomyosarcoma in a teenage girl: a rare form of uterine leiomyosarcoma. J Taibah Univ Med Sci. 2022;17(3):523-528.
doi pubmed pmc - Tirumani SH, Deaver P, Shinagare AB, Tirumani H, Hornick JL, George S, Ramaiya NH. Metastatic pattern of uterine leiomyosarcoma: retrospective analysis of the predictors and outcome in 113 patients. J Gynecol Oncol. 2014;25(4):306-312.
doi pubmed pmc - D’Angelo E, Prat J. Uterine sarcomas: a review. Gynecol Oncol. 2010;116(1):131-139.
doi pubmed - Liu J, Wang Z. Advances in the preoperative identification of uterine sarcoma. Cancers (Basel). 2022;14(14):3517.
doi pubmed pmc - Zak K, Zaremba B, Rajtak A, Kotarski J, Amant F, Bobinski M. Preoperative differentiation of uterine leiomyomas and leiomyosarcomas: current possibilities and future directions. Cancers (Basel). 2022;14(8):1966.
doi pubmed pmc - Genevois A-L, Carton M, Jean-Denis M, Cyrta J, Corradini N, Berlanga P, Chemin-Airiau C, et al. Leiomyosarcoma and liposarcoma in young patients: The national netsarc+ network experience. EJC Paediat Oncol. 2023;2:100026.
- Ozcan J, Dulger O, Kupelioglu L, Gonenc AI, Ersahin A. Uterine sarcoma in a 14 year-old girl presenting with uterine rupture. Gynecol Oncol Rep. 2014;10:44-46.
doi pubmed pmc - Mbatani N, Olawaiye AB, Prat J. Uterine sarcomas. Int J Gynaecol Obstet. 2018;143(Suppl 2):51-58.
doi pubmed - Felix AS, Cook LS, Gaudet MM, Rohan TE, Schouten LJ, Setiawan VW, Wise LA, et al. The etiology of uterine sarcomas: a pooled analysis of the Epidemiology of Endometrial Cancer Consortium. Br J Cancer. 2013;108(3):727-734.
doi pubmed pmc - Kohler G, Vollmer M, Nath N, Hessler PA, Dennis K, Lehr A, Koller M, et al. Benign uterine mass-discrimination from leiomyosarcoma by a preoperative risk score: a multicenter cohort study. Arch Gynecol Obstet. 2019;300(6):1719-1727.
doi pubmed - Nagai T, Takai Y, Akahori T, Ishida H, Hanaoka T, Uotani T, Sato S, et al. Novel uterine sarcoma preoperative diagnosis score predicts the need for surgery in patients presenting with a uterine mass. Springerplus. 2014;3:678.
doi pubmed pmc - Zhang G, Yu X, Zhu L, Fan Q, Shi H, Lang J. Preoperative clinical characteristics scoring system for differentiating uterine leiomyosarcoma from fibroid. BMC Cancer. 2020;20(1):514.
doi pubmed pmc - Brohl AS, Li L, Andikyan V, Obican SG, Cioffi A, Hao K, Dudley JT, et al. Age-stratified risk of unexpected uterine sarcoma following surgery for presumed benign leiomyoma. Oncologist. 2015;20(4):433-439.
doi pubmed pmc - Perez-Fidalgo JA, Ortega E, Ponce J, Redondo A, Sevilla I, Valverde C, Isern Verdum J, et al. Uterine sarcomas: clinical practice guidelines for diagnosis, treatment, and follow-up, by Spanish Group for Research on Sarcomas (GEIS). Ther Adv Med Oncol. 2023;15:17588359231157645.
doi pubmed pmc - Harter P, El-Khalfaoui K, Heitz F, du Bois A. Operative and conservative treatment of uterine sarcomas. Geburtshilfe Frauenheilkd. 2014;74(3):267-270.
doi pubmed pmc - Schoffski P, Kubickova M, Wozniak A, Blay JY, Strauss SJ, Stacchiotti S, Switaj T, et al. Long-term efficacy update of crizotinib in patients with advanced, inoperable inflammatory myofibroblastic tumour from EORTC trial 90101 CREATE. Eur J Cancer. 2021;156:12-23.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
