| Journal of Clinical Gynecology and Obstetrics, ISSN 1927-1271 print, 1927-128X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Gynecol Obstet and Elmer Press Inc |
| Journal website https://www.jcgo.org |
Case Report
Volume 13, Number 2, June 2024, pages 54-57
Rare Fetal Case of Absent Ductus Venosus With Extrahepatic Drainage Into the Coronary Sinus
Nicole Seyfrieda, c, Yevgenia Y. Fominaa, Mansi Gaitondeb, Rafael Levya, Elaine Duryeaa
aDivision of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX, USA
bDivision of Pediatric Cardiology, Department of Pediatrics, University of Texas Southwestern Medical Center, Dallas, TX, USA
cCorresponding Author: Nicole Seyfried, Division of Maternal-Fetal Medicine, Department of Obstetrics and Gynecology, University of Texas Southwestern Medical Center, Dallas, TX 75235, USA
Manuscript submitted April 11, 2024, accepted June 17, 2024, published online June 30, 2024
Short title: Fetal ADV With Drainage Into the CS
doi: https://doi.org/10.14740/jcgo970
| Abstract | ▴Top |
Absent ductus venosus (ADV) is a rare anomaly that is often associated with significant fetal anomalies and chromosomal aneuploidies. Prognosis depends on the pattern of drainage of the umbilical vein and the associated anomalies. The ductus venosus carries oxygenated blood from the umbilical vein to the inferior vena cava and prevents fetal high output heart failure by providing a resistor against placental blood flow. This case demonstrates echocardiographic delineation of a rare variant of ADV, with extrahepatic drainage from the left superior vena cava into the coronary sinus.
Keywords: Absent ductus venosus; Umbilical vein; Coronary sinus
| Introduction | ▴Top |
The ductus venosus (DV) is an embryologic vessel carrying placental oxygenated blood to the fetal right heart. It branches from the umbilical vein, traverses the liver, and empties into the inferior vena cava (IVC) [1]. Within fetal circulation, oxygenated blood flows from the placenta through the umbilical vein to the DV [2]. The DV contains smooth muscle, elastic connective tissue and a sphincter at the origin of the DV that serves as a fetal resistor against placental blood flow. While the true incidence of an absent ductus venosus (ADV) is unknown, it is identified in approximately 0.6% of fetuses referred for a fetal echocardiogram [3]. In cases of ADV, the insertion of the umbilical vein may be described as intrahepatic or extrahepatic [4]. In most patients, the umbilical vein drains directly into the right atrium, however, the umbilical vein may also drain into other vessels such as the IVC, iliac vein, renal vein, or portal circulation [5]. While these alternate pathways may still provide some degree of resistance to combat placental overcirculation, extrahepatic drainage of the umbilical vein has been associated with high-output heart failure, hydrops fetalis, and portal agenesis [6]. Reported infant outcomes are variable and may be dependent on the course of the aberrant drainage. Here, we present a rare case of ADV that highlights the fetal and postnatal course of ADV with a posterior extrahepatic course to a left superior vena cava, ultimately draining into the coronary sinus.
| Case Report | ▴Top |
Investigations
A 19-year-old woman, gravida 1, presented to our facility for a routine obstetric ultrasound at 25 weeks of gestation. ADV with suspected extrahepatic drainage to a dilated coronary sinus was diagnosed, and the patient was referred for evaluation by maternal fetal medicine and fetal cardiology. Fetal echocardiography at 30 weeks demonstrated ADV with an extrahepatic, aberrant course of the umbilical vein posteriorly within the fetal abdomen. Careful interrogation of the large vessel demonstrated no significant resistance to flow as it coursed superiorly along the posterior leftward thorax, draining into the left superior vena cava and finally into the profoundly dilated coronary sinus (Fig. 1). There was severe cardiomegaly with right atrial and right ventricular dilation. The right ventricular systolic function appeared low normal with a Tei index of 0.54, the left ventricular systolic function was mildly diminished with a Tei index of 0.67 and shortening fraction of 17%. There was severe high-output heart failure with a combined cardiac output calculation around 900 mL/min. There were no significant effusions, biphasic tricuspid valve inflow Doppler, and normal umbilical artery and venous Dopplers, though there was periodically visualized retrograde flow in the aortic isthmus The umbilical cord had an unusual thickened, dilated appearance on ultrasound.
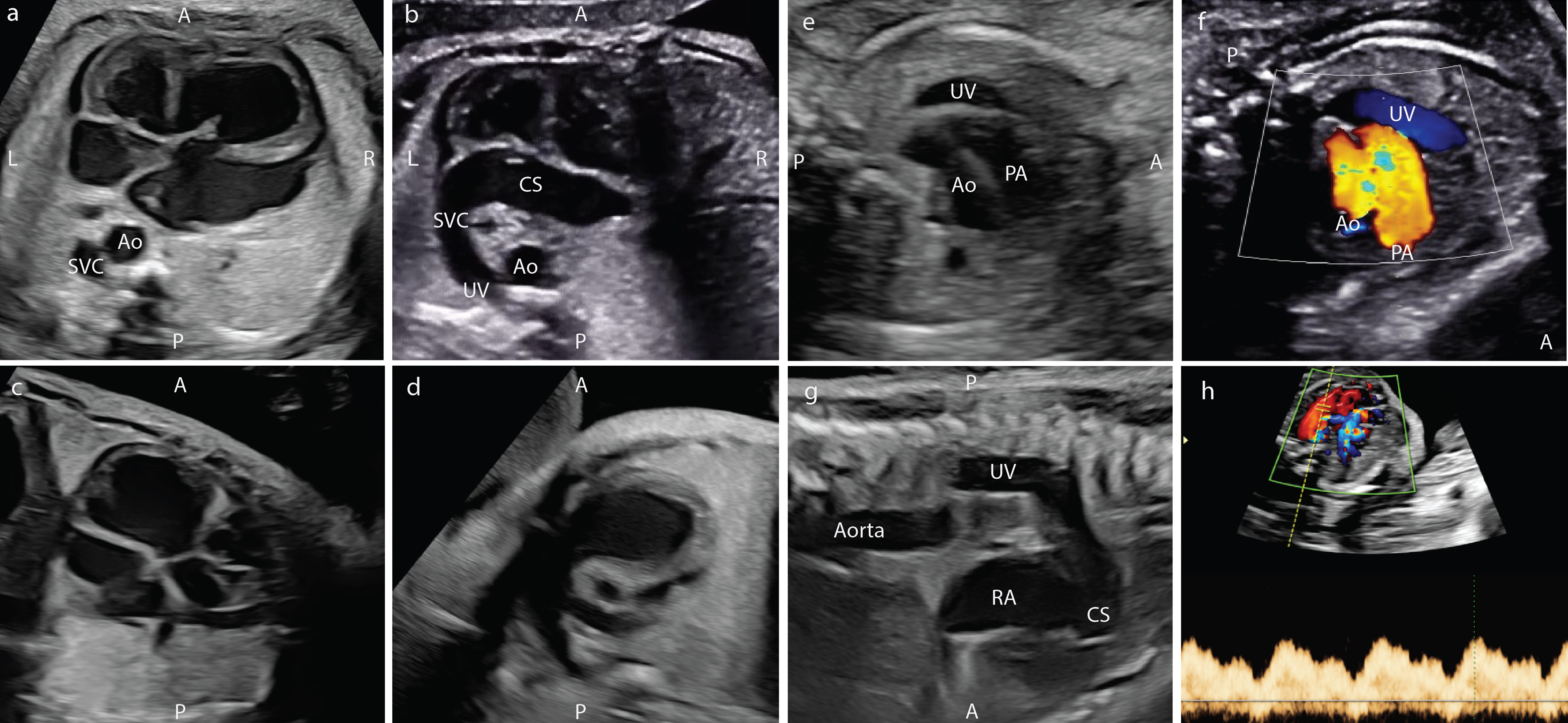 Click for large image | Figure 1. Fetal ultrasound findings in the patient with absent ductus venosus. (a) Four-chamber view with dilated right atrium and ventricle and left SVC. (b) Four-chamber view with dilated coronary sinus connected to left SVC. (c) Left ventricular outflow tract. (d) Right ventricular outflow tract. (e) Three-vessel trachea view with umbilical vein. (f) Three-vessel trachea view with umbilical vein in color Doppler. (g) Sagittal view of left SVC. (h) Doppler of umbilical vein. A: anterior; Ao: aorta; CS: coronary sinus; L: left; LV: left ventricle; PA: pulmonary artery; P: posterior; R: right; RV: right ventricle; SVC: superior vena cava; UV: umbilical vein. |
Diagnosis
The case was discussed in a multidisciplinary conference with fetal cardiology, obstetrics, maternal fetal medicine, social work, genetics, and neonatal intensive care. Antenatal surveillance included weekly outpatient sonographic monitoring for hydrops fetalis, bi-weekly fetal echocardiograms, and monthly rate of growth sonograms. At 26 weeks of gestation, an amniocentesis was performed demonstrating a normal karyotype analysis (46, XY) and a 24 kb deletion in the AH1 gene located on 6q23.3 on chromosomal microarray, corresponding with a fetal carrier status for autosomal recessive Joubert syndrome. No neurologic anomalies were appreciated on ultrasonography and molar tooth sign was not present. The patient denied family history. Follow-up targeted sonograms demonstrated normal fetal growth.
Treatment
At 32 weeks of gestation, maternal digoxin therapy was trialed with the intent to augment fetal cardiac function. Digoxin load was initiated but terminated prematurely when maternal heart block was noted on telemetry. Fetal echocardiography at 34 weeks of gestation demonstrated increasing high output heart failure, with combined cardiac output calculation around 1,200 mL/min. The right ventricular systolic function now appeared mildly diminished and dyskinetic with a monophasic tricuspid valve inflow Doppler pattern and development of trivial pericardial effusion and ascites. Due to these findings, delivery was recommended to prevent progression of fetal hydrops.
The patient underwent induction of labor with resultant uncomplicated spontaneous vaginal delivery. Pediatric resuscitation team was present for delivery of the 2,494 g male infant with Apgar of 8 and 9 at 1 and 5 min of life respectively. No positive pressure ventilation or supplementary oxygen was required. Cord gas pH was 7.27 with a base excess of 6.1. The newborn did not display any obvious signs of malformation. The placenta was grossly normal. Placental pathology revealed a hypercoiled umbilical cord, focal villous edema and increased syncytial knots.
Follow-up and outcomes
A transthoracic echocardiogram was obtained shortly after birth with findings that were consistent with severe cardiomegaly and severe dilation of the right atrium, right ventricle, and coronary sinus. Due to moderately decreased right ventricular systolic function and mild tachypnea, the infant was started on enteral digoxin and furosemide therapy. Within 1 week, the biventricular function appeared to improve, and the finding of a persistent left superior vena cava was confirmed. There was some concern that the tachypnea was also related to pulmonary underdevelopment from the severe cardiomegaly in utero. After 3 weeks of inpatient optimization, the infant was discharged home on room air and outpatient heart failure therapy with enteral digoxin and Lasix. At the 6-month follow-up, the infant had normal growth and milestone development with the plan to wean off cardiac medications.
| Discussion | ▴Top |
Comprehensive review of the literature revealed several retrospective and prospective studies evaluating the incidence, pattern of drainage and associated anomalies and chromosomal abnormalities associated with ADV. A 2006 retrospective review by Berg et al reported that intrahepatic drainage was more common than extrahepatic drainage and had a significantly more favorable prognosis. Additionally, 65% of fetuses with ADV had associated anomalies, and 52% had fetal hydrops [7]. In contrast, Contratti et al found that extrahepatic drainage was more common than intrahepatic drainage. The incidence of major anomalies including chromosomal abnormalities was 24%, and the incidence of hydrops was 33% [8]. Associated anomalies across all reviewed studies included increased nuchal translucency (NT), dilated IVC, polyhydramnios, oligohydramnios, intrauterine growth restriction, cardiomegaly, Dandy-Walker malformation, esophageal atresia, enlarged cisterna magna, Ebstein anomaly, Noonan syndrome, and prune belly syndrome [4, 5, 8-11].
Several prior studies have reported that associated anomalies worsen the prognosis in a fetus with ADV. A 2018 systematic review of 340 ADV cases with associated anomalies concluded increased outcomes of pregnancy termination, intrauterine fetal demise or neonatal death [10]. Similarly, a retrospective study by Thomas et al found that none of the identified cases of isolated ADV had perinatal loss or termination of pregnancy. They conclude that isolated ADV has a favorable outcome regardless of intra- versus extrahepatic pattern of umbilical vein drainage [6]. In 2011, a prospective screening study was performed that looked at the first trimester ultrasound NT measurements. They found that 57% had a NT greater than the 95th percentile. Almost half (42%) were found to have an aneuploidy, the most common being Turner syndrome. Interestingly, 81% of cases with AVD and a normal NT resulted in a live healthy birth [9]. A 2014 review by Chaoui et al described findings associated with a poor prognosis as aneuploidies, syndromes, structural or functional cardiac lesions, hydrops and extrahepatic course of the umbilical vein [12].
In our case, careful attention was given to echocardiographic assessment of ventricular systolic function using the Tei or myocardial performance index, as well as the use of two-dimensional and M-mode calculations of ventricular shortening fraction. With the current fetal echocardiographic ultrasound technology, there are limitations and no clear established guidelines for fetal cardiac functional assessment compared to postnatal transthoracic echocardiography; however, these multiple described echocardiographic methods above can be utilized in the fetus to complement subjective assessments. In addition, calculations of left ventricular, right ventricular, and combined cardiac outputs can better quantify the progression of high-output heart failure throughout pregnancy. It is well known that during most pregnancy, the right ventricular output is dominant, providing around 60% of the combined cardiac output. There is an expected fetal physiologic transition as pregnancy progresses with decline in the relative placental blood flow as the third trimester progresses. The impact of this expected transition in a fetus with ADV is unclear but may suggest that a fetus in the third trimester is at highest risk for development of fetal hydrops.
While there is no fetal interventional treatment for ADV beyond delivery, antepartum medical therapy to assist with fetal contractility can be considered. Digoxin has an unclear specific mechanism for fetal tachyarrhythmias and heart failure but has been shown to improve ventricular contractility in both in infants and adults [13]. Thus far, there have been no cases in the literature evaluating the use of maternal digoxin therapy to treat fetal heart failure secondary to ADV. In this fetus, the trial of maternal digoxin therapy was unable to be adequately evaluated as the therapy was terminated prematurely due to the development of maternal heart block. A retrospective case series published in 2018 revealed that maternal arrhythmias, including heart block, occurred in 18.2% of women undergoing treatment with digoxin alone or with another antiarrhythmic [14]. Ultimately, late preterm delivery appeared to be the best option for this fetus once ventricular dyskinesis, pericardial effusion, and ascites were noted. Fortunately, the infant did not appear to develop any significant complications related to the prematurity status, with a good outcome from a thorough multidisciplinary evaluation and follow-up.
ADV is a rare anomaly that is often associated with significant fetal anomalies and chromosomal aneuploidies. Prognosis depends on the pattern of drainage of the umbilical vein and the associated anomalies. Careful echocardiographic and ultrasound screening should be done with regular attention to markers of worsening fetal heart failure and development of fetal hydrops. Earlier delivery may be necessary to provide the best postnatal outcome for the fetus, with hopes that eventual cardiac recovery will occur over time once the fetal heart is removed from the high output environment.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Nicole Seyfried wrote the manuscript and literature search. Yevgenia Fomina contributed to manuscript editing. Mansi Gaitonde wrote the manuscript. Rafael Levy contributed to image acquisition and interpretation. Elaine Duryea, as a senior author, contributed to manuscript editing.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Cunningham FG KJL, Dashe JS, Hoffman BL, Spong CY, Casey BM. Williams Obstetrics. 26th ed. New York: McGraw Hill; 2022.
- Sidhu PS, Lui F. Embryology, ductus venosus. In: StatPearls. Treasure Island (FL). 2024.
pubmed - Acherman RJ, Evans WN, Galindo A, Collazos JC, Rothman A, Mayman GA, Luna CF, et al. Diagnosis of absent ductus venosus in a population referred for fetal echocardiography: association with a persistent portosystemic shunt requiring postnatal device occlusion. J Ultrasound Med. 2007;26(8):1077-1082.
doi pubmed - Perles Z, Nir A, Nadjari M, Ergaz Z, Raas-Rothschild A, Rein AJ. Absent ductus venosus in the fetus: review of the literature and first report of direct umbilical venous drainage to the coronary sinus. Fetal Diagn Ther. 2003;18(4):247-251.
doi pubmed - Maruotti GM, Saccone G, Ciardulli A, Mazzarelli LL, Berghella V, Martinelli P. Absent ductus venosus: case series from two tertiary centres. J Matern Fetal Neonatal Med. 2018;31(18):2478-2483.
doi pubmed - Thomas JT, Petersen S, Cincotta R, Lee-Tannock A, Gardener G. Absent ductus venosus—outcomes and implications from a tertiary centre. Prenat Diagn. 2012;32(7):686-691.
doi pubmed - Berg C, Kamil D, Geipel A, Kohl T, Knopfle G, Hansmann M, Gembruch U. Absence of ductus venosus-importance of umbilical venous drainage site. Ultrasound Obstet Gynecol. 2006;28(3):275-281.
doi pubmed - Contratti G, Banzi C, Ghi T, Perolo A, Pilu G, Visentin A. Absence of the ductus venosus: report of 10 new cases and review of the literature. Ultrasound Obstet Gynecol. 2001;18(6):605-609.
doi pubmed - Staboulidou I, Pereira S, Cruz Jde J, Syngelaki A, Nicolaides KH. Prevalence and outcome of absence of ductus venosus at 11(+0) to 13(+6) weeks. Fetal Diagn Ther. 2011;30(1):35-40.
doi pubmed - Pacheco D, Brandao O, Montenegro N, Matias A. Ductus venosus agenesis and fetal malformations: what can we expect? - a systematic review of the literature. J Perinat Med. 2018;47(1):1-11.
doi pubmed - Sau A, Sharland G, Simpson J. Agenesis of the ductus venosus associated with direct umbilical venous return into the heart—case series and review of literature. Prenat Diagn. 2004;24(6):418-423.
doi pubmed - Chaoui R, Heling KS, Karl K. Ultrasound of the fetal veins part 1: the intrahepatic venous system. Ultraschall Med. 2014;35(3):208-228.
doi pubmed - Vergani P, Mariani E, Ciriello E, Locatelli A, Strobelt N, Galli M, Ghidini A. Fetal arrhythmias: natural history and management. Ultrasound Med Biol. 2005;31(1):1-6.
doi pubmed - Moatassim S, Touleimat S, Hazelzet T, Brasseur MD, Diguet A, Durand I, Verspyck E. Maternal complications induced by digoxin treatment of fetal tachycardia: A retrospective series of 18 cases. J Gynecol Obstet Hum Reprod. 2018;47(2):35-38.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Gynecology and Obstetrics is published by Elmer Press Inc.
